Abstract
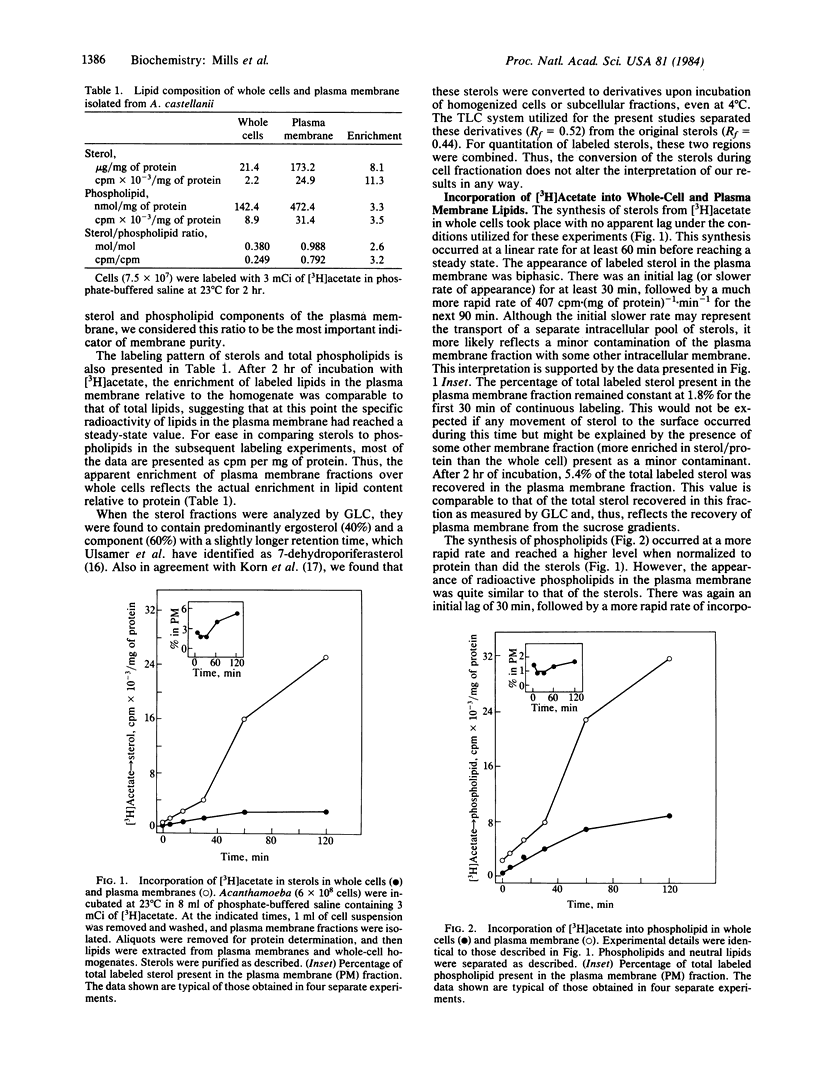
We examined the transfer of sterols and phospholipids from their site of synthesis to the plasma membrane of Acanthamoeba castellanii. Cells were labeled with [3H]acetate, and plasma membrane fractions were isolated under conditions that minimize the nonspecific exchange of lipids between subcellular membrane fractions. Sterols and phospholipids were purified from both whole-cell homogenates and isolated plasma membrane. In whole cells, 3H-labeled lipids were formed, with no apparent time lag, in a linear manner up to 1 hr. Labeled sterol and phospholipids appeared in the plasma membrane, after a 30-min lag, at approximately the same rate. However, the ratio of newly synthesized sterol to phospholipid was significantly enriched in the plasma membrane relative to the whole cell, even at the earlier time points. Pulse-chase experiments indicated that sterols and phospholipids are turned over in the plasma membrane with similar, rather short half-lives. The results of these studies suggest that, although sterols and phospholipids are transported to the cell surface with similar kinetics, some sorting of the lipids must occur at an early stage in membrane biogenesis. The data are consistent with a model of lipid translocation by vesicular transport.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bowers B., Olszewski T. E. Pinocytosis in Acanthamoeba castellanii. Kinetics and morphology. J Cell Biol. 1972 Jun;53(3):681–694. doi: 10.1083/jcb.53.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlapowski F. J., Band R. N. Assembly of lipids into membranes in Acanthamoeba palestinensis. II. The origin and fate of glycerol- 3 H--labeled phospholipids of cellular membranes. J Cell Biol. 1971 Sep;50(3):634–651. doi: 10.1083/jcb.50.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- De Silva N. S., Siu C. H. Vesicle-mediated transfer of phospholipids to plasma membrane during cell aggregation of Dictyostelium discoideum. J Biol Chem. 1981 Jun 10;256(11):5845–5850. [PubMed] [Google Scholar]
- DeGrella R. F., Simoni R. D. Intracellular transport of cholesterol to the plasma membrane. J Biol Chem. 1982 Dec 10;257(23):14256–14262. [PubMed] [Google Scholar]
- Korn E. D. The isolation of the amoeba plasma membrane and the use of latex beads for the isolation of phagocytic vacuole (phagosome) membranes from amoebae including the culture techniques for amoebae. Methods Enzymol. 1974;31:686–698. doi: 10.1016/0076-6879(74)31074-9. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Ulsamer A. G., Weihing R. R., Wetzel M. G., Wright P. L. The enzymatic aromatization of the B ring of delta5,7-sterols. Biochim Biophys Acta. 1969 Dec 17;187(4):555–563. doi: 10.1016/0005-2760(69)90053-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morré D. J., Kartenbeck J., Franke W. W. Membrane flow and intercoversions among endomembranes. Biochim Biophys Acta. 1979 Apr 23;559(1):71–52. doi: 10.1016/0304-4157(79)90008-x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight R. G., Pagano R. E. Rapid appearance of newly synthesized phosphatidylethanolamine at the plasma membrane. J Biol Chem. 1983 Aug 10;258(15):9050–9058. [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Ulsamer A. G., Smith F. R., Korn E. D. Lipids of Acanthamoeba castellanii. Composition and effects of phagocytosis on incorporation of radioactive precursors. J Cell Biol. 1969 Oct;43(1):105–114. doi: 10.1083/jcb.43.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsamer A. G., Wright P. L., Wetzel M. G., Korn E. D. Plasma and phagosome membranes of Acanthamoeba castellanii. J Cell Biol. 1971 Oct;51(1):193–215. doi: 10.1083/jcb.51.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W. Transfer of phospholipids between membranes. Biochim Biophys Acta. 1974 Sep 16;344(2):95–117. doi: 10.1016/0304-4157(74)90001-x. [DOI] [PubMed] [Google Scholar]



