Abstract
Purpose
To compare the efficacy of topical cefazolin-gentamicin versus vancomycinceftazidime for treatment of bacterial corneal ulcers.
Methods
This randomized double-masked clinical trial was performed on consecutive patients with bacterial corneal ulcers referred to Feiz Hospital, Isfahan, Iran from 2004 to 2005. Patients were randomly assigned to cefazolin-gentamicin or vancomycin-ceftazidime eye drops in a masked fashion. Outcome measures included time for resolution of stromal infiltration, re-epithelization of the epithelial defect, and clearance of anterior chamber inflammation as well as culture results and complications.
Results
The study included 89 eyes of 89 patients with bacterial corneal ulcers consisting of 57 (64%) male and 32 (36%) female subjects. Specimens were culturenegative in 46% of cases. Forty-one eyes received cefazolin-gentamicin and 48 eyes were treated with vancomycin-ceftazidime. Time for resolution of stromal infiltration was 17.7± 4.3 days versus 13.8± 3.6 days (P=0.04), time to complete re-epithelization was 13.2± 3.1 days versus 9.6± 2.7 days (P=0.01) and time for clearing of the anterior chamber was 11.6± 2.9 days versus 8.1± 2.3 days (P=0.04) in the cefazolin-gentamicin and vancomycin-ceftazidime groups, respectively. The most common complaint related to the medications was ocular burning in 73.1% of patients treated with cefazolingentamicin and 62.9% of cases receiving vancomycin-ceftazidime (P=0.007).
Conclusion
Vancomycin-ceftazidime eye drops seem to be more effective than cefazolin-gentamicin eye drops for the treatment of bacterial corneal ulcers and are probably better tolerated locally.
Keywords: Corneal Ulcer, Cefazolin, Gentamicin, Vancomycin, Ceftazidime
INTRODUCTION
Bacterial keratitis is a sight threatening condition which may rapidly progress to corneal perforation or endophthalmitis if left untreated and is considered an ocular emergency.1 Risk factors for bacterial keratitis include contact lens wear, trauma, contaminated eye drops,and defects in defense mechanisms or anatomical disorders of the cornea.2
The major micro-organisms associated with bacterial keratitis in normal corneas include Pseudomonas aeruginosa, Streptococcus pneumoniae, Moraxella catarrhalis, beta-hemolytic Streptococcus and Klebsiella pneumoniae;however, S.aureus is the most common patho gen in compromised corneas. The most prevalent organisms in contact lens wearers are P. aeruginosa, S. aureus and S. epidermidis.3
Cephalosporins are semi-synthetic, penicillin-like bactericidal agents which interfere with bacterial cell wall synthesis. They act against staphylococci and penicillinase-producing Streptococci as well as some gramnegative bacteria especially Escherichia coli,Proteus and Klebsiella. Third and fourth generation cephalosporins are also effective against Pseudomonas species.3Ceftazidime is the only third generation cephalosporin which demonstrates good activity against Pseudomonas species.2 Gentamicin is also an effective agent against Staphylococci and some species of Streptococci other than S. pneumoniae. It inhibits bacterial protein synthesis via binding to RNA polymerase.1
This study was undertaken to compare the efficacy of vancomycin-ceftazidime eye drops with that of cefazolin-gentamicin eye drops for treatment of bacterial corneal ulcers.
METHODS
This randomized double-blind clinical trial was conducted on consecutive patients with a clinical diagnosis of bacterial corneal ulcers referred to Feiz Hospital, Isfahan, Iran from April 2005 to April 2006. After obtaining bacteriological specimens, the patients were randomly assigned to receive either vancomycinceftazidime or cefazolin-gentamicin eye drops.Patients suspected of herpetic or fungal ulcers based on clinical impression or lack of response to antibacterial therapy, those with non-bacterial ulcers documented by microbiologic tests, patients who developed drug reactions or complications such as corneal perforation or descemetoceles,and those who underwent additional therapeutic measures such as subconjunctival antibiotics or a conjunctival flap were excluded from the study.
Samples were obtained for bacteriological studies including direct smear and culture on blood agar, chocolate agar and trypticase soy broth (TSB) media from both eyes before initiation of treatment. Specimens were obtained from the lid margins and inferior fornix as well as from the container and solution in contact lens wearers. Following instillation of tetracaine 0.5% eye drops, a tissue specimen was obtained from the corneal ulcer using Kimura platinum spatula or a bent #27 needle. The latter specimen was obtained for gram staining and culturing on blood agar, chocolate agar and TSB media. The direct smear was stained immediately after sampling by one of the authors (FF). A laboratory technician evaluated bacterial cultures following 24 hours of incubation at 37°C and 48 hours thereafter, if no growth had occurred. Fungal cultures were incubated for 4 additional days at room temperature.
All patients were hospitalized for the diagnostic procedures, initiation of treatment and monitoring response to therapy. The concentration of the antibiotic drops was 50 mg/ml for vancomycin, ceftazidime and cefazolin, and 14 mg/ml for gentamicin. Following an initial loading dose of 1–2 drops every 5 minutes for the first 30 minutes, the drops were administered every 1 hour up to 3 days, every 2 hours on days 4 and 5, and every 4 hours from days 6 to 14. The drops were instilled by experienced staff and the patients were trained to digitally compress the medial canthus for 2 minutes after instillation of the eye drops. Treatment was stopped when the epithelial defect was completely healed, and the stromal infiltration and anterior chamber inflammatory reaction had resolved. Patients were examined daily during the admission period and every other day thereafter until corneal epithelium was healed. Study data included age, sex, drug history, previous history of ocular or systemic disease, best-corrected visual acuity (BCVA),site and depth of the ulcer, size of epithelial defect, and anterior chamber reaction.
RESULTS
During the study period, 134 patients with corneal ulcers including 82 male (61.2%) and 52 female (38.8%) subjects were enrolled, of whom 45 patients including 24 (53.3%) subjects in the cefazolin-gentamicin group and 21 (46.7%) cases in the vancomycin-ceftazidime group were excluded. Eventually, data from 89 eyes of 89 patients consisting of 41 eyes of 41 patients including 25 (61.0%) male and 16 (36.0%) female subjects in the cefazolin-gentamicin group, and 48 eyes of 48 patients including 32 (67.0%) male and 16 (33.0%) female subjects in the vancomycin-ceftazidime group were included for final analysis.
Predisposing factors for bacterial corneal ulcers included corneal surface disorders such as neurotrophic conditions, bullous keratopathy and dry eye (46.0%), contact lens wear (24.7%), prior corneal surgery (7.8%), and trauma (6.7%). However, no predisposing factor was present in 14.8%.
Mean BCVA (when refraction was feasible) was 0.2± 0.04 logMAR (counting fingers to 7/10) in the cefazolin-gentamicin group and 1.8± 0.04 logMAR (counting fingers to 9/10) in the vancomycin-ceftazidime group (P=0.11).
The corneal ulcer involved less than 1/3 of corneal thickness in 39 (43.8%) patients, 1/3 to 1/2 of the cornea in 41 (46%) cases and more than 1/2 of the corneal thickness in 9 (10.2%) subjects. Mean depth of stromal infiltration was 38%± 13% in the cefazolin-gentamicin group versus 41%± 15% in the vancomycin-ceftazidime group (P=0.79). The distribution of corneal ulcers by size (P=0.786) and degree of anterior chamber inflammation was comparable in the study groups (Table 1 and Table 2).
Table 1.
Corneal ulcer size in the study groups
| size (mm) | Groups:No(%) | ||
|---|---|---|---|
|
| |||
| Cefazolin-Gentamicin | Vancomycin-Ceftazidime | Total | |
| <3 | 11 (26.8) | 16 (33.3) | 27 (30.3) |
| 3–5 | 15 (36.6) | 17 (35.4) | 32 (36.0) |
| 5–7 | 10 (24.4) | 8 (16.7) | 18 (20.0) |
| ≥7 | 5 (12.2) | 7 (14.6) | 12 (13.5) |
|
| |||
| Total | 41 (100) | 48 (100) | 89(100) |
Chi-square test, P=0.786
Table 2.
Degree of anterior chamber inflammation in the studygroup
| Groups:No(%) | ||
|---|---|---|
|
| ||
| Cefazolin-Gentamicin | Vancomycin-Ceftazidime | |
| Reaction | ||
| 1+ to 3+ | 14 (34.2) | 16 (33.3) |
| 4+ | 10 (24.4) | 13 (27.1) |
| Hypopyon | ||
| <1 mm | 8 (19.5) | 8 (16.7) |
| 1–3 mm | 6 (14.6) | 7 (14.6) |
| >3 mm | 3 (7.3) | 4 (8.3) |
|
| ||
| Total | 41 (100) | 48 (100) |
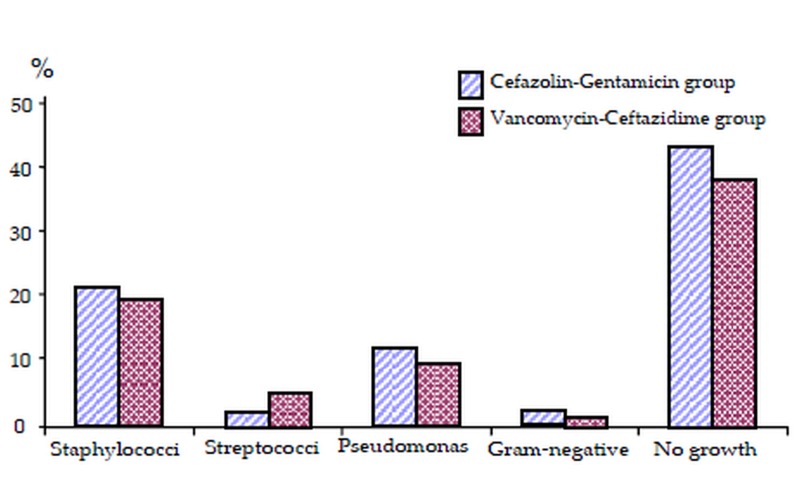
Overall 53.9% of enrolled eyes, including 51.1% in the cefazolin-gentamicin group and 56.2% in the vancomycin-ceftazidime group (P=0.63), were culture-positive. The most common isolated organisms were S. aureus (24.7%) and Pseudomonas (14.6%) (Fig. 1).
Figure 1.
Isolated bacterium from the culture specimens.
Treatment with vancomycin-ceftazidime was superior to cefazolin-gentamicin in terms of epithelial healing, resolution of stromal infiltration and clearing of anterior chamber reaction.Mean time for epithelial healing was 13.2± 3.1 ( 6–23) days versus 9.6± 2.7 (range 3–17) days (P=0.01); mean time for resolution of the stromal infiltrate was 17.7± 4.3 (range 9–29) days and 13.8± 3.6 (range 6–22) days (P=0.021); and clearing of the anterior chamber took a mean of 11.6± 2.9 (range 6–20) days versus 8.1± 2.3 (range 3–14) days (P=0.04) in the cefazolin-gentamicin and vancomycin-ceftazidime groups, respectively.
The most common adverse effect of treatment was burning sensation which was reported by 73% and 63% of patients in the cefazolingentamicin and vancomycin-ceftazidime groups, respectively (P=0.007).
DISCUSSION
The most common micro-organisms isolates in the current study were S. aureus (24.7%) and Pseudomonas (14.6%) which is similar to the results reported by Fazel et al4 (S. aureus 41% and Pseudomonas 27%), and Foroutan et al5 (S.aureus 43%). However, the most common pathogen has been S. epidermidis in studies by Nejabat et al6 (43.3%), and Jahadi et al7 (50%).According to the above-mentioned studies Staphylococci and Pseudomonas seem to be the most common pathogens associated with bacterial corneal ulcers.
Several studies have evaluated the sensitivity of these micro-organisms to different antibiotics, however we are unaware of any clinical trial comparing different combinations of antibiotics in the treatment of corneal ulcers.Wang et al8 demonstrated that vancomycinceftazidime is the most effective combination in terms of coverage against bacteria. In a study by Jahadi et al7 isolated bacteria from corneal ulcers were sensitive to gentamicin in 65%, to cefazolin in 62.5% and to one of them in 85%.The same authors reported that 40% of isolated Pseudomonas species were resistant to both cefazolin and gentamicin. Diaze Valle and coworkers9 reported a case of corneal abscess due to Pseudomonas which was resistant to ciprofloxacin but was successfully treated using ceftazidime eye drops. Robinson et al10 reported 12 patients with corneal ulcers due to Pseudomonas resistant to fortified cefazolingentamicin,but all were successfully treated using ceftazidime-gentamicin. They concluded that replacing cefazolin by ceftazidime in combination with gentamicin is very effective for treatment of keratitis due to Pseudomonas resistant to aminoglycosides. Eiferman et al11 reported 3 cases of corneal ulcers due to methicillin-resistant S. aureus (MRSA) which were resistant to fortified aminoglycoside and cephalosporin drops and only sensitive to vancomycin.They recommended using vancomycin eye drops in cases of MRSA and S. epidermidis.Aliprandis et al12 found that vancomycin (50 mg/ml) is as effective as moxifloxacin against MRSA.
As seen in our series and many others,Pseudomonas is among the principal pathogens associated with corneal ulcers and ceftazidime is the only third generation cephalosporins with good coverage against it. Therefore it is prudent to combine ceftazidime with other antibiotics for treatment of corneal ulcers. The present study revealed that vancomycin-ceftazidime is more effective than cefazolin-gentamicin for treatment of bacterial corneal ulcers such that the mean duration of treatment was significantly (4 days) shorter with the former regimen. Mean duration of treatment for bacterial corneal ulcers with cefazolin-gentamicin has been reported to be 17.3± 9.5 days by Karimian et al,13 which is comparable to our study, and 15.5± 3.9 days using cefazolintobramycin in the study by Khokhar et al.14
In summary, vancomycin-ceftazidime eye drops seem to be superior to cefazolin-gentamicin eye drops for treatment of bacterial corneal ulcers in terms of epithelial healing,resolution of stromal infiltrates and clearing of anterior chamber inflammation. Considering the fact that the principal pathogens associated with bacterial corneal ulcers, namely S. aureus and Pseudomonas, are more sensitive to vancomycin and ceftazidime respectively, it is prudent to use this combination instead of cefazolin-gentamicin as empiric therapy for presumed bacterial corneal ulcers.
REFERENCES
- 1.Arffa RC. Gragson’s disease of cornea. 4th ed. St.Louis: Mosby; mosby; New York: 1997. [Google Scholar]
- 2.American Academy of Ophthalmology. Basic and clinical science course:external disease and cornea. Philadelphia: The Acaademy; 2002–2003. pp. 168–174. [Google Scholar]
- 3.Nicola F. Non-viral infectious keratitis: predisposing factors, etiologic agents and laboratory diagnosis. Rev Argent Microbiol. 2005;37:229–239. [PubMed] [Google Scholar]
- 4.Fazel F, Ramezanzadeh M. Comparision of ciprofloxacin with fortified gentamicin-cefazolin drugs in treatment of bacterial keratitis. Bina J Ophthalmol. 1998;4:226–334. [Article in Farsi] [Google Scholar]
- 5.Forutan AR, Parvaresh MM, Arz-Peyma S, Mirsamadi M, Modarreszadeh M, Ansari MR. Review of predisposing, etiologic and epidemiologic factors of patients with corneal ulcer in Rasool Akram hospital. Bina J Ophthalmol. 1998;3:210–217. [Article in Farsi] [Google Scholar]
- 6.Nejabat M, Razaghinejad MR, Alborzi A. Comparison of medical effect of ciprofloxacin 3% versus cefazolin-gentamicin eye drop for bacterial corneal ulcers. Feiz Periodical. 2002;6:1–9. [Article in Farsi] [Google Scholar]
- 7.Jahadi-Houseini HR, Ghasemi Z, Attarzadeh A, Katbab A, Khoshniat H, Movahhedan H, et al. In Vitro Susceptibility of Bacterial Keratitis Pathogens to Five Antibiotics. Bina J Ophthalmol. 2002;1:22–32. [Article in Farsi] [Google Scholar]
- 8.Wang AG, Wu CC, Liu JH. Bacterial corneal ulcer: a multivariate study. Ophthalmologica. 1998;212:126–132. doi: 10.1159/000027291. [DOI] [PubMed] [Google Scholar]
- 9.Díaz Valle D, Alós Cortés JI, Arteaga Sánchez A, Toledano Fernández N, Poza Morales Y, Díaz-Valle T. Pseudomonas aeruginosa corneal abscess refractory to fluoroquinolones. Arch Soc Esp Oftalmol. 2002;77:397–399. [PubMed] [Google Scholar]
- 10.Robinson A, Kremer I, Avisar R, Gaton D, Savir H, Yassur Y. The combination of topical ceftazidime and aminoglycosides in the treatment of refractory pseudomonal keratitis. Graefes Arch Clin Exp Ophthalmol. 1999;237:177–180. doi: 10.1007/s004170050215. [DOI] [PubMed] [Google Scholar]
- 11.Eiferman RA, O,Neill KP, Morrison NA. Methicillin-resistant Staphylococcus aureus corneal ulcers. Ann Ophthalmol. 1991;23:414–415. [PubMed] [Google Scholar]
- 12.Aliprandis E, Ciralsky J, Lai H, Herling I, Katz HR. Comparative efficacy of topical moxifloxacin versus ciprofloxacin and vancomycin in the treatment of P.aeruginosa and ciprofloxacin-resistant MRSA keratitis in rabbits. Cornea. 2005;24:201–205. doi: 10.1097/01.ico.0000134462.88535.d0. [DOI] [PubMed] [Google Scholar]
- 13.Karimian F, Javadi MA, Babai M, Einollahi B, Zare M. Comparison of ciprofloxacin 0.3% with fortified topical gentamicin and cefazolin in treatment of bacterial keratitis. Bina J Ophthalmol. 1998;4:235–242. [Article in Farsi] [Google Scholar]
- 14.Khokhar S, Sindhu N, Mirdha BR. Comparison of topical 0.3% ofloxacin to fortified tobramycincefazolin in the therapy of bacterial keratitis. Infection. 2000;28:149–152. doi: 10.1007/s150100050068. [DOI] [PubMed] [Google Scholar]