Abstract
The ocular surface consists of two distinct types of epithelial cells; conjunctival and corneal. Although anatomically continuous, these epithelia comprise two distinct cell populations. Corneal stem cells are located at the limbus. The microenvironment of the limbus is important in maintaining “stemness” of the stem cells and also acts as a barrier to conjunctival epithelial cells preventing them from migration onto the corneal surface.Damage to the limbus results in varying degrees of limbal stem cell deficiency with characteristic clinical features including conjunctivalization of the cornea. Regenerative management of corneal conjunctivalization utilizing stem cells comprises of two approaches; limbal auto- or allografts by using existing stem cells and induction and regeneration of ocular tissues from embryonic stem cells. Herein, we review stem cells and limbal stem cells in particular, types of epithelial cells in the cornea, markers of corneal epithelial cells in different stages, as well as the current approach to corneal epithelial regeneration.
Keywords: Stem Cells, Limbus Corneae, Epithelium, Corneal
INTRODUCTION
There are several examples in the animal kingdom of the ability to regenerate tissues following injury. The newt, with its remarkable capacity to regenerate whole limbs, is one of the best illustrations. As humans we are unable to regenerate whole organs; however, we are able to replace certain tissues lost during normal wear and tear such as our hair and epidermis.We are also able to initiate a wound healing response following trauma and if the initial injury is not too severe, we can even replace parts of our liver, small intestine and blood cells. Our ability to replace these tissues is largely related to a small population of stem cells that have the capacity to self-renew and differentiate along specified molecular pathways lifelong. Stem cells are the keys to the maintenance of tissue integrity throughout the body,including the eye and cornea.1
The corneal surface is the most specialized of the body surfaces and in a constant state of cell renewal and regeneration. Cells in the outermost layer of the corneal epithelium are continuously desquamated and must be replaced by cell proliferation. There are 3 major cell types in the cornea: epithelial cells, corneal fibroblasts and endothelial cells.2
Corneal epithelium is non-keratinized, stratified and squamous, and of ectodermal origin.Together with the peripheral limbal epithelium and conjunctival epithelium, it covers the ocular surface. The main function of the corneal epithelium is to serve as a protective barrier against fluid loss and penetration of pathogens.3,4
The corneal stroma plays a major role in all main functions of the mature cornea: protecttion,and transmittance and refraction of light.All these functions are related to the highly organized extracellular matrix which is formedprimarily by aligned bundles of collagen termed lamellae which are stacked in an orthogonal pattern.5
The corneal endothelium functions to maintain the stroma in a relatively dehydrated state.This function depends on the presence of a barrier formed by focal tight junctions preventing fluid flow and on a pumping action provided by Na+/K+ ATPase and Mg++ dependent bicarbonate enzymes present in the lateral membranes of the endothelial cells.6
CORNEAL ORGANOGENESIS IN HUMANS
The development of cornea as a tissue initiates by derivation from the ectoderm overlying the crystalline lens as early as five weeks in the human embryo. In brief, a primitive two-cell layer thick epithelium is first apparent at about five weeks which is contiguous with the surface ectoderm. During the next one to two weeks,the epithelium stratifies to 3 to 4 cell layers, the lens completes its formation and detaches from the ectoderm, and the eyelids form and fuse.Almost immediately after separation of the lens from the corneal epithelium, waves of neural crest cells migrate into the space between the lens and epithelium. These cells become the corneal endothelium and the stromal keratocytes.Corneal development continues gradually until the time of eyelid opening, which is associated with major developmental changes.7
At the time of birth, approximately 20% of epithelial and stromal cells and 12% of endothelial cells are actively progressing through the cell cycle. By the time of eyelid opening, the number of cells actively proliferating in both the stroma and endothelium has decreased to nearly zero. This low level of proliferation will be maintained throughout life. In contrast, the level of proliferation increases noticeably in the corneal epithelium and peaks after eyelid opening with almost 75% of the basal cells actively proliferating. The burst of proliferation correlates well with the stratification of the epithelium.8
STEM CELLS IN THE EYE
Several unique inherent properties, discussed below, enable stem cells to accomplish their important task to maintain tissue integrity throughout the body.
Error-free proliferation:Error-free mitosis is essential since any genetic error at the level of the stem cell will continuously and permanently pass on to the whole clone of cells,resulting in abnormal differentiation and cellular dysfunction. To minimize any error produced during stem cell mitosis, several protective mechanisms have been developed. First, stem cells are relatively quiescent during the state of steady growth. They leave the job of active DNA synthesis and cell number amplification to transient amplifying cells (TACs). So that even if an error is made at the TAC level it will be self-limited because all cells except stem cells have a limited lifespan. In cell kinetic terms, stem cells have a longer cell cycle time (180 vs 90 hours) and a shorter S-phase duration (2–3 vs 9–21 hours) as compared to TACs in the case of skin epidermis.9 Secondly, Potten et al10 demonstrated that there is asymmetrical DNA segregation during stem cell mitosis,suggesting that stem cells retain their original genetic message during mitosis, passing only the new copy onto TACs.
Poor differentiation:It has been emphasized that the concept of “stemness” excludes further differentiation as a necessary property.Therefore, it has long been recognized that the cytoplasm of stem cells appears primitive and contains few, if any, differentiation products. If differentiation is envisioned as reprogramming of the genome, then the process of differentiation also means removal of cells from the stem cell population. Therefore, a stem cell having responded to a differentiation stimulus is out of the stem cell population. Two possible mechanisms can explain how a differentiation event is induced. First, a full mitotic cell cycle is needed to produce an asymmetrical cell division into two different daughter cells. One will remain a stem cell (self-renewal), while the other is destined for cellular differentiation.Second, a full cell cycle may not occur; instead,differentiation stimuli affect stem cells at the G0 state, in which most stem cells are during steady state growth.10 ,11 Affected cells will be removed from the stem cell population by virtue of the change induced by the differentiation process, and will not enter the stem cell mitotic cell cycle.
Long lifespan:The stem cell lifespan may be equivalent to the lifetime of the organism in which they reside.12
Long cell cycle time or slow cycling:This property indicates low mitotic activity.Although stem cells are capable of high proliferative potential, under steady-state conditions,they exhibit extremely low rates of proliferation.12
-
Symmetric and asymmetric division:In terms of daughter cell fate, stem cell division is either symmetric or asymmetric. When cell division is obligatorily asymmetric, one of the daughter cells remains similar to its parent and serves to replenish the stem cell pool, whereas the other daughter cell is destined to divide and differentiate with acquisition of features that characterize the specific tissue. Such a cell is called a TAC and is less primitive than its parent stem cell. On the other hand, the asymmetry in division perhaps induces otherwise similar daughter cells to behave differently.Finally, all divisions of the stem cell may be symmetric, but are self-renewing only half of the time.13
Two opposing theories exist about the origin of the corneal epithelium. One states that it is derived from the adjacent conjunctiva by conjunctival transdifferentiation while the other proposes that the origin of the corneal epithelium is corneal stem cells in the limbal basal epithelium.In the first theory, conjunctival epithelial ingrowths into denuded cornea following large epithelial wounds14 differentiates into corneal epithelium; the process is described by the term “conjunctival transdifferentiation”.15 However, there are several pieces of experimental evidence against the above mentioned assumption: Wei et al16 demonstrated in culture media that corneal and limbal cells synthesize identical keratins, including large amounts of K3 and K12 markers of corneal-type differentiation. By contrast, the conjunctival epithelium produced another keratin pattern with large amounts of simple epithelial keratins but only minimal amounts of K3-K12 keratin,indeed, the glycogen content and several other biochemical properties of conjunctiva-derived corneal epithelium remain abnormal long after completion of transdifferentiation.17 The conjunctiva-derived corneal epithelium can respond to corneal vascularization by forming goblet cells and by expressing immunoglobulin A secretory component; two markers of normal conjunctival epithelium.18 Although conjunctiva-derived corneal epithelium appears normal on light microscopic studies, electron microscopic studies showed that the epithelium has much wider intercellular spaces than the corneal epithelium.9 It was also found that, in humans, the conjunctiva-derived corneal epithelium is frequently associated with persistent epithelial defects, recurrent erosions, stromal vascularization, necrosis and slow healing rates.14
Taken together, these data strongly suggest that limbal-corneal epithelium and conjunctival epithelium represent two separate sets of cell lineage that are essentially different. These data also indicate that conjunctival transdifferentiation does not represent a true conversion of a differentiated corneal phenotype but rather describes an environmental modulation of the conjunctival epithelium.14
CORNEAL STEM CELLS LOCALIZATION
Due to the lack of a stem cell marker, the localization of limbal stem cells has been based on clinical and laboratory evidence supporting the location of corneal epithelial stem cells at the limbal region. There are six sources of evidence that identify the basal layer of limbal epithelium as the one harboring stem cells for the corneal epithelium:
Pigment migration studies:In 1971, for the first time, it was proposed that the papillary structures (palisades of Vogt) at the basal layer of the limbal epithelium are generative organs for corneal epithelial cells. They demonstrated that in guinea pig eyes, where the basal layer of the limbal epithelium is pigmented, the cornea healed with pigmented epithelium when the normally non-pigmented corneal epithelium was removed.19,20
Radiolabelled thymidine studies:Using this method in 1989, Cotsarelis and co-workers20 demonstrated that basal cells of the limbal epithelium are normally slow cycling in nature, but can be made to cycle much more rapidly when the central corneal epithelium is damaged.
Immunohistochemical studies:Initial immunohistochemical data suggested that limbal basal cells have the lowest level of differentiation among all corneal epithelial cells. Keratins are a group of water-insoluble cytoskeletal proteins that form the desmosome-associated 10-nm intermediate filament in almost all epithelia.21 Perhaps the most important keratin type for ocular surface epithelia is the K3–K12 pair, which is synthesized by corneal and some oral mucosal epithelium but only in small amounts in conjunctival epithelia.19,22
High proliferative capacity of limbal epithelium in cultures:Limbal basal epithelial cells have higher proliferative potential in cell cultures than central and peripheral corneal epithelial cells. Limbal basal cells respond to central corneal wounds and to tumor-promoting agents by undergoing greater proliferation than central corneal epithelial cells, which terminate proliferation-initiating differentiation.14
Corneal conjunctivalization:When the limbal epithelium is partially or completely removed,a spectrum of corneal surface abnormalities occur which are characterized by conjunctival epithelial ingrowth (conjunctivalization),vascularization, and chronic inflammation,which can be explained by limbal stem cell deficiency.23
Corneal epithelial neoplasm:The limbal location of corneal epithelial stem cells could account for the relative preponderance of limbal neoplasms and the scarcity of corneal epithelial tumors.3
-
Corneal regeneration:A mathematical analysis of the kinetics of maintenance of corneal epithelial mass confirms that the corneal epithelium can be maintained by centripetal migration of epithelial cells originating from the limbus without contribution by adjacent conjunctiva.24
The above sources of evidence point to the 1.5–2 mm wide area of basal layer in the limbal epithelium as the region harboring stem cells for corneal epithelium. These cells are a small sub-population of the total tissue and have been estimated to make up from 0.5% or less to 10% of the total cell population located in palisades of Vogt (Fig. 1).7,25,26
Figure 1.

The limbal palisades of Vogt.Palisades of Vogt (arrow) are easily recognized in the human limbus (A). Such a unique pigmented structure can be identified on the flat mount preparation of dispase-isolated human limbal epithelial sheets (B). In dark-skin donors, palisades of Vogt are pigmented (C, arrow). Under high magnification, these areas of limbus appear undulated (D, stars). Hematoxylin staining highlights higher stratification and further undulation of the limbal epithelium, and the underlying limbal stroma demonstrates high cellularity and vascularity (E, arrow shows blood vessel). The bar represents 500 μm in A and B, 200 μm in C and 50 μm in D.26
THE LIMBUS AS LIMBAL STEM CELL NICHE
What maintains the stemness of a stem cell is not well understood. In addition to characteristics inherent to stem cells, extrinsic influences from surrounding microenvironment may also play a role.27 Schofield28 suggested for the first time that this property of stem cells is maintained by extrinsic factors in their microenvironment.Upon stem cell division, one daughter cell remains a stem cell and returns to the niche to replenish the stem cell pool while the other becomes a TAC that will eventually terminally differentiate.29 The microenvironment of the limbus differs from that of the cornea; one of the most striking differences is the presence of blood supply at the limbus. The blood vessels form an undulating network in the palisades of Vogt. This arrangement allows close approximation between blood vessels and the epithelium, potentially providing increased levels of nutrients and blood-borne cytokines to the cells at the limbus.1
The limbal basement membrane differs from that of the cornea in that it undulates with pegs of stroma extending upward interconnected with anchoring fibrils linked to the basement membrane. This could provide resident stem cells with an adherence niche, protecting them from injury and movement within their microenvironment. Corneal basement membrane is composed of collagen type IV (;3, α4, and α5 chains), collagen type VII, laminin (Ln-α1, Ln-α3, Ln-β1, Ln-β3, Ln-γ1 and Lnγ2 chains), fibronectin, and heparan sulfate proteoglycans.Limbal basement membrane possesses additional laminin α2 and β2 chains together with α1 and α2 chains of collagen type IV but no collagen type XII and collagen type IV (α3, α4, and α5 chains). Although not proven, the differences in the composition of the basement membrane in limbal and corneal epithelium might be at least partially responsible for different cell phenotypes and proliferative behaviors of these two distinct cell poulations.29Future studies are needed to clarify the role of stromal microenvironment in the regulation of stem cell function.
Another difference between the limbus and the cornea is the expression of several proteins at higher concentrations in the basal cells of limbal epithelium as compared to central corneal epithelium, such as cytochrome oxidase,30,31 Na+/K+ ATPase and carbonic anhydrase.26 It is not clear whether any of these proteins is involved in the maintenance and regulation of stem cells. There seem to be regional differences in the distribution and concentration of various regulatory factors, such as retinoic acid in human limbal and corneal epithelium,as well as in the underlying stroma and fibroblasts26 which affect stemness properties.Other important differences include different expressions of cytokines in the limbus and cornea.Three types of cytokines have been determined in limbus and cornea:
Type I cytokines:These include interleukin 1β,which is produced by both limbal and corneal fibroblasts and is increased following injury to the corneal epithelium.
Type II cytokines:Including transforming growth factor β1, produced by both limbal and corneal fibroblasts.
Type III cytokines:Keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF) are released by fibroblasts. KGF, predominantly produced by limbal fibroblast,causes proliferation of limbal epithelial cells and HGF, produced by corneal fibroblast,causes migration of epithelial cells onto the corneal surface.32,33
TYPES AND FUNCTIONS OF CORNEAL EPITHELIAL CELLS
Stem Cells
Stem cells are few in number but have high capacity for self-renewal and large proliferative potential. In healthy tissues, they have a tendency to remain quiescent. They divide and undergo differentiation to form TACs when stimulated and are located in the basal layer of the limbal epithelium.29
Transient Amplifying Cells (TACs)
It is most likely that limbal basal epithelium consists not only of stem cells but also of TACs which are located in the basal layer of the limbus and peripheral corneal epithelium. They have an important role in wound healing.29 TACs in the limbus and peripheral cornea display some characteristics of stem cells such as a long life, slow cycling with low mitotic activity, and less differentiation in the normal steady state. In contrast, TACs in the cornea have a short lifespan, are rapid cycling, and can amplify cell mass effectively through limited rounds of mitosis. At a critical point, TACs stop mitosis and differentiate into corneal suprabasal post-mitotic cells and terminally differentiated cells (TDCs).34,35 Although the exact mechanism remains unknown, many studies have indicated that the control of mitotic kinetics in TACs for limbal stem cells is different from that for corneal cells. The presence of TACs in the limbus and cornea provides advantages including:(1) amplifying each stem cell division and minimizing the need for stem cell proliferation and conserving stem cell energy, (2) minimizing the chance for introducing replicative DNA errors into the stem cell population, and (3) providing new cells that are much closer to the terminally differentiated functional cellular compartment, for example, the epithelium that covers the central cornea.12
Terminally Differentiated Cells (TDCs) and Post-Mitotic Cells (PMCs)
The division of TACs results in non-dividing PMCs, which differentiate and migrate toward the central cornea and specifically take on the final corneal cell phenotype as TDCs. These cells have no capacity for self-renewal and no proliferative potential. In normal healthy tissues they are continuously being shed and replaced because of their limited lifespan. They are located in the superficial part of the central cornea and have many tight junctions.29 Fig. 2 demonstrates cell contents of the limbal stem cell niche.
Figure 2.
Hypothetical diagram of the limbal stem cell niche. Limbal epithelial stem cells are located at the basement membrane of the limbus. In this epithelial level,there are several other cell types in the vicinity such as the immediate progeny, i.e., early transient amplifying cells (eTACs), melanocytes, and Langerhans’ cells. It remains to be determined whether these cell types act as niche cells. It is believed that eTACs are destined for progeny production by differentiating into late TACs located at the basement membrane of the cornea which consecutively differentiate into suprabasal post-mitotic cells, and finally into superficial terminally differentiated cells. The limbal basement membrane separates the epithelium from the underlying stroma. Limbal stroma contains mesenchymal cells, which may also serve as niche cells. Limbal stroma is highly innervated and vascularized; however the role of nerves and blood vessels in the niche remains to be defined.26
CORNEAL AND LIMBAL MARKERS
The ideal stem cell marker should not only be able to pinpoint the location of stem cells within the epithelium but should also allow for isolation,enrichment, and molecular characterization of viable stem cells. To date several putative stem cell markers have been proposed,although no single specific molecular marker has been identified. This has significantly limited our capacity to study the characteristics and behavior of these cells. Continuous administration of titrated thymidine (3H-TdR) for a prolonged period labels all dividing cells.Slow cycling cells that remain labeled for a long time are termed "label retaining cells" and are believed to be stem cells. Another characteristic of stem cells is their capacity to remain highly proliferative in vitro. Table 1 summarizes some stem cell markers in human ocular surface epithelia.36
Table 1.
Semiquantitative immunohistochemical localization of putative stem cell markers in human corneal and limbal epithelia
| Markers | Limbal Epithelia | Corneal Epithelia | |||
|---|---|---|---|---|---|
|
| |||||
| Basal | Suprabasal | Basal | Suprabasal | ||
| Cytoplasmic/Nuclear | |||||
| Cytokeratin K5/K14 | + + + | + + + | ± | – | |
| Keratin K3/K12 | – | + | + + + | + + + | |
| Cytokeratin K19 | + + + | – | – | – | |
| Vimentin | + + + | + | – | – | |
| Enolase-α | + + + | + | + | – | |
| Metalothionein | + | + + + | – | + + + | |
| P63 | + + + | + | + | – | |
| Nestin | – | – | + + + | + + + | |
| Cell Surfaces | |||||
| Connexin 43 | – | + | + | + + + | |
| E-Cadherin | + | +++ | +++ | +++ | |
| P-Cadherin | ± | – | + | – | |
| β Catenin | + + + | + + + | + + + | + + + | |
| Integrin α2 | ± | + | + + + | + | |
| Integrin α3 | ± | + | + + + | + | |
| Integrin α6 | ± | + | + + + | + | |
| Integrin β1 | + + + | + | + + + | + | |
| Integrin β2 | + | + | + | + | |
| Integrin β4 | + + + | + | ± | + | |
| Integrin β5 | + | – | + | + | |
| EGFR | +++ | + | +++ | ++ | |
| ABCG2 | + + + | – | – | – | |
| Involucrin | – | + + + | – | + + + | |
–, undetectable; ±, weak positivity; +, moderate positivity; +++, strong positivity.EGFR, epithelial growth factor recptor; ABCG, ATP binding cassette G.
Glycolytic enzyme α-enolase is a multifunctional protein, which may exert a variety of cellular functions in addition to its primary role in the glycolytic pathway.13,37 Zieske et al38 demonstrated that the expression of α-enolase increases in mitotically active cells, but remains almost at an undetectable level in quiescent cells. They also detected increased concentrations of α-enolase in limbal epithelial cells and proposed this as a potential marker for corneal epithelial stem cells. Expression of α-enolase has been also reported to be elevated during corneal epithelial migration from the limbal basal cell population after epithelial debridement.39 However, a more recent study using human corneas showed that α-enolase was not only expressed by basal epithelial cells but also by suprabasal epithelial cells at the limbus and occasionally by corneal basal epithelial cells.40 Other enzymes such as cytochrome oxidase,30,31 Na+/K+ ATPase30,31 and carbonic anhydrase26 which are associated with cellular metabolic functions have also been found in higher concentrations in the limbal basal epithelium than in the central corneal basal epithelium.
Aldehyde dehydrogenase (ALDH) and transketolase (TKT) have been identified as major cytosolic proteins in the corneal epithelium,but are completely absent (ALDH) or only minimally expressed (TKT) in the murine limbus.41,42 The corneal zone expresses a dramatic accumulation of large quantities of globular metabolic enzymes, in particular ALDH and TKT.43 The expression of these enzymes may be correlated with corneal transparency and its refractive properties. In the rabbit, the copious expression of ALDH class 1 is limited to the corneal domain.44 The magnitude of changes in phenotype at the limbo-corneal demarcation has also been suggested to be due to differences in shape, size and intracellular complexity of basal cells in both domains in rodents and humans.45
Lectin staining of corneal sections has yielded unique insights to changes occurring within basal cells in their transition across the limbal-corneal margin in the rabbit.46,47 Limbal epithelial cells have been shown to express on their cell surface unsialylated galactose residues that are recognized by peanut lectin and lack any sialic acid bound through α-2,3 bonds.Differentiation of the cells causes sialylation of these residues and the appearance of α-2,3 sialic acid residues, suggest the expression or activation of α-2,3-sialyltransferase.
Gap junctions are communicating cellcell junctions consisting of six transmembrane proteins called connexins (Cx). The gap junction channels allow diffusion of ions, low molecular weight metabolites and second messengers between cells and thus, determine the extent of cell metabolic synchrony or cooperation within a population (Fig. 3 A,B).48–50 The presence of Cx26, Cx30, Cx43 and Cx50 gap junctions have been confirmed at the protein level by immunohistology in the corneal epithelium of the human, rat and rabbit.51,52 Cx43 and Cx50 are abundantly expressed in the corneal epithelium with Cx50 being expressed throughout all layers and Cx43 being mainly confined to the basal cell layer (Fig. 4).37,47 In contrast, both connexins were absent at the limbal basal layer in human, mouse, chicken, and neonatal rabbit eyes, whereas suprabasal limbal cells showed slightly positive membranous staining (Fig. 3C).49,51,53,54
P63, a transcription factor expressed in the nucleus, has been suggested as a new marker for limbal stem cells (Fig. 5). This protein is known to be involved in tumor suppression and morphogenesis, and belongs to a family that includes p53 and p73.55,56 P63 is consistently expressed in basal cells of stratified epithelia on the nucleous, and is essential for epithelial development and differentiation.57 Originally,epithelial cells expressing p63 were found specifically in the basal layer of the limbus but not in the corneal epithelium in humans.55
Cytokeratines (CK) together with microfilaments and microtubules form the cytoskeleton of all vertebrate cells.58 There are a total of approximately 30 keratins that can be divided into an acidic (type I) and neutral-to-basic (type II) subfamilies. It is notable that each basic keratin tends to co-express with a particular acidic keratin, forming a so-called keratin pair.In addition, each keratin pair tends to be expressed in a tissue-restricted and differentiation-dependent fashion.16 Using a monoclonal antibody (AE5) it was demonstrated that K3 is expressed suprabasally in limbal epithelium but uniformly in the central corneal epithelium (Fig. 6). This finding implies that the K3-positive basal cells in the central corneal epithelium may have attained a more advanced state of differentiation than the K3-negative basal cells of the limbal epithelium. Also, immunohistochemistry has demonstrated that cytokeratine CK3/12 identifies more differentiated cells in the corneal epithelium. This CK dimmer is therefore absent in limbal stem cells and early TACs in the limbal epithelium.19
PAX6 plays critical roles in mature tissue.59 In humans, heterozygous loss of PAX6 function results in multiple ocular developmental defects, the major one being aniridia.7 The corneal epithelium of PAX6 +/+/PAX6 −/− mouse chimeras, exhibits a decrease in the expression of adherence proteins and keratin K12,and shows conjunctival invasion consistent with limbal cell deficiency.60,61 Eventually,PAX6 −/− cells are excluded from the corneal epithelium, pointing to the acute dependence of the phenotype on PAX6 expression.62 Basal cells in the limbal epithelium of the rat eye have been reported to express the transcription factor PAX6, which is present in the developing central nervous system and plays a central role in eye development.63 PAX6 has also been shown to be strongly expressed in the nuclei of all cells within the corneal, limbal, and conjunctival epithelia of adult mouse and monkey eyes, and might be necessary for the maintenance or proliferation of corneal stem cells.64
Cadherins are a family of Ca++ dependent transmembrane receptors that mediate cell–cell adhesion.65 E-cadherin mediated cell–cell contacts result in cell activation and an increase in key signaling molecules that are involved in cell proliferation and survival.66 In the rat cornea, E-cadherin was immunolocalized to all cell layers of the corneal epithelium.67 In human corneas, the expression pattern of E-cadherin in corneal and limbal epithelia (Fig. 7) resembled that of Cx43, with positive membrane staining in suprabasal layers and negative staining in basal layers at the limbus. In contrast, basal cells of human ocular surface epithelia showed preferential membrane associated immunoreactivity for P-cadherin with small clusters of negative cells among positive cells in the limbal basal epithelium (Fig. 7). Beta-catenin is a central component of the cadherin cell adhesion complex and, as an essential molecule in the Wnt signaling pathway, a key regulator of epithelial differentiation and proliferation. Therefore, it has been shown to be essential for the maintenance of keratinocyte stem cells.68 In rat and rabbit corneas, β-catenin was strongly positive in the basal layer of the limbal epithelium, but relatively weakly positive in the basal layer of the cornea.69
Integrins: Immunohistochemical studies have identified several subunits of integrins,such as integrins α2, α3, α4, α5, α6, and αv as well as β1, β4, and β5 to be present in the human corneal epithelium, whereas integrins α1 and β3 were not detected.35 Integrins β1, β4,α2, α3, α6, and αv were mainly expressed in the basal epithelial cell layer; integrins α6 and β4,as components of hemidesmosomes, were localized specifically to the basal membrane of basal cells (Fig. 8). Some integrins have been suggested to be markers for epidermal stem cells, such as integrins β1 and α6. Integrin β1 was abundantly expressed in corneal and limbal epithelia with a much higher levels of expression in limbal basal versus limbal suprabasal cells.70 These observations suggest that limbal basal cells deficient in Cx43, P-cadherin,and integrins α2, α3, α6, and β4 are thought to represent corneal stem cells. The lack of intercellular communication (connexins) and adhesion molecules (cadherins, integrins) may be an inherent feature of limbal stem cells reflecting their need for independence and the uniqueness of their microenvironment.
Transferring receptors: Different expressions of the transferring receptor CD71 have been observed in limbal and corneal epithelia.In contrast to basal cells in the corneal epithelium which stain intensely with an anti-CD71 antibody, the limbal basal cells are negative.35 However, in a more recent study, anti-CD71 antibody stained not only the cell membranes of most corneal epithelial cells but also some basal epithelial cells in the human limbus.70,71
Figure 3.
Types of cell junctions (A), electron-microscopic location of cell junctions in cells (B),48–50 and immunostaining of connexin-43 in limbal stem cell culture (C).54
Figure 4.

Immunofluorescent staining for connexin 43 in the cornea and limbus (arrows) (×100).37
Figure 5.
Expression of p63 in the epithelium of rabbit limbus (A) and cornea (B).56
Figure 6.
Immunostaining for cytokeratine 3 (K3) in the center of the cornea (Ebrahimi et al 2006; unpublished).K3 is expressed in the suprabasal (green) but not in the basal layer of the limbus or cornea.
Figure 7.
Immunofluorescent staining for E-cadherin and P-cadherin expression on frozen sections of the human limbus. The arrows point to positively or negatively labeled basal cells at the limbus (×100).37
Figure 8.

Immunofluorescent staining for integrins expression on frozen sections of the human limbus. The arrows point to positively or negatively labeled basal cells at the limbus (×100).37
FACTORS REGULATING LIMBAL STEM CELL PROLIFERATION
Many regulatory factors affect the proliferation of limbal stem cells in both fetal and adult life.
1) Growth factor receptors (GFRs): GFRs consists of epithelial (EGFR), keratinocyte (KGFR) and hepatocyte (HGFR) which preferentially localize to cell membranes of limbal basal cells. Undifferentiated cells in the limbal basal epithelium have been originally reported to contain higher levels of GFRs than suprabasal cells in the rat cornea (Fig. 9).9,72,73 Although a strong expression of GFRs in limbal basal cells was also confirmed in human corneas,there was no clear difference in staining intensity between the basal cells of the limbus,cornea, and conjunctiva.40,74 It has been suggested that high levels of GFRs may inhibit differentiation by signaling the cells to maintain their proliferation potential.73
2) ABCG2 is a member of the ATP binding cassette (ABC) transporters, localizes predominantly to the plasma membrane and has been proposed as a universal and conserved marker for stem cells from a wide variety of tissues.75,76 ABCG2, also known as breast cancer resistant protein 1 (BCRP1), causes resistance to certain chemotherapeutic drugs. Immunolocalization experiments have consistently demonstrated ABCG2-positive cells in basal77 and also frequently in suprabasal layers of the limbal epithelium,70 but not in the corneal epithelium (Fig. 9).
Figure 9.
Immunofluorescent staining for Growth factor receptors on frozen sections of the human limbus. The arrows point to positively or negatively labeled basal cells at the limbus (×100).37
LIMBAL STEM CELL DEFICIENCY (LSCD)
LSCD may occur due to a deficient stromal microenvironment supporting the stem cells,such as aniridia, congenital erythrokeratodermia,keratitis associated with multiple endocrine deficiencies, neurotrophic keratopathy and chronic limbitis; or more commonly following external insults which destroy the limbal stem cells such as chemical or thermal injuries,Stevens-Johnson syndrome (SJS), ocular cicatricial pemphigoid (OCP), multiple surgeries or cryotherapies, contact lens wear, and severe microbial infections (Table 2 ).78 Limbal stem cell deficiency can be diffuse (total) or sectoral (partial). In the latter case, conjunctivalization of the cornea is limited to deficient areas (Fig. 10). In some patients, limbal deficiency may be subclinical at the time of the insult, this may eventually progress to overt deficiency as the stem cell population further depletes over time.13,14,37,79–83
Table 2.
Human conditions causing limbal stem cell deficiency
| Category I: Destruction of limbal stem cell population | ||
| a. Chemical or thermal injuries | ||
| b. Multiple surgeries or cryotherapies of the limbus | ||
| c. Stevens-Johnson syndrome | ||
| d. Contact-lens-induced keratopathy | ||
| e. Severe microbial infection | ||
|
| ||
| Category II: Dysfunction of the stromal microenvironment of limbal stem cells | ||
| a. Aniridia (hereditary) | ||
| b. Keratitis associated with multiple endocrine deficiencies (hereditary) | ||
| c. Neurotrophic keratopathy (neuronal or ischemic) | ||
| d. Chronic limbitis or peripheral corneal inflammation and ulceration | ||
| e. Pterygium and pseudopterygium | ||
| f. Idiopathic keratopathy | ||
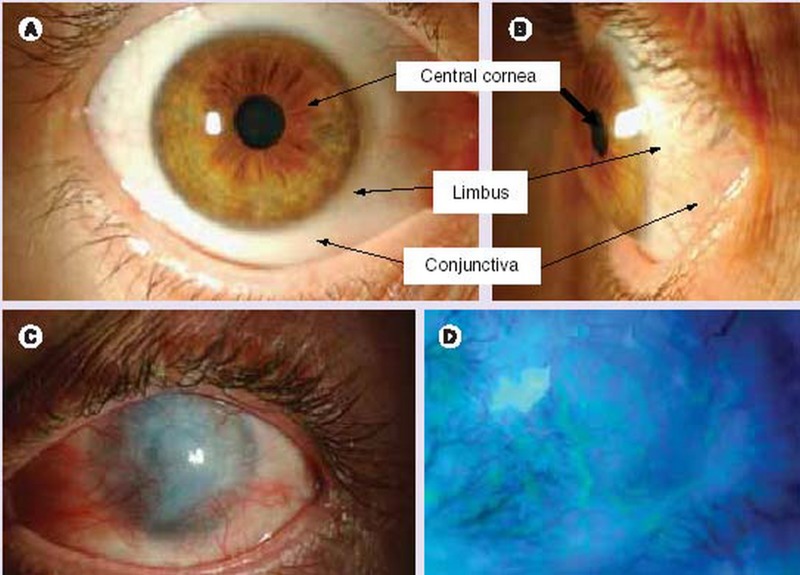
Figure 10.
Appearance of the human ocular surface in a healthy (A & B) eye and in limbal stem cell deficiency (C & D). Anterior view (A) and side view (B) of the anterior segment of the human eye. Clinical signs of limbal stem cell deficiency (C), and delayed epithelial staining and areas of epithelial defects (D).
Some conditions such as aniridia, keratitis associated with multiple endocrine deficiencies,neurotrophic keratopathy, and pterygium/pseudopterygium represent milder forms of subtotal limbal stem cells deficiency in which a gradual loss of stem cells, or poor TAC generation and amplification occurs. Such patients may experience severe photophobia,pain, reduced vision, and even blindness.84–87 The common pathogenic features of this seemingly diverse group of diseases is depletion of the stem cell population from the limbus88 which results in conjunctivalization or ingrowths of conjunctival elements onto the surface of the cornea. These patients are generally poor candidates for conventional corneal transplantation for several reasons: (1) with this technique only corneal TACs, not stem cells,are transplanted, (2) pre-existing corneal vascularization and inflammation increase the risk of allograft rejection (3) these eyes tend to develop recurrent conjunctivalization due to stem cell dysfunction.84
TREATMENT OF LIMBAL STEM CELL DEFICIENCY
Limbal stem cell transplantation is the only available treatment for LSCD. The success of this procedure depends on a variety of factors and may be adversely affected by concomitant lid pathology, dry eye and uncontrolled systemic disease. Hence, the management of LSCD necessitates addressing associated adnexal problems, management of dry eye and control of systemic diseases.89
Theoretically it is possible to restore stem cell function by expanding the stem cell population through modulations in the microenvironment,or by inducing TACs mitosis with the use of appropriate growth factors.79 Limbal stem cells may be obtained from the fellow eye (autograft), a cadaver (allograft) or a living relative (allograft). Limbal stem cells are harvested with a carrier which may be conjunctiva (conjunctival-limbal grafts) or cornea (Keratolimbal grafts)90 A variety of methods have been employed for preparing transplant sheets, e.g. a sheet of corneal epithelial cells alone, a sheet of corneal epithelial cells cultured on a polymer substrate, collagen or fibrin,91 or on biomaterial such as amniotic membrane.92,93 Surgical procedures used for treatment of partial or total limbal stem cell deficiency are discussed below.
Amniotic Membrane Transplantation
The human amniotic membrane is the innermost layer of the placenta. Histologically the amnion is 0.02 mm to 0.5 mm in thickness, composed of three basic layers; the epithelial monolayer,the thick basement membrane and the avascular hypocellular stromal matrix.94 The structural integrity, transparency and elasticity of amniotic basement membrane make it currently the most widely accepted tissue replacement for ocular surface reconstruction. Amniotic membrane is known to promote epithelial cell migration, adhesion and differentiation.It is an ideal substrate for supporting the growth of epithelial progenitor cells by prolonging their lifespan, maintaining their clonigenicity and preventing epithelial cell apoptosis (Table 3) .95,96
Table 3.
Amniotic membrane (AM) characteristics mimicking stem cell niche and promoting stem cell expansion
| AM characteristics | Potential mechanism for ex vivo expansion of limbal stem cells |
|---|---|
| Amniotic epithelium | |
| Cytokines (major: EGF, KGF, HGF, bFGF, NGF, and minor: TGF-α, TGF-β1, TGF,β2) |
Prevents early contact with ECM-components |
| Tissue inhibitors of metalloproteinases Thrombospondin-1 |
Provides cytokines affecting the cell cycle and cell survival |
| Amniotic basement memrane | |
| Collagens IV (α chain), and VII Laminin 1 and 5 |
Facilitates cell migration and ECM adhesion |
| Fibronectin | Triggers signaling pathways through integrins |
| Amniotic stroma | |
| Cytokines (major: NGF, HGF, KGF, and minor: TGF−α, TGF−β1+2, EGF, bFGF) |
Provides a non-inflamed microenvironment |
| Tissue inhibitors of metalloproteinases Thrombospondin-1 |
Provides cytokines for major signaling pathways known to be involved in stromal and limbal epithelial communication |
EGF, epithelial growth factor; KGF, keratinocyte growth factor; HGF, hepatocyte growth factor; bFGF, basic fibroblast growth factor; NGF, nerve growth factor; TGF, tumor growth factor; ECM, extracellular matrix.
Amniotic membrane transplantation (AMT) was initially reported for corneal surface reconstruction in a rabbit model of total limbal deficiency.97 Tsubota et al98 have used this technique,combined with allograft limbal transplantation,to effectively reconstruct the corneal surface in patients with severe dry eye caused by OCP and SJS. It has been reported that AMT alone is sufficient to restore the corneal surface in eyes with partial LSCD, suggesting that AMT may help expand the remaining limbal epithelial stem cells in vivo.99,100
Studies have shown that the amniotic membrane contains high levels of EGF, KGF,HGF, TGF (tumor growth factor), and bFGF (basic fibroblast growth factor) which are potentially involved in epithelial-stromal interactions of the human ocular surface including epithelialization, and modulation of proliferation and differentiation of stromal fibroblasts.101 Therefore the amniotic epithelium might provide cytokines, which play a crucial role in the microenvironmental niche of limbal progenitor cells. In addition, the basement membrane of the amniotic membrane contains types IV, V,and VII collagen, Ln1, Ln5, and fibronectin that play an important role in corneal epithelial cell adhesion and migration.94 The stromal matrix also suppresses the expression of certain inflammatory cytokines that originate from ocular surface epithelia, including interleukin 1α(IL-1α), IL-2, IL-8, interferon γ, tumor necrosis factor-β, basic fibroblast growth factor, and platelet derived growth factor.102 The amniotic membrane attracts and sequesters inflammatory cells infiltrating the ocular surface and contains various forms of protease inhibitors which explain some of its anti-inflammatory properties.103
Amniotic membrane stroma contains high amounts of nerve growth factor which plays a key role in epithelial integrity and stem cell survival. When rabbit corneas were covered by a layer of human amniotic membrane after excimer laser ablation, the acute inflammatory reaction was markedly reduced, evidenced by rapid apoptosis of polymorphonuclear neutrophils.This finding was also supported in human patients with acute burns where lymphocytes were trapped by amniotic membrane and exhibited apoptosis. When alkali burns are created in rabbit corneas, amniotic membrane transplantation used as a temporary patch reduces acute and severe inflammation evidenced by a smaller amount of infiltration by polymorphonuclear neutrophils. These antiinflammatory properties help explain how a non-inflamed stroma created by AMT is a prerequisite for successful limbal stem cell transplantation and survival. The above mentioned biological effects of amniotic membrane may explain how it facilitates preservation of the normal phenotypes of human conjunctival and corneal epithelial cells in culture, and provides an ideal stromal niche for stem cell expansion.104
Complications of AMT
AMT does not entail major complications, however minor events may follow surgery. In the immediate postoperative period a hematoma may form under the membrane.105 This blood usually absorbs, but if excessive may need drainage by a small incision in the graft. Occasionally,a residual subepithelial membrane persists and opacifies the visual axis. The incidence of post-AMT microbial infections is as low as 1.6%.106 This figure is much lower than the 8% rate reported with the use of fresh amniotic membrane; gram-positive organisms are the most frequent isolates.107 Gabler et al108 reported a case of sterile hypopyon after repeat AMT. Calcification occurs in about 12.8% of cases and white plaques of ciprofloxacin deposits may occur. The key to reducing postoperative complications is meticulous selection of donors and recipients and maintaining high standards of quality.109
Limbal Auto-/Allograft Transplantation
In total LSCD, autologous or allogeneic limbal epithelial stem cells need to be transplanted.This technique was first introduced by Kenyon and Tseng in 1989, and subsequently by many others for treating patients with focal or unilateral LSCD in different clinical settings.Donor tissue can be harvested from the healthy fellow eye (conjunctival limbal autograft),from a living related donor (conjunctival limbal allograft) or from a cadaver eye (keratolimbal allograft).110–112
Limbal grafting involves transplantation of large pieces of healthy limbus from the donor.Each of the varieties of the technique has drawbacks;in the case of autografting and allografting from living related donors, there is a limit to the amount of limbal tissue that can be harvested, due to the risk of producing iatrogenic LSCD in the donor eye.23 Allografts from living related or cadaveric donors entail the risk of tissue rejection and their survival depends on aggressive systemic immunosuppression113,114 which is associated with significant morbidity and reduction in quality of life.
Ex Vivo Limbal Stem Cell Transplantation
Recently, with the increased knowledge on stem cell biology, techniques have been developed to expand small biopsies of limbal tissue in culture for subsequent transplantation, this overcomes some of the main hurdles entailed by whole limbal tissue transplants.115 The concept of culturing stem cells was derived from the use of cultured human epidermal cells as autologous grafts in patients with burns and in plastic and reconstructive surgery.116 Currently,culturing corneal epithelial stem cells is the most exciting and promising technique in limbal transplantation. It is possible to culture stem cells using a small amount of tissue thereby minimizing damage to the donor and depletion of its stem cell reserve. With this technique only epithelial cells (not Langerhans’cells and blood vessels) are transplanted,therefore theoretically reducing the possibility of rejection.
There are 3 main techniques for culturing limbal epithelium. The first involves the coculture of limbal epithelium with mitotically inactivated 3T3 mouse fibroblasts, the second entails the use of human amniotic membrane as a substrate for cultivating limbal epithelium and the third combines the mentioned methods.
The original method of culturing limbal epithelium on amniotic membrane involves taking a small limbal biopsy (approximately 1 mm) or explants, and culturing it in the center of the amniotic membrane (Fig. 11). The ex vivo expanded limbal epithelium grows out from the explant onto the amniotic membrane. When the amniotic membrane is sufficiently covered by ex vivo expanded limbal cells, it is transplanted to the eye with LSCD (Fig. 12).117 Amniotic membrane promotes epithelialization,118 reduces inflammation and scarring,119 preserves and maintains existing limbal stem cells,and serves as a natural substrate on which limbal stem cells can grow and proliferate. It also enables easier handling of the cultured limbal stem cells. It is believed that the amniotic membrane may act as a barrier to immune cells, diminishing the immune response by inhibiting IL-1β and IL8 expression, and may also produce anti-angiogenic proteins.119
Figure 11.

Technique for limbal epithelial culture using limbal explants on denuded amniotic membrane.116
Figure 12.
Transplantation of cultured limbal cells onto rabbit corneas with limbal stem cell deficiency. The conjunctival overgrowth was removed by scraping 5 mm beyond the limbus. Human amniotic membrane (HAM) carrying cultured human limbal cells on the basement membrane surface (colored) are sutured on the denuded region and covered by a second HAM with the basement membrane surface oriented toward the cornea. Orientation of the HAM basement membrane is shown by arrows.
Fibrin substrate has also been used to culture limbal stem cells.91 The culture system may be maintained for 14–28 days and either transferred to the recipient bed or subjected to air-lifting in order to promote epithelial tight junction formation and stratification.120
CONCLUSION
The concept of limbal stem cells has greatly improved our understanding of corneal epithelial proliferation, migration, and regeneration. This has also contributed directly to improved medical and surgical management of a wide range of ocular surface disorders. Many questions however remain. Clinically, the most important is the issue of limbal allograft rejection and the long term survival of limbal transplants and that of improving immunosuppressive regimens.In terms of stem cell biology, unanswered questions include: How is “stemness” of stem cells maintained? Which factors regulate the asymmetric division of stem cells? What are the external and internal modulators influencing stem cells? What is the role of the microenvironment in stem cell function and regulation?
REFERENCES
- 1.Daniels JT, Dart JK, Tuft SJ, Khaw PT. Corneal stem cells in review. Wound Repair Regen. 2001;9:483–494. doi: 10.1046/j.1524-475x.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 2.Germain L, Carrier P, Auger FA, Salesse C, Guerin SL. Can we produce a human corneal equivalent by tissue engineering? Prog Retin Eye Res. 2000;19:497–527. doi: 10.1016/s1350-9462(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 3.Chung EH, Bukusoglu G, Zieske JD. Localization of corneal epithelial stem cells in the developing rat. Invest Ophthalmol Vis Sci. 1992;33:2199–2206. [PubMed] [Google Scholar]
- 4.Moore JE, McMullen CB, Mahon G, Adamis AP. The corneal epithelial stem cell. DNA Cell Biol. 2002;21:443–451. doi: 10.1089/10445490260099737. [DOI] [PubMed] [Google Scholar]
- 5.Espana EM, Ti SE, Grueterich M, Touhami A, Tseng SC. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br J Ophthalmol. 2003;87:1509–1514. doi: 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 7.Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981–991. doi: 10.1387/ijdb.041876jw. [DOI] [PubMed] [Google Scholar]
- 8.Zieske JD. Corneal development associated with eyelid opening. Int J Dev Biol. 2004;48:903–911. doi: 10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]
- 9.Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 10.Potten C, Lajtha L. Stem cells versus stem lines. Ann N Y Acad Sci. 1982;10:49–61. doi: 10.1111/j.1749-6632.1982.tb43416.x. [DOI] [PubMed] [Google Scholar]
- 11.Technau G. A single cell approach to problems of cell lineage and commitment during embryogenesis of Drosophila melanogaster. Development. 1987;100:1–12. doi: 10.1242/dev.100.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 13.Morrison SJ, Shah NM. Anderson DJ Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 14.Sangwan VS. Limbal stem cells in health and disease. Biosci Rep. 2001;21:385–405. doi: 10.1023/a:1017935624867. [DOI] [PubMed] [Google Scholar]
- 15.Kruse FE, Chen JJ, Tsai RJ, Tseng SC. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990;31:1903–1913. [PubMed] [Google Scholar]
- 16.Wei ZG, Wu RL, Lavker RM, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix,and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993;34:1814–1828. [PubMed] [Google Scholar]
- 17.Thoft RA, Wiley LA, Sundarraj N. The multipotential cells of the limbus. Eye. 1989;3(Pt 2):109–113. doi: 10.1038/eye.1989.17. [DOI] [PubMed] [Google Scholar]
- 18.Huang AJ, Tseng SC, Kenyon MR. Morphogenesis of rat conjunctival goblet cells. Invest Ophthalmol Vis Sci. 1988;29:969–975. [PubMed] [Google Scholar]
- 19.Schermer A, Galvin S, Sun TT. Differentiationrelated expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 21.Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. J Investig Dermatol Symp Proc. 2004;9:202–207. doi: 10.1111/j.1087-0024.2004.09311.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurpakus M, Daneshvar C, Davenport J, Kim A. Human corneal epithelial cell adhesion to laminins. Curr Eye Res. 1999;19:106–114. doi: 10.1076/ceyr.19.2.106.5330. [DOI] [PubMed] [Google Scholar]
- 23.Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219–2233. [PubMed] [Google Scholar]
- 24.De Luca M, Pellegrini G, Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med. 2006;1:45–57. doi: 10.2217/17460751.1.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Kenyon K, Tseng S. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 26.Wei L, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996;23:47–58. doi: 10.1007/BF00357072. [DOI] [PubMed] [Google Scholar]
- 28.Schofield R. The stem cell system. Biomed Pharmacother. 1983;37:375–380. [PubMed] [Google Scholar]
- 29.Ahmad S, Figueiredo F, Lako M. Corneal epithelial stem cells: characterization, culture and transplantation. Regen Med. 2006;1:29–44. doi: 10.2217/17460751.1.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espana EM, Kawakita T, Romano A, Di Pascuale M, Smiddy R, Liu CY, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- 32.Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelialfibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 33.Li DQ, Tseng SC. Differential regulation of cytokine and receptor transcript expression in human corneal and limbal fibroblasts by epidermal growth factor, transforming growth factor-alpha,platelet-derived growth factor B, and interleukin-1 beta. Invest Ophthalmol Vis Sci. 1996;37:2068–2080. [PubMed] [Google Scholar]
- 34.Lauweryns B, van den Oord JJ, De Vos R, Missotten L. A new epithelial cell type in the human cornea. Invest Ophthalmol Vis Sci. 1993;34:1983–1990. [PubMed] [Google Scholar]
- 35.Lauweryns B, van den Oord JJ, Missotten L. The transitional zone between limbus and peripheral cornea.An immunohistochemical study. Invest Ophthalmol Vis Sci. 1993;34:1991–1999. [PubMed] [Google Scholar]
- 36.SchlÖtzer-Schrehardt U, Kruse F. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Chee KY, Kicic A, Wiffen SJ. Limbal stem cells: the search for a marker. Clin Experiment Ophthalmol. 2006;34:64–73. doi: 10.1111/j.1442-9071.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 38.Zieske JD, Bukusoglu G, Yankauckas MA. Characterization of a potential marker of corneal epithelial stem cells. Invest Ophthalmol Vis Sci. 1992;33:143–152. [PubMed] [Google Scholar]
- 39.Chung EH, DeGregorio PG, Wasson M, Zieske JD. Epithelial regeneration after limbus-to-limbus debridement.Expression of alpha-enolase in stem and transient amplifying cells. Invest Ophthalmol Vis Sci. 1995;36:1336–1343. [PubMed] [Google Scholar]
- 40.Chen W, Cao L, Hara K, Yoshitomi T. Effect of immunosuppression on survival of allograft limbal stem cells. Jpn J Ophthalmol. 2004;48:440–447. doi: 10.1007/s10384-004-0098-3. [DOI] [PubMed] [Google Scholar]
- 41.Guo J, Sax CM, Piatigorsky J, Xu FX. Heterogeneous expression of transketolase in ocular tissues. Curr Eye Res. 1997;16:467–474. doi: 10.1076/ceyr.16.5.467.7042. [DOI] [PubMed] [Google Scholar]
- 42.Kays W, Piatigorsky J. Aldehyde dehydrogenase class 3 expression: identification of a corneapreferred gene promoter in transgenic mice. Proc Natl Acad Sci U S A. 1997;94:13594–1359. doi: 10.1073/pnas.94.25.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sax C, Kays WT, Salamon C, Chervenak MM, Xu YS, Piatigorsky J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea. 2000;19:833–841. doi: 10.1097/00003226-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Dong Y, Roos M, Gruijters T, Donaldson P, Bullivant S, Beyer E, et al. Differential expression of two gap junction proteins in corneal epithelium. Eur J Cell Biol. 1994;64:95–100. [PubMed] [Google Scholar]
- 45.Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;44:5125–5129. doi: 10.1167/iovs.03-0628. [DOI] [PubMed] [Google Scholar]
- 46.Wolosin JM, Wang Y. Alpha-2,3 sialylation differentiate the limbal and corneal epithelial cell phenotypes. Invest Ophthalmol Vis Sci. 1995;36:2277–2286. [PubMed] [Google Scholar]
- 47.Wolosin JM, Xiong X, Schutte M, Stegman Z, Tieng A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000;19:223–255. doi: 10.1016/s1350-9462(99)00005-1. [DOI] [PubMed] [Google Scholar]
- 48.Kumar N, Gilula N. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, Evans WH, Pflugfelder SC, Li DQ. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells. 2006;24:1265–1273. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res. 2003;76:303–314. doi: 10.1016/s0014-4835(02)00314-7. [DOI] [PubMed] [Google Scholar]
- 51.Matic M, Petrov IN, Chen S, Wang C, Dimitrijevich SD, Wolosin JM. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation. 1997;61:251–260. doi: 10.1046/j.1432-0436.1997.6140251.x. [DOI] [PubMed] [Google Scholar]
- 52.Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, et al. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matic M, Evans WH, Brink PR, Simon M. Epidermal stem cells do not communicate through gap junctions. J Invest Dermatol. 2002;118:110–116. doi: 10.1046/j.0022-202x.2001.01623.x. [DOI] [PubMed] [Google Scholar]
- 54.Baharvand H, Ebrahimi M, Javadi MA. Comparison of characteristics of cultured limbal cells on denuded amniotic membrane and fresh conjunctival, limbal and corneal tissues. Develop.Growth Differ. 2007;49:241–251. doi: 10.1111/j.1440-169X.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 55.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang DY, Hsueh YJ, Yang VC, Chen JK. Propagation and phenotypic preservation of rabbit limbal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:4698–4704. doi: 10.1167/iovs.03-0272. [DOI] [PubMed] [Google Scholar]
- 57.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 58.Strelkov S, Herrmann H, Aebi U. Molecular architecture of intermediate filaments. Bioessays. 2003;25:243–251. doi: 10.1002/bies.10246. [DOI] [PubMed] [Google Scholar]
- 59.Sivak JM, West-Mays JA, Yee A, Williams T, Fini ME. Transcription factors Pax6 and AP-2alpha interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol Cell Biol. 2004;24:245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis J, Duncan MK, Robison WG Jr, Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003;116:2157–2167. doi: 10.1242/jcs.00441. [DOI] [PubMed] [Google Scholar]
- 61.Ramaesh K, Dhillon B. Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction. Eur J Ophthalmol. 2003;13:515–524. doi: 10.1177/112067210301300602. [DOI] [PubMed] [Google Scholar]
- 62.Collinson JM, Chanas SA, Hill RE, West JD. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6 (+/−) mouse. Invest Ophthalmol Vis Sci ,2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- 63.Zhao X, Das AV, Thoreson WB, James J, Wattnem TE, Rodriguez-Sierra J, et al. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev Biol. 2002;250:317–331. [PubMed] [Google Scholar]
- 64.Koroma B, Yang J, Sundin O. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997;38:108–120. [PubMed] [Google Scholar]
- 65.Nose A, Tsuji K, Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990;61:147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- 66.Scott R, Lauweryns B, Snead DM, Haynes RJ, Mahida Y, Dua HS. E-cadherin distribution and epithelial basement membrane characteristics of the normal human conjunctiva and cornea. Eye. 1997;11:607–612. doi: 10.1038/eye.1997.163. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki S. Structural and functional diversity of cadherin superfamily: are new members of cadherin superfamily involved in signal transduction pathway? 1996;61:531-542. J Cell Biochem. 1996;61:531–542. doi: 10.1002/(sici)1097-4644(19960616)61:4<531::aid-jcb6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 68.Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 69.Hsueh YJ, Wang DY, Cheng CC, Chen JK. Agerelated expressions of p63 and other keratinocyte stem cell markers in rat cornea. J Biomed Sci. 2004;11:641–651. doi: 10.1007/BF02256130. [DOI] [PubMed] [Google Scholar]
- 70.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen YX, Krull CE, Reneker LW. Targeted gene expression in the chicken eye by in ovo electroporation. Mol Vis. 2004;10:874–883. [PubMed] [Google Scholar]
- 72.Zieske JD, Wasson M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J Cell Sci. 1993;106:145–152. doi: 10.1242/jcs.106.1.145. [DOI] [PubMed] [Google Scholar]
- 73.Zieske JD. Perpetuation of stem cells in the eye. Eye. 1994;8:163–169. doi: 10.1038/eye.1994.41. [DOI] [PubMed] [Google Scholar]
- 74.Liu JJ, Wilson SE. Characterization of human and mouse angiopoietin-like factor CDT6 promoters. Invest Ophthalmol Vis Sci. 2001;42:2776–2783. [PubMed] [Google Scholar]
- 75.Zhou X, Qu J, Xie R, Wang R, Jiang L, Zhao H, et al. Normal development of refractive state and ocular dimensions in guinea pigs. Vision Res. 2006;46:2815–2823. doi: 10.1016/j.visres.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 76.Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HPbeta-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. 2002;233:159–167. doi: 10.1016/s0378-5173(01)00933-4. [DOI] [PubMed] [Google Scholar]
- 77.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kruse FE. Stem cells and corneal epithelial regeneration. Eye. 1994;8:170–183. doi: 10.1038/eye.1994.42. [DOI] [PubMed] [Google Scholar]
- 79.Akpek EK, Foster CS. Limbal stem-cell transplantation. Int Ophthalmol Clin. 1999;39:71–82. doi: 10.1097/00004397-199903910-00009. [DOI] [PubMed] [Google Scholar]
- 80.Samson CM, Nduaguba C, Baltatzis S, Foster CS. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology. 2002;109:862–868. doi: 10.1016/s0161-6420(02)00994-6. [DOI] [PubMed] [Google Scholar]
- 81.Wylegala E, Tarnawska D, Wroblewska EM. Limbal stem cell transplantation from HLAcompatible living donors.Long term observation. Klin Oczna. 2003;105:378–83. [Article in Polish] [PubMed] [Google Scholar]
- 82.Shimazaki J, Shimmura S, Tsubota K. Limbal stem cell transplantation for the treatment of subepithelial amyloidosis of the cornea (gelatinous drop-like dystrophy) Cornea. 2002;21:177–180. doi: 10.1097/00003226-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Fernandes M, Sangwan VS, Rao SK, Basti S, Sridhar MS, Bansal AK, et al. Limbal stem cell transplantation. Indian J Ophthalmol, 2004;52:5–22. [PubMed] [Google Scholar]
- 84.Djalilian AR, Holland EJ, Schwartz GS. Limbal stem cell deficiency. Ophthalmology. 2003;110:2071–2072. doi: 10.1016/S0161-6420(03)00918-7. author reply 2071. [DOI] [PubMed] [Google Scholar]
- 85.Sauder G, Jonas JB. Limbal stem cell deficiency after subconjunctival mitomycin C injection for trabeculectomy. Am J Ophthalmol. 2006;141:1129–1130. doi: 10.1016/j.ajo.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 86.Fernandes M, Sangwan VS, Vemuganti GK. Limbal stem cell deficiency and xeroderma pigmentosum: a case report. Eye. 2004;18:741–743. doi: 10.1038/sj.eye.6700717. [DOI] [PubMed] [Google Scholar]
- 87.Ellies P, Anderson DF, Touhami A, Tseng SC. Limbal stem cell deficiency arising from systemic chemotherapy. Br J Ophthalmol. 2001;85:373–374. doi: 10.1136/bjo.85.3.371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 89.Alfonso EC. Treatment of severe ocular-surface disorders with corneal epithelial stem cell transplantation. Arch Ophthalmol. 2000;118:123–124. doi: 10.1001/archopht.118.1.123. [DOI] [PubMed] [Google Scholar]
- 90.Tseng SC, Tsai RJ. Limbal transplantation for ocular surface reconstruction-a review. Fortschr Ophthalmol. 1991;88:236–242. [PubMed] [Google Scholar]
- 91.Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 92.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 93.Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–2513. [PubMed] [Google Scholar]
- 94.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of sub-chains of the basement membrane components type IV collagen and laminin among the amniotic membrane,cornea and conunctiva. Cornea. 1999:73–79. [PubMed] [Google Scholar]
- 95.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120:783–790. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 96.Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- 98.Tsubota K. Ocular surface management in corneal transplantation, a review. Jpn J Ophthalmol. 1999;43:502–508. doi: 10.1016/s0021-5155(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 99.Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–575. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anderson DF. Amniotic membrane transplantation after the primary surgical management of band keratopathy. Cornea. 2001;20:354–361. doi: 10.1097/00003226-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 101.Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–240. doi: 10.1097/01.icu.0000172827.31985.3a. [DOI] [PubMed] [Google Scholar]
- 102.Solomon A, Ellies P, Anderson DF, Touhami A, Grueterich M, Espana EM, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–1166. doi: 10.1016/s0161-6420(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 103.Shimmura S, Tsubota K. Surgical treatment of limbal stem cell deficiency: are we really transplanting stem cells? Am J Ophthalmol. 2008;146:154–155. doi: 10.1016/j.ajo.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 104.Sangwan VS. Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007;55:251–260. doi: 10.4103/0301-4738.33036. [DOI] [PubMed] [Google Scholar]
- 105.Dua H, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 106.Marangon F, Alfonso EC, Miller D, Remonda NM, Muallem MS, Tseng SC. Incidence of microbial infection after amniotic membrane transplantation. Cornea. 2004;23:264–269. doi: 10.1097/00003226-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 107.Panda A, Ghose S, Khokhar S, Das H. Surgical outcomes of epibulbar dermoids. J Pediatr Ophthalmol Strabismus. 2002;39:20–25. doi: 10.3928/0191-3913-20020101-06. [DOI] [PubMed] [Google Scholar]
- 108.Gabler B, Lohmann C. Hypopyon after repeated transplantation of human amniotic membrane onto the corneal surface. Ophthalmology. 2000;107:1344–1346. doi: 10.1016/s0161-6420(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 109.Anderson NJ, Hardten DR, McCarty TM. Penetrating keratoplasty and keratolimbal allograft transplantation for corneal perforations associated with the ectodermal dysplasia syndrome. Cornea. 2003;22:385–388. doi: 10.1097/00003226-200305000-00022. [DOI] [PubMed] [Google Scholar]
- 110.Basti S, Mathur U. Unusual intermediate-term outcome in three cases of limbal autograft transplantation. Ophthalmology. 1999;106:958–963. doi: 10.1016/S0161-6420(99)00516-3. [DOI] [PubMed] [Google Scholar]
- 111.Basti S, Rao SK. Current status of limbal conjunctival autograft. Curr Opin Ophthalmol. 2000;11:224–232. doi: 10.1097/00055735-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Rao SK, Rajagopal R, Sitalakshmi G, Padmanabhan P. Limbal allografting from related live donors for corneal surface reconstruction. Ophthalmology. 1999;106:822–828. doi: 10.1016/S0161-6420(99)90173-2. [DOI] [PubMed] [Google Scholar]
- 113.Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506)in the management of high-risk corneal and limbal grafts. Ophthalmology. 2001;108:1838–1844. doi: 10.1016/s0161-6420(01)00759-x. [DOI] [PubMed] [Google Scholar]
- 114.Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125–130. doi: 10.1016/s0161-6420(02)01451-3. [DOI] [PubMed] [Google Scholar]
- 115.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 116.Kolli S, Lako M, Figueiredo F, Mudhar H, Ahmad S. Loss of corneal epithelial stem cell properties in outgrowths from human limbal explants cultured on intact amniotic membrane. Regen Med. 2008;3:329–342. doi: 10.2217/17460751.3.3.329. [DOI] [PubMed] [Google Scholar]
- 117.Grueterich M, Espana EM, Touhami A, Ti SE, Tseng SC. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 2002;109:1547–1552. doi: 10.1016/s0161-6420(02)01105-3. [DOI] [PubMed] [Google Scholar]
- 118.Lee S, Tseng CS. Rose Bengal staining and cytologic characteristics associated with lipid tear deficiency. Am J Ophthalmol. 1997;124:736–750. doi: 10.1016/s0002-9394(14)71690-3. [DOI] [PubMed] [Google Scholar]
- 119.Avila M, Espana M, Moreno C, Pena C. Reconstruction of ocular surface with heterologous limbal epithelium and amniotic membrane in a rabbit model. Cornea. 2001;20:414–420. doi: 10.1097/00003226-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 120.Han B, Schwab IR, Madsen TK, Isseroff RR. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea. 2002;21:505–510. doi: 10.1097/00003226-200207000-00013. [DOI] [PubMed] [Google Scholar]