The diagnostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and conventional CT regarding the ability to detect the primary tumor site in patients with extracervical metastases from carcinoma of unknown primary site was evaluated prospectively. 18F-FDG PET/CT was not shown to provide a clear advantage.
Keywords: Carcinoma of unknown primary tumor site, CUP, CT, 18F-FDG PET/CT
Learning Objectives
After completing this course, the reader will be able to:
Compare the diagnostic performances of 18F-FDG PET/CT and conventional CT with respect to their ability to detect primary tumor sites in carcinoma of unknown primary patients with extracervical metastases.
Describe the rate of identification of primary tumor sites using 18F-FDG PET/CT and conventional CT.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
The aim of the present study was to evaluate prospectively the diagnostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and conventional CT regarding the ability to detect the primary tumor site in patients with extracervical metastases from carcinoma of unknown primary (CUP) site.
Patients and Methods.
From January 2006 to December 2010, 136 newly diagnosed CUP patients with extracervical metastases underwent 18F-FDG PET/CT.
A standard of reference (SR) was established by a multidisciplinary team to ensure that the same set of criteria were used for classification of patients, that is, either as CUP patients or patients with a suggested primary tumor site. The independently obtained suggestions of primary tumor sites using PET/CT and CT were correlated with the SR to reach a consensus regarding true-positive (TP), true-negative, false-negative, and false-positive results.
Results.
SR identified a primary tumor site in 66 CUP patients (48.9%). PET/CT identified 38 TP primary tumor sites and CT identified 43 TP primary tumor sites. No statistically significant differences were observed between 18F-FDG PET/CT and CT alone in regard to sensitivity, specificity, and accuracy.
Conclusion.
In the general CUP population with multiple extracervical metastases 18F-FDG PET/CT does not represent a clear diagnostic advantage over CT alone regarding the ability to detect the primary tumor site.
Introduction
Carcinoma of unknown primary (CUP) represents a heterogeneous group of metastatic malignancies for which no primary site of the tumor can be identified following a thorough medical history, careful clinical examination, and extensive diagnostic workup. CUP accounts for 3%–5% of all cancer diagnoses [1, 2]. Several favorable CUP subsets, representing only ∼15% of all cases, have been recognized based on specific clinical and pathological features. These favorable subsets require organ-specific recommended treatment strategies, which may translate into better outcomes [3, 4].
Unfortunately, the majority of CUP patients do not fit into any of the above favorable subsets. These patients form an extremely heterogeneous group with a few common features, including the presence of multiple metastases, early dissemination, uncommon metastatic sites, and usually a poor prognosis [5]. It remains a diagnostic challenge to identify the primary tumor site in these patients. Despite the fact that the conventional diagnostic workup has improved over the years, a primary tumor is identified in <30% of CUP patients ante mortem [2].
In a recent review, it was estimated that a primary tumor site could be identified in 73% of CUP patients by postmortem examinations, most often in the lung, pancreas, or hepatobiliary tree [6]. Therefore, more sensitive diagnostic tools could lead to identification of more primary tumor sites in CUP patients.
18F-fluorodeoxyglucose positron emission tomography/computed tomography 18F-FDG PET/CT scanning, which combines metabolic and anatomical information, may provide the additional sensitivity required to detect the primary tumor sites. 18F-FDG PET/CT has been successfully used in staging and treatment monitoring for several solid tumor types [7]. As an example, in non-small cell lung cancer patients, 18F-FDG PET/CT has significantly better accuracy in staging and positively affects the therapeutic management, when compared with 18F-FDG PET or CT alone [8, 9]. Also, 18F-FDG PET and 18F-FDG PET/CT have been of great importance in the detection of the primary tumor site in the favorable CUP subset with cervical lymph node metastases and subsequent treatment planning [10–13]. The value of 18F-FDG PET/CT is less well studied in CUP patients with extracervical metastases. The available studies are mainly retrospective and small [14, 15]. In addition, no recent studies have evaluated conventional CT as a diagnostic tool in CUP patients.
The aim of the present study was to evaluate prospectively the diagnostic value of 18F-FDG PET/CT and conventional CT regarding their ability to detect the primary tumor site in CUP patients with extracervical metastases. The study was conducted at a single center to ensure the same PET/CT procedure and quality.
Patients and Methods
From January 2006 to December 2010, 136 newly diagnosed CUP patients were enrolled in this study at Copenhagen University Hospital Rigshospitalet, Denmark. The trial was approved by the local ethics committee (KF 01233694), and patients gave written informed consent. The study was reported on ClinicalTrials.gov (identifier, NCT00269373).
Eligibility
Newly diagnosed CUP patients aged ≥18 years with normal plasma creatinine referred for further diagnostic workup and treatment were included. Patients were considered to have CUP when a diagnostic workup as recommended by the European Society of Medical Oncology (ESMO) failed to identify the primary tumor site [16].
Exclusion criteria were: (a) a history of previous malignancy within 5 years except nonmelanoma skin cancer or in situ carcinoma of the cervix, (b) diabetes mellitus, (c) claustrophobia, (d) severe obesity (>150 kg), and (e) allergy to contrast media. In addition, the following favorable subsets of patients were excluded: (a) patients with squamous cell carcinoma or poorly differentiated carcinoma involving only the cervical lymph nodes, (b) patients with poorly differentiated carcinoma consistent with a germ cell tumor, (c) patients with neuroendocrine carcinoma, and (d) women with adenocarcinoma involving only the axillary lymph nodes.
After study completion, one patient was excluded because subsequent diagnostic workup identified three different primary tumor sites (ovarian cancer, cervical cancer, and sarcoma).
All referral hospitals had performed routine pathological evaluation of the CUP biopsies, including light microscopic evaluation and immunohistochemical stainings depending on clinical and pathological features. At Rigshospitalet, one pathologist (B.L.P.) reviewed all the CUP biopsies and a broad panel of antibodies was applied, including antibodies against site-specific antigens, mucin antigens, and intermediary filaments, to help suggest the primary tumor site. Poorly differentiated carcinomas were routinely stained with markers specific for lymphoma, malignant melanoma, germ cell tumor, sarcoma, neuroendocrine tumor, lung cancer, and prostate cancer.
PET/CT Scanning Procedure
Four integrated PET/CT scanners (GE Discovery LS PET/CT [General Electric Medical Systems, Milwaukee, WI]; Siemens Biograph Sensation 16; Siemens Biograph 40, TruePoint; and Siemens Biograph 64, TruePoint [Siemens Medical Solutions, Knoxville, TN]) were used. 18F-FDG PET/CT scans were carried out according to standard procedures. Patients fasted for ≥6 hours prior to i.v. injection of ∼400 MBq 18F-FDG. Imaging acquisition started ∼60 minutes after the administration of 18F-FDG. In order to minimize bladder activity, patients were asked to void right before image acquisition. The examination was performed with the patient positioned supine with the arms placed over the head, and patients were scanned from the base of the skull to the thigh.
The CT component of the PET/CT scan was performed as a diagnostic CT with the use of oral contrast medium (ioxitalamat solution, 12.6 mg I/mL, in 500 mL water) 30 minutes before image acquisition, and i.v. contrast enhancement (75–125 mL, 300 mg I/mL, Optiray™, Covidien Pharmaceuticals, Hazelwood, MO) was injected followed by 100 mL saline. The administration rate and delay varied depending on the scanner. The CT scan was performed immediately prior to the PET scan with a multidetector CT scanner (four- to 64-slice CT scans); CT parameters were 120–140 kV, reference 225 mAs. The PET scan followed immediately with acquisition times of 2.5–4 minutes per bed position depending on the size and weight of the patient. The PET scan was reconstructed by ordered-subset expectation-maximization, with data from the CT scan used for attenuation correction. The CT contrast did occasionally cause a PET artifact, but none of these led to false-positive (FP) findings.
Image Interpretation
Routine Procedure
Experienced radiologists (A.K.B., 15 years of experience; J.C.C., 3 years of experience) and nuclear medicine physicians (A.L., 18 years of experience; J.G., 11 years of experience; C.B.C., 3 years of experience), in teams of one radiologist and one nuclear medicine physician, evaluated the PET, fused PET/CT, and CT images side by side and a consensus was reached, which included suggestion of a potential primary tumor site, if possible, and the number of metastatic sites. Primary tumor site assessment was based on the appearance of a contrast-enhanced mass, necrosis, and/or invasiveness on the CT scan and/or the detection of focal pathologically higher 18F-FDG uptake on the PET scan. Thus, a PET-negative but obviously malignant-looking tumor seen on the CT part of the PET/CT scan would be defined as a positive lesion and an 18F-FDG PET-positive lesion without clear anatomical CT substrate was classified as negative. A written report with the PET and CT findings as well as the reached PET/CT consensus was sent to the Department of Oncology. Potential primary tumor sites suggested by 18F-FDG PET/CT were further investigated using other imaging techniques and/or a new biopsy or were correlated with clinicopathological features. According to the above findings, treatment was decided as: (a) organ-specific treatment if the primary tumor site was detected or (b) a CUP regimen if the primary tumor site remained unknown.
Standard of Reference and Classification of Study Results
To avoid potential variability introduced into daily clinical practice by different observers, one team consisting of an experienced oncoradiologist (A.K.B., 15 years of experience) and nuclear medicine physician (A.L., 18 years of experience) subsequently reviewed all PET/CT images together (PET, CT, and fused PET/CT images). All CT images from PET/CT scans were retrieved from the picture archiving and communication system and reviewed separately and independently by another experienced oncoradiologist (K.D.P., >30 years of experience) at a workstation in the Department of Radiology without knowledge of the PET/CT findings or the PET images. All observers were provided with the same information concerning the medical history but were blinded to the initial written PET/CT reports and treatment decisions. CUP patients were categorized as follows: (a) primary tumor site identified and (b) unknown primary tumor site. In addition, the number of metastatic sites was depicted and noted independently by the two teams.
It is inherently difficult to validate a diagnostic tool in CUP patients because the primary tumor site remains unknown in the majority of patients during life time. As a consequence, the independently obtained suggestions of primary tumor sites using PET/CT and CT alone were correlated with a Standard of Reference (SR) that was established by an experienced pathologist (B.L.P.) and two experienced oncologists (G.D. and K.P.). The SR reviewed all clinical and pathological information (patient demographics, metastatic pattern, results of clinical and laboratory tests, imaging data—including the initial, routine PET/CT report—and the results from pathological evaluations of the biopsies or autopsy) from each patient within a 2-month follow-up period after the performed PET/CT scan. Based on the above information, the SR either classified the patient as having CUP or suggested a primary tumor site. The clinical follow-up period was limited to 2 months after the PET/CT scan to avoid introduction of incorrect false-negative (FN) scan results. A longer follow-up period might have allowed for tumor progression to a stage in which a primary tumor site that initially was undetectable when the PET/CT scan was performed had become visible (e.g., using new imaging procedures, biopsies, or autopsies).
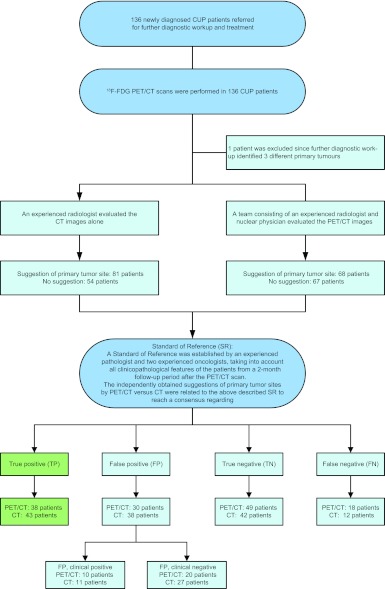
The independently obtained suggestions of primary tumor sites using PET/CT versus CT scans were correlated with the above described SR to reach a consensus regarding true-positive (TP), true-negative (TN), FN, and FP results. In some patients with an FP result, the SR identified a different primary tumor site. FP results were therefore divided into: (a) FP, clinical positive (FPCP), that is, FP results for which the SR identified a different primary tumor site, and (b) FP, clinical negative (FPCN), that is FP results for which the primary tumor site remained unknown (Fig. 1).
Figure 1.
Consort diagram.
Abbreviations: CT, computed tomography; CUP, carcinoma of unknown primary; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
Data Analysis
The diagnostic performance (sensitivity, specificity, and accuracy) of each imaging modality was calculated using the following formulas: sensitivity = TP/(TP + FPCP + FN), specificity = TN/(TN + FPCN), and accuracy = TP + TN/(TP + TN + FP + FN).
Differences in diagnostic performance between the independently obtained 18F-FDG PET/CT and CT results were tested for significance using McNemar's test (two-sided level of significance <.05) and are reported with a 95% confidence interval. Statistical analyses were carried out using SPSS version 18 (SPSS Inc., Chicago, IL) and SAS version 9.2 (SAS Institute Inc., Cary, NC) software.
Results
Patient Characteristics
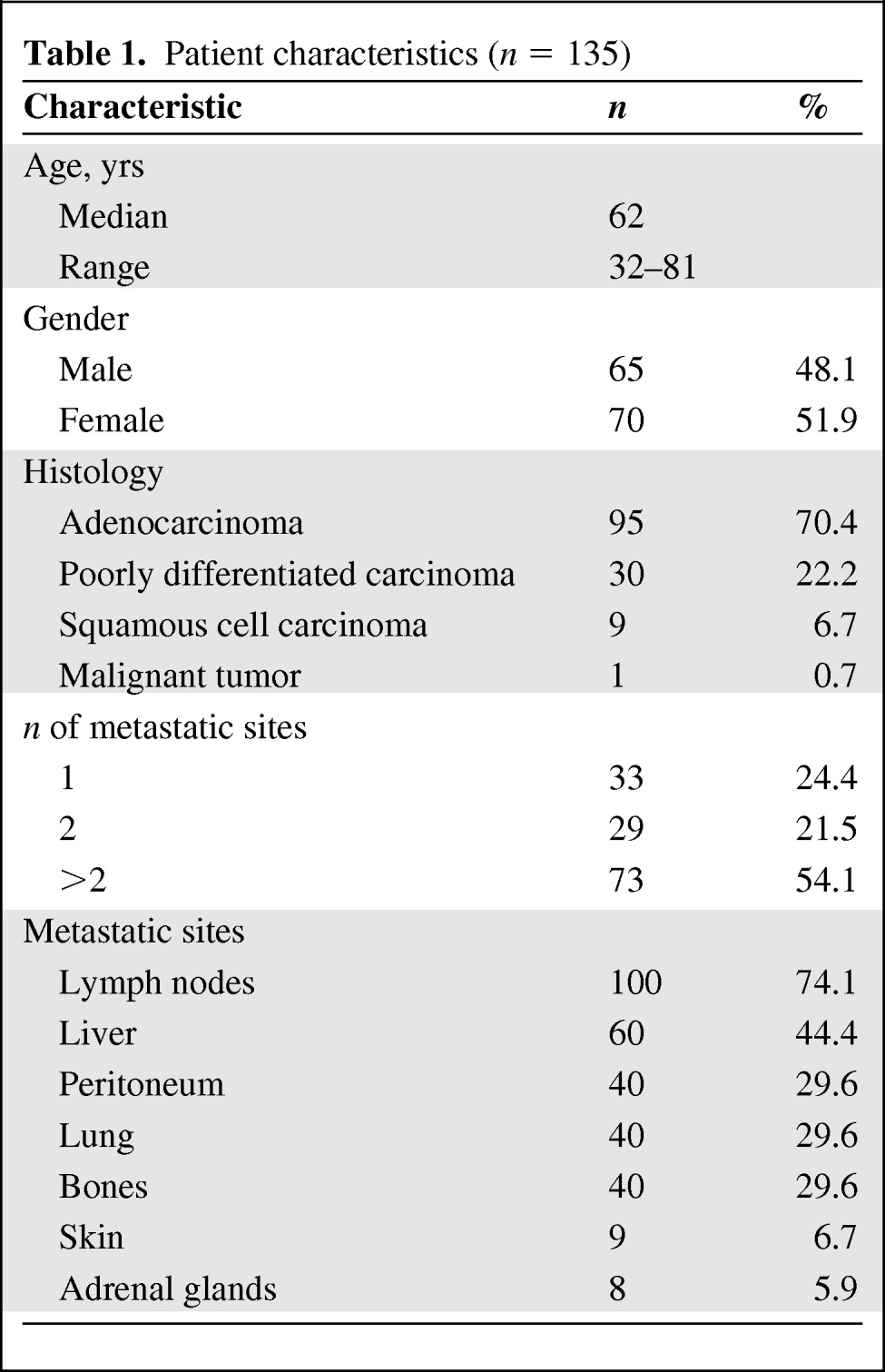
The characteristics of the 135 CUP patients are summarized in Table 1.
Table 1.
Patient characteristics (n = 135)
Because of the extended recruitment period (5 years), new PET/CT scanners were introduced during the study period. The PET/CT scans were performed with 4- or 16-slice CT scanners for the first 93 CUP patients (68.9%). In the remaining 42 patients (31.1%), the PET/CT was performed with either 40- or 64-slice CT scanners. Of note, there were no statistical differences in the diagnostic accuracy (TN + TP) between the results obtained with 4- or 16-slice CT scanners and those obtained with 40- or 64-slice CT scanners.
Baseline Examinations
An overview of the diagnostic procedures performed prior to the 18F-FDG PET/CT scan is provided in Table 2.
Table 2.
Baseline examinations (n = 135)
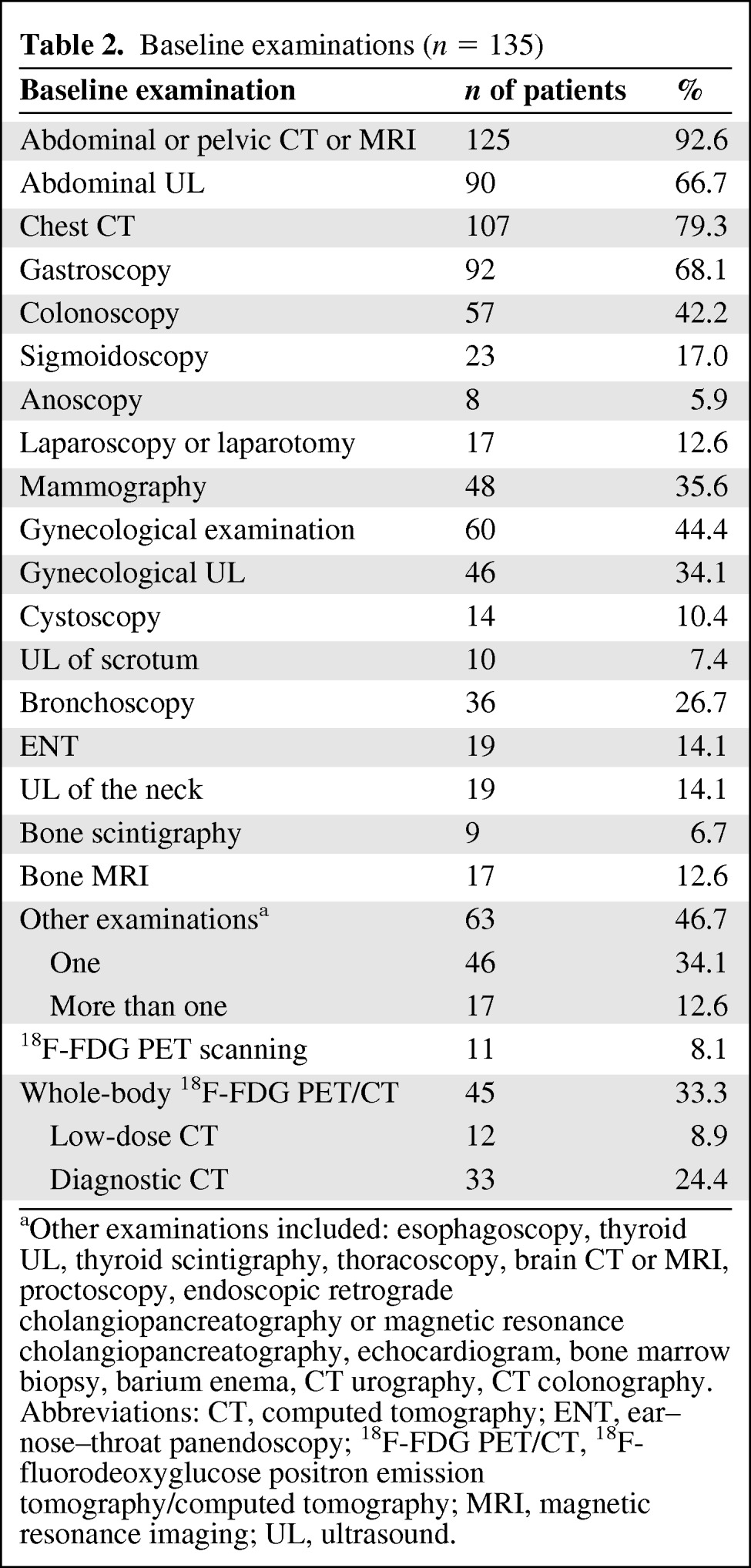
aOther examinations included: esophagoscopy, thyroid UL, thyroid scintigraphy, thoracoscopy, brain CT or MRI, proctoscopy, endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography, echocardiogram, bone marrow biopsy, barium enema, CT urography, CT colonography.
Abbreviations: CT, computed tomography; ENT, ear–nose–throat panendoscopy; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; MRI, magnetic resonance imaging; UL, ultrasound.
The median number of days between the conventional cross-sectional diagnostic workup at the referral hospital and the 18F-FDG PET/CT scan at Rigshospitalet was 32 days (range, 1–120 days). An abdominal CT or magnetic resonance imaging (MRI) scan and a chest CT scan were performed in the majority of patients (n = 125 patients, 92.6% and n = 107, 79.3%, respectively). The 10 patients without an earlier abdominal CT or MRI scan had all previously undergone a whole-body contrast-enhanced 18F-FDG PET/CT scan, whereas 17 of the 28 CUP patients without an earlier chest CT scan had a whole-body 18F-FDG PET/CT scan. The 11 patients without a prior chest CT or whole-body PET/CT all had a normal chest x-ray and were therefore not excluded from the study. In addition, exclusion of the 11 patients without a prior chest CT or 18F-FDG PET/CT scan from the analysis did not change the overall conclusions (data not shown).
Independent Findings Using 18F-FDG PET/CT and CT
The primary tumor sites identified by the SR are summarized in Table 3 and supplemental online Tables 1 and 2. In 66 patients (48.9%), a primary tumor site was identified, and in 69 patients the primary tumor site remained undetected (51.1%). The identified primary tumor sites were, in most cases, based on correlation of imaging data and histopathological information (43 patients) (Table 3). For five patients, the primary tumor sites were identified at autopsy within the 2-month follow-up period, and for 13 patients they were identified following confirmation by a new biopsy or surgery. Furthermore, biopsy revision and additional immunohistochemistry alone identified five primary tumor sites (two goblet cell carcinoids, one disseminated skin cancer [basocellular carcinoma], one angiosarcoma, and one desmoplastic small round cell tumor) (Table 3).
Table 3.
Identified primary tumor sites by the standard of reference
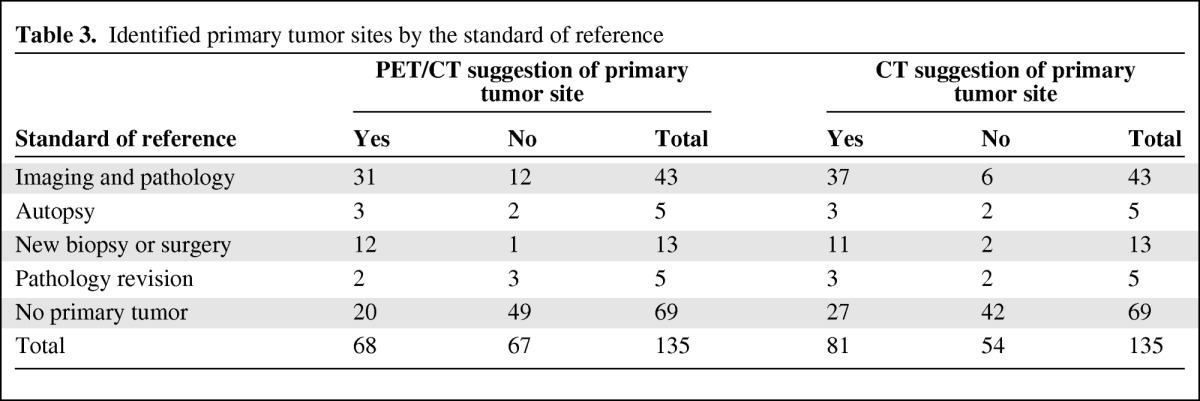
The PET/CT team suggested a primary tumor site in 68 of the 135 patients (50.4%).These suggestions were found to be TP in 38 patients and FP in 30 patients (Fig. 1 and supplemental online Table 1). The CT analysis by the independent oncoradiologist suggested a primary tumor site in 81 patients (60%), and 43 of these patients were found to be TP whereas 38 were found to be FP (Fig. 1 and supplemental online Table 2). In 25 patients, the diagnoses were found to be TP using both imaging modalities. An overview of the results using 18F-FDG PET/CT and CT is provided in supplemental online Tables 1 and 2, respectively.
Of the 67 patients with a negative PET/CT scan, the PET/CT diagnoses were TN in 49 patients, whereas CT alone resulted in a TN finding in 42 of 54 patients. Of note, similar patterns of FN tumor sites were obtained with the two imaging methods (supplemental online Tables 1 and 2).
In ∼20 patients with multiple metastases and an FP result with PET/CT and/or CT, biopsy-verified cancer tissue was found in the suggested sites. However, histopathological assessment indicated that these lesions were metastases and not of primary origin.
PET/CT identified significantly more CUP patients with bone metastases than CT alone (28.1% versus 20%; McNemar's test p = .0045).
Comparison of 18F-FDG PET/CT and CT
For PET/CT, the specificity, sensitivity, and diagnostic accuracy were 71%, 57.6%, and 64.4%, respectively. For CT, the specificity, sensitivity, and diagnostic accuracy were 60.9%, 65.2%, and 63%, respectively. No statistically significant differences were observed between the two imaging modalities in regard to these parameters (Table 4).
Table 4.
Sensitivity, specificity, and accuracy of 18F-FDG PET /CT and CT
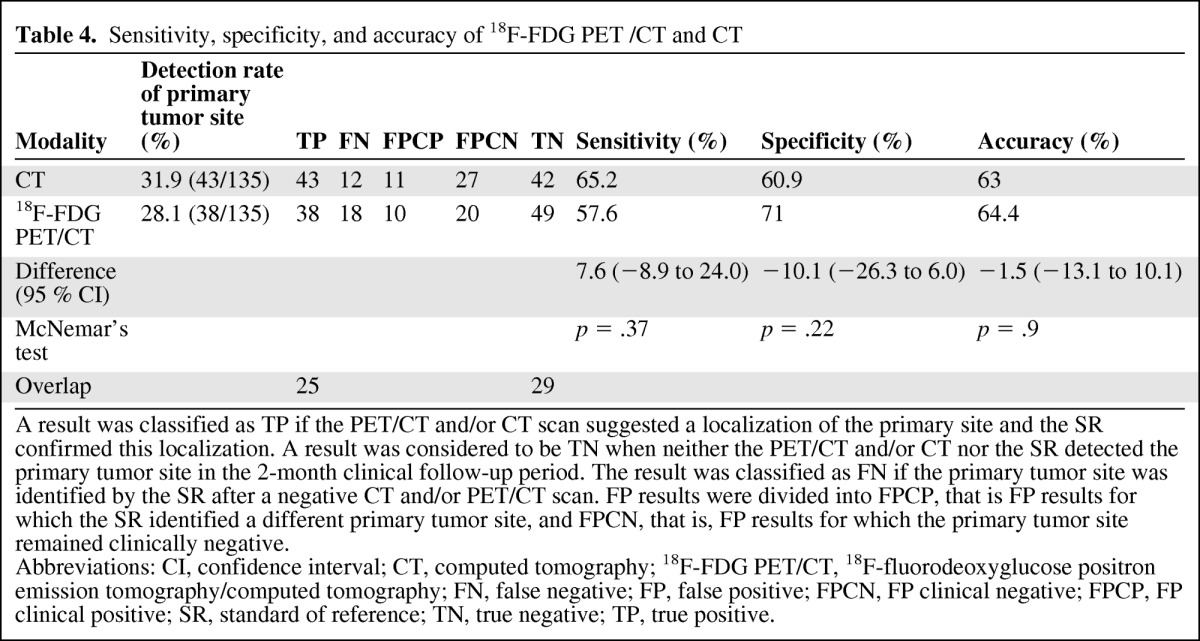
A result was classified as TP if the PET/CT and/or CT scan suggested a localization of the primary site and the SR confirmed this localization. A result was considered to be TN when neither the PET/CT and/or CT nor the SR detected the primary tumor site in the 2-month clinical follow-up period. The result was classified as FN if the primary tumor site was identified by the SR after a negative CT and/or PET/CT scan. FP results were divided into FPCP, that is FP results for which the SR identified a different primary tumor site, and FPCN, that is, FP results for which the primary tumor site remained clinically negative.
Abbreviations: CI, confidence interval; CT, computed tomography; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; FN, false negative; FP, false positive; FPCN, FP clinical negative; FPCP, FP clinical positive; SR, standard of reference; TN, true negative; TP, true positive.
Discussion
The majority of CUP patients are characterized by widely disseminated disease and a poor prognosis [2]. Diagnostic tools for prompt diagnosis of the primary tumor site are highly needed. In the present prospective study, we compared the diagnostic performances of 18F-FDG PET/CT and CT in 135 newly diagnosed CUP patients with extracervical metastases referred for further diagnostic work-up and treatment. In 66 CUP patients (48.9%), a primary tumor site was identified using the SR. The number of identified primary tumor sites using the SR in our study is comparable with those obtained in most other 18F-FDG PET/CT studies in CUP patients with extracervical metastases (mean, 45%; range, 37%–51%) [15].
Lung cancer was the most commonly identified primary tumor site using the SR (12 patients). All these patients had undergone a chest CT and 10 patients had a bronchoscopy prior to the 18F-FDG PET/CT scan. Thus, the number of identified lung cancers in the present study does not represent patients with an inappropriate diagnostic workup prior to 18F-FDG PET/CT.
Assessment and comparison of overall survival outcomes between patients in whom a primary tumor site was detected and patients in whom the primary tumor site remained unknown were not performed in the present study. Of note, in a study by Yapar et al. [17] identification of the primary tumor site did not result in a better survival outcome in 90 CUP patients.
In the present study, PET/CT identified 38 (28.1%) TP primary tumor sites and CT identified 43 (31.9%) TP primary tumor sites. No statistically significant differences were observed between the two imaging modalities in regard to sensitivity, specificity, and accuracy.
Although numerically similar results were obtained with 18F-FDG PET/CT and CT, there were considerable differences in regard to the suggested primary tumor sites (Figs. 2 and 3). Among the TP results obtained with PET/CT and CT, only 25 cases were overlapping. Similarly, only 29 overlapping TN results were obtained. All 18F-FDG PET/CT images were evaluated by a single team, and the CT images from the PET/CT scans were evaluated by a single, independent observer. This experimental setup may have introduced interobserver variability between 18F-FDG PET/CT and CT results. Therefore, in the present study, a direct, unbiased comparison of the two imaging methods is not possible, and the limited overlap may, to a large extent, be a result of this unavoidable interobserver variability.
Figure 2.
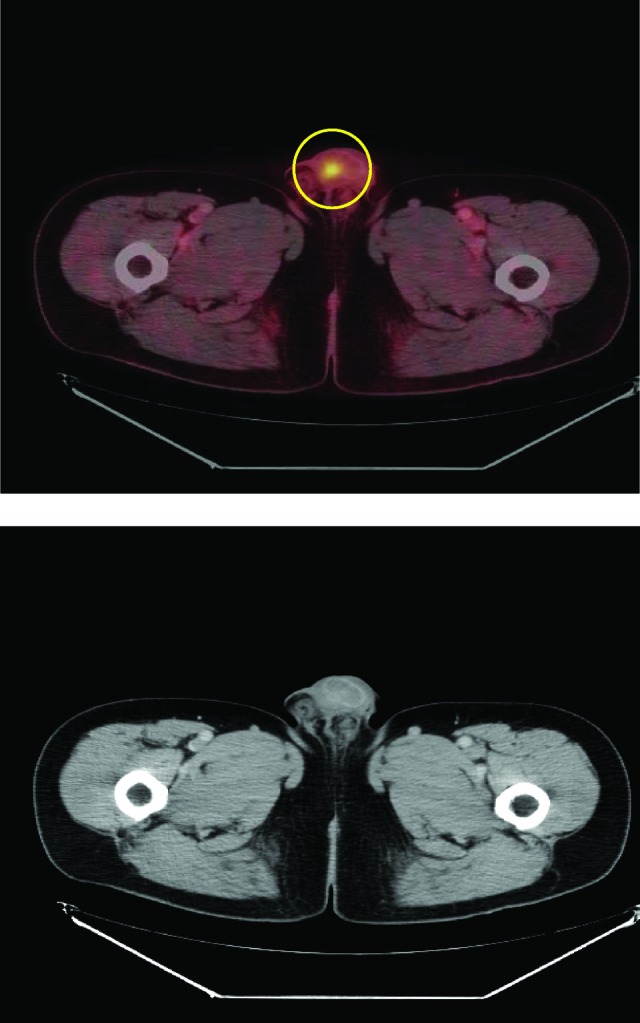
A 59-year-old man with squamous cell carcinoma in inguinal lymph nodes. The primary tumor site in the urethra was only visible on the combined positron emission tomography/computed tomography scan but not with computed tomography alone.
Figure 3.
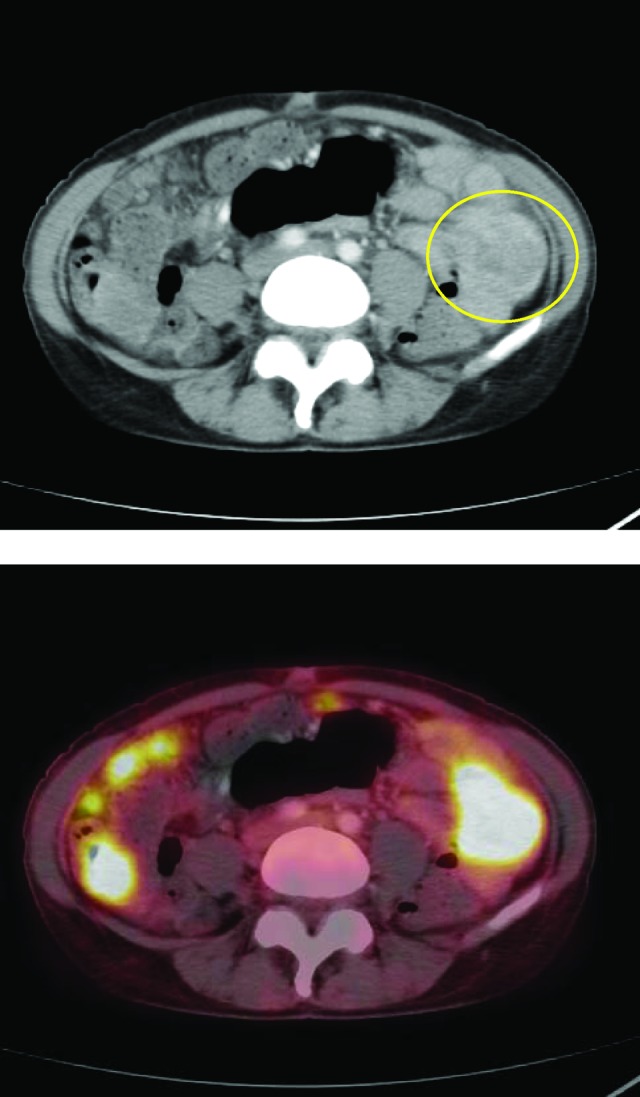
A 51-year-old woman with adenocarcinoma of the peritoneal cavity. The histopathological suggestion of the primary tumor site was the lower gastrointestinal tract. The primary tumor site in the small intestine was only visible with computed tomography alone. On the combined positron emission tomography/computed tomography scan, the primary tumor site was misinterpreted as peritoneal carcinomatosis.
Several studies have evaluated and compared the diagnostic performance of 18F-FDG PET/CT, 18F-FDG PET, and CT using different study designs to minimize interobserver variability among imaging modalities. One approach is to use the same group of observers evaluating the 18F-FDG PET, CT, and fused 18F-FDG PET/CT images [18, 19]. However, this approach may introduce recognition bias even when adding a time interval between the different reading sessions [18].
The majority of the included patients in this study presented with two or more metastatic sites. Because the primary tumor site might be small compared with the metastases and the metastases might present in an unusual pattern or organ, it may be difficult to discriminate between metastases and primary tumor sites using imaging alone (Fig. 4). This may explain some of the interobserver variability and the rather low sensitivity, specificity, and overall accuracy in this heterogeneous group of cancer patients. It is not possible to set a clear definition of how to discriminate a primary tumor from a metastasis. This is often an observer-dependent decision. There are no guidelines concerning FDG uptake for this kind of discrimination. However, the findings of an expansive, less well-defined, and irregular lesion on CT images in the lung parenchyma, pancreas, gastrointestinal tract, or urinary bladder raise the suspicion of a primary tumor and indicate the need for an additional image-guided biopsy in patients with proven metastatic disease at other sites.
Figure 4.
A 61-year-old man with adenocarcinoma in multiple organs (lung, liver, bone, lymph nodes). The histopathological suggestion of the primary tumor site was the lower gastrointestinal tract. The primary tumor was not identified with either positron emission tomography/computed tomography or computed tomography alone.
To our knowledge, no other prospective studies comparing 18F-FDG PET/CT with CT have been conducted in patients with extracervical metastases of CUP. Thus, only four retrospective studies comprising 152 CUP patients have addressed this important diagnostic issue [17, 18, 20, 21]. 18F-FDG PET/CT detected the primary tumor site in 60 patients with extracervical CUP (39.5%). Selection bias might be a problem in these studies because of their retrospective nature. Furthermore, all the included studies had rather low quality scores [14, 15]. Only one of the above studies compared the diagnostic performance of 18F-FDG PET alone, CT alone, 18F-FDG PET and CT side by side, and fused 18F-FDG PET/CT images [18]. Although, fused 18F-FDG PET/CT images revealed more primary tumor sites than the other modalities, the differences were not statistically significant.
In the present study, PET/CT revealed significantly more patients with bone metastases than CT alone (28.1% versus 20%; p = .0045), but the detection of this additional metastatic site using PET/CT did not change the treatment decision in any of these patients because all patients had multiple metastases.
Despite the limitations regarding the interobserver variability that is inherent in the current study design, it seems appropriate to conclude that, in the general CUP population with multiple extracervical metastases, 18F-FDG PET/CT does not represent a clear diagnostic advantage over CT alone. In a newly published study, Park et al. [22] obtained similar results and reached similar conclusions. Therefore, 18F-FDG PET/CT cannot be recommended as a routine examination in CUP patients because the accuracy is similar to that of CT alone and 18F-FDG PET/CT is associated with higher costs and a longer examination time than CT alone and cannot be performed in all hospitals. In CUP patients with multiple extracervical metastases, we suggest a high-quality contrast-enhanced multidetector CT scan of the chest, abdomen, and pelvis for the standard initial diagnostic workup [23], which is also the recommendation of the ESMO guidelines [16]. Further diagnostic workup should depend on specific signs and clinical and pathological features. However, for CUP patients presenting with a solitary metastasis or a single metastatic site, a contrast-enhanced PET/CT scan may be superior to CT alone to define definitive locoregional therapy, including confirmation of the solitary nature of the disease. Prospective studies are required to confirm the above hypothesis.
Using all relevant diagnostic tools, a multidisciplinary team (SR) was only able to reach a putative primary tumor site diagnosis in 66 of the 135 (48.9%) newly diagnosed CUP patients with extracervical metastases. Gene-expression profiling may help to identify the primary tumor in CUP patients, especially when used in concert with histopathology and imaging techniques [24–26]. This approach is of particular interest in the half of the CUP patients in whom the primary tumor site remains unknown.
In conclusion, a primary tumor site can be identified in ∼50% of CUP patients by a multidisciplinary team. The results from this study indicate that 18F-FDG PET/CT does not represent a clear diagnostic advantage over CT alone regarding the ability to detect the primary tumor site in CUP patients with multiple extracervical metastases.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Anne Kirstine H. Møller, Gedske Daugaard, Annika Loft, Anne K. Berthelsen, Jesper Graff
Provision of study material or patients: Anne Kirstine H. Møller, Gedske Daugaard, Annika Loft, Anne K. Berthelsen, Jesper Graff, Karen D. Pedersen, Bodil L. Petersen
Collection and/or assembly of data: Anne Kirstine H. Møller, Gedske Daugaard, Katharina Perell, Annika Loft, Anne K. Berthelsen, Jesper Graff, Karen D. Pedersen, Charlotte B. Christensen, Bodil L. Petersen
Data analysis and interpretation: Anne Kirstine H. Møller, Gedske Daugaard, Junia C. Costa, Katharina Perell, Annika Loft, Anne K. Berthelsen, Jesper Graff, Karen D. Pedersen, Charlotte B. Christensen, Bodil L. Petersen, Lene T. Skovgaard
Manuscript writing: Anne Kirstine H. Møller, Gedske Daugaard, Junia C. Costa, Katharina Perell, Annika Loft, Anne K. Berthelsen, Jesper Graff, Karen D. Pedersen, Charlotte B. Christensen, Bodil L. Petersen, Lene T. Skovgaard
Final approval of manuscript: Anne Kirstine H. Møller, Gedske Daugaard, Junia C. Costa, Katharina Perell, Annika Loft, Anne K. Berthelsen, Jesper Graff, Karen D. Pedersen, Charlotte B. Christensen, Bodil L. Petersen, Lene T. Skovgaard
References
- 1.Pavlidis N, Briasoulis E, Hainsworth J, et al. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39:1990–2005. doi: 10.1016/s0959-8049(03)00547-1. [DOI] [PubMed] [Google Scholar]
- 2.Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP) Crit Rev Oncol Hematol. 2009;69:271–278. doi: 10.1016/j.critrevonc.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Fizazi K. Treatment of patients with specific subsets of carcinoma of an unknown primary site. Ann Oncol. 2006;17(suppl 10):x177–x180. doi: 10.1093/annonc/mdl256. [DOI] [PubMed] [Google Scholar]
- 4.Hainsworth JD, Fizazi K. Treatment for patients with unknown primary cancer and favorable prognostic factors. Semin Oncol. 2009;36:44–51. doi: 10.1053/j.seminoncol.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Daugaard G, Moller AK, Petersen BL. Cancer of unknown primary site. In: Cavalli F, Hansen H, Kaye S, et al., editors. Textbook of Medical Oncology. Fourth Edition. London, United Kingdom: Informa Healthcare; 2009. pp. 313–322. [Google Scholar]
- 6.Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: From autopsy to microarray. Eur J Cancer. 2007;43:2026–2036. doi: 10.1016/j.ejca.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Poeppel TD, Krause BJ, Heusner TA, et al. PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol. 2009;70:382–392. doi: 10.1016/j.ejrad.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 9.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 10.Johansen J, Buus S, Loft A, et al. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck. 2008;30:471–478. doi: 10.1002/hed.20734. [DOI] [PubMed] [Google Scholar]
- 11.Keller F, Psychogios G, Linke R, et al. Carcinoma of unknown primary in the head and neck: Comparison between positron emission tomography (PET) and PET/CT. Head Neck. 2011;33:1569–1575. doi: 10.1002/hed.21635. [DOI] [PubMed] [Google Scholar]
- 12.Roh JL, Kim JS, Lee JH, et al. Utility of combined (18)F-fluorodeoxyglucose-positron emission tomography and computed tomography in patients with cervical metastases from unknown primary tumors. Oral Oncol. 2009;45:218–224. doi: 10.1016/j.oraloncology.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101:2641–2649. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 14.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: Systematic review and meta-analysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Møller AK, Loft A, Berthelsen AK, et al. 18F-FDG PET/CT as a diagnostic tool in patients with extracervical carcinoma of unknown primary site: A literature review. The Oncologist. 2011;16:445–451. doi: 10.1634/theoncologist.2010-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fizazi K, Greco FA, Pavlidis N, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(suppl 6):vi64–vi68. doi: 10.1093/annonc/mdr389. [DOI] [PubMed] [Google Scholar]
- 17.Yapar Z, Kibar M, Yapar AF, et al. The value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in carcinoma of an unknown primary: Diagnosis and follow-up. Nucl Med Commun. 2010;31:59–66. doi: 10.1097/MNM.0b013e328332b340. [DOI] [PubMed] [Google Scholar]
- 18.Gutzeit A, Antoch G, Kḧl H, et al. Unknown primary tumors: Detection with dual-modality PET/CT—initial experience. Radiology. 2005;234:227–234. doi: 10.1148/radiol.2341031554. [DOI] [PubMed] [Google Scholar]
- 19.Veit-Haibach P, Luczak C, Wanke I, et al. TNM staging with FDG-PET/CT in patients with primary head and neck cancer. Eur J Nucl Med Mol Imaging. 2007;34:1953–1962. doi: 10.1007/s00259-007-0564-5. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosini V, Nanni C, Rubello D, et al. 18F-FDG PET/CT in the assessment of carcinoma of unknown primary origin. Radiol Med. 2006;111:1146–1155. doi: 10.1007/s11547-006-0112-6. [DOI] [PubMed] [Google Scholar]
- 21.Pelosi E, Pennone M, Deandreis D, et al. Role of whole body positron emission tomography/computed tomography scan with 18F-fluorodeoxyglucose in patients with biopsy proven tumor metastases from unknown primary site. Q J Nucl Med Mol Imaging. 2006;50:15–22. [PubMed] [Google Scholar]
- 22.Park JS, Yim JJ, Kang WJ, et al. Detection of primary sites in unknown primary tumors using FDG-PET or FDG-PET/CT. BMC Res Notes. 2011;4:56. doi: 10.1186/1756-0500-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchal G, Vogl TJ, Heiken JP, Rubin GD, editors. Milan, Italy: Springer; 2005. Multidetector-Row Computed Tomography: Scanning and Contrast Protocols; pp. 1–130. [Google Scholar]
- 24.Greco FA, Spigel DR, Yardley DA, et al. Molecular profiling in unknown primary cancer: Accuracy of tissue of origin prediction. The Oncologist. 2010;15:500–506. doi: 10.1634/theoncologist.2009-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morawietz L, Floore A, Stork-Sloots L, et al. Comparison of histopathological and gene expression-based typing of cancer of unknown primary. Virchows Arch. 2010;456:23–29. doi: 10.1007/s00428-009-0867-y. [DOI] [PubMed] [Google Scholar]
- 26.Varadhachary GR, Talantov D, Raber MN, et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008;26:4442–4448. doi: 10.1200/JCO.2007.14.4378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.