In this longitudinal study among colorectal cancer patients who received curative treatment, greater patient engagement with clinicians about cancer-related information was found to improve patients' subsequent adherence to recommended surveillance.
Keywords: Information-seeking behavior, Colorectal neoplasms, Early detection of cancer, Neoplasm recurrence, Guideline adherence, Follow-up studies
Abstract
Introduction.
Follow-up surveillance after curative treatment for colorectal cancer (CRC) patients is recommended to detect early cancer recurrences and improve survival outcomes. However, a substantial proportion of CRC patients do not undergo cancer surveillance. Several demographic and disease-related factors have been associated with cancer surveillance adherence. Thus far, patient-centered communication has not been studied as a determinant for undergoing cancer surveillance. The purpose of this study is to determine whether patient–clinician information engagement (PCIE) influences patients' self-reported adherence to recommended CRC surveillance procedures.
Methods.
The study was a longitudinal survey among Pennsylvanian patients diagnosed with CRC in 2005. CRC patients who were eligible for surveillance and participated in both the baseline and 1-year follow-up surveys were included in this analysis (n = 305). The main outcome measure was self-reported adherence to physical examination, carcinoembryonic antigen testing, and colonoscopy according to recommended guidelines.
Results.
Controlling for potential confounders, higher PCIE at baseline predicted a higher odds for CRC patients reporting adherence to recommended surveillance 1 year later by 2.8 times. Other significant predictors of adhering to recommended surveillance were a higher education level and having received systemic therapy.
Discussion.
In this longitudinal study among CRC patients who received curative treatment, greater patient engagement with clinicians about cancer-related information was found to improve patients' subsequent adherence to recommended surveillance. This finding provides support for encouraging greater patient–physician communication among CRC patients.
Introduction
Routine postoperative surveillance represents an integral part of the follow-up care of patients diagnosed with colorectal cancer (CRC) who undergo potentially curative resection [1–3]. Cancer surveillance is aimed at detecting recurrences of the cancer amenable to further curative treatment, screening for new tumors or polyps, and detecting metastatic sites before patients present with symptoms. Two meta-analyses reported that conducting post-treatment surveillance through procedures including serum carcinoembryonic antigen (CEA) testing, endoscopy, physical examination, and computed tomography (CT) imaging was associated with the beneficial outcomes of a lower overall mortality rate, earlier detection of recurrences, and better chances of curative re-resection [4, 5]. However, a substantial proportion of CRC patients do not undergo routine cancer surveillance following curative surgery [6–11].
Understanding the determinants of adherence to CRC surveillance, especially modifiable ones, is therefore important for improving patient health and survival outcomes. Studies showed that ethnicity, income, age, and cancer stage were associated with a greater likelihood of receiving surveillance testing according to recommended guidelines. For instance, studies based on the Surveillance, Epidemiology, and End Results (SEER)–Medicare database reported that CRC patients who were younger, were white, had regional stage cancers, or had poorly differentiated tumors were more likely to adhere to guideline-recommended surveillance testing [6, 11]. Research in a managed care setting showed that CRC patients who were white or lived in neighborhoods with higher median household incomes were more likely to undergo colon examination and CEA testing [9]. Unfortunately, the above sociodemographic and clinical predictors of surveillance testing are often not modifiable.
Research suggests that patients' active engagement in cancer-related information seeking from various sources—in particular their physicians—may be an important factor influencing cancer surveillance adherence. First, we know that CRC patients are interested in obtaining information about a variety of topics related to their disease from myriad sources and are reported as frequently seeking for health information [12–15]. To illustrate, most CRC survivors reported needing more information related to tests and treatments (e.g., follow-up tests and procedures that they should have), health promotion (e.g., nutrition and diet information), and side effects of treatment or symptoms to alert their doctors to [12]. Cancer patients in general frequently cited health professionals as an information source and, more specifically within this category, physicians were the most often identified information source [13]. Furthermore, we know that patient-centered communication plays an important role in influencing the quality of patient–clinician relationships, compliance with cancer screening and treatment guidelines, as well as short- and longer-term patient outcomes [16]. For instance, individuals' perceived quality of communication with their physicians is significantly associated with greater adherence to cancer screening tests in healthy populations [17–21]. Among cancer patients, information engagement between patients and their physicians significantly predicts treatment decision satisfaction [22], patients' perceived health status, and self-reported quality of life [23–25].
In light of the above evidence, this study investigated the relationship between one form of patient-centered communication—patient–clinician information engagement (PCIE)—and CRC patients' adherence to recommended cancer surveillance in the form of physical examinations, CEA testing, and colonoscopy or sigmoidoscopy. We conducted a longitudinal survey among a population-based sample of CRC patients in the post-treatment period to assess their baseline PCIE and subsequent cancer surveillance 1 year later. The study findings will inform CRC patients, their caregivers, and their clinicians on the role of active engagement with health care professionals as a determinant of adherence to cancer surveillance testing.
Materials and Methods
Study Population and Procedure
The overall study population was a randomly selected sample of patients diagnosed with breast cancer, prostate cancer, or CRC between January 2005 and December 2005 as reported to the Pennsylvania Cancer Registry (PCR). The sampling frame included all patients diagnosed with one of these cancers who were listed in the PCR before data collection began in September 2006. This accounted for ∼95% of all incident cases of these cancers in Pennsylvania in 2005 that would be reported to the PCR. To ensure sufficient statistical power for planned subgroup analyses, we oversampled cancer patients with stage IV disease and African-American patients. Further details of the overall study population and sample selection are described elsewhere [25, 26].
We developed the questionnaire following a literature review, expert consultation, and a pilot study with 29 cancer patients. Appropriate revisions to the survey were included following the pilot testing. We mailed survey questionnaires to participants using Dillman's tailored design method for mail surveys [27]. We first sent a notice letter to sampled participants informing them of the study objectives and instructions for opting out. The survey, a small monetary incentive, and a stamped return envelope were then sent to participants. Those who did not indicate their wish to opt out and did not return the survey within 2 weeks were sent an additional letter and survey. Instructions for completing the survey indicated that participation was voluntary and submitting a completed questionnaire implied informed consent. The University of Pennsylvania Institutional Review Board approved the study procedure and materials.
Outcome Variable: Adherence to Recommended Surveillance
The outcome measure was a binary variable representing patients' adherence to recommended follow-up surveillance procedures during the 12 months preceding the survey in round 2 (∼2 years after being diagnosed with CRC—survey participants were diagnosed with CRC in January to December 2005, the baseline survey was conducted in September 2006, and the follow-up survey was conducted in September 2007). The criteria for adherence were adopted from Cooper and colleagues [6], and being adherent was defined as having received all the following: (a) two or more office visits or physical examinations in the last year, (b) two or more CEA tests in the last year, and (c) one colonoscopy within the last year. These criteria represent a composite of the minimum level of surveillance procedures consistent with the prevailing recommended procedures in 2005 that were common across major professional societies (e.g., the American Society of Clinical Oncology [ASCO], National Comprehensive Care Network [NCCN], and American Society of Colon and Rectal Surgeons) [1–3].
We assessed participants' frequency of undergoing cancer surveillance procedures by asking participants in round 2: “How often have you done the following things in the past 12 months, as part of your routine cancer follow-up? Do not include the times that you have done things because of a new symptom or health concern.” (These emphases were included in the original survey questionnaire.) Participants indicated the frequency, ranging from zero times to five or more times, of: (a) a doctor visit and physical exam, (b) a CEA blood test, and (c) a colonoscopy or flexible sigmoidoscopy.
Independent Variable: PCIE
PCIE is conceptualized as the process of information engagement between cancer patients and their clinicians, primarily operationalized as patients' reports of active seeking of cancer-related information from clinician sources. To a smaller extent, PCIE included patients' recall of physician-initiated communication behaviors (i.e., recommending patients obtain information from other sources). This analysis used the PCIE scale as described by Martinez and colleagues [22]. The scale comprised eight survey items that asked participants to think back to the first few months of their cancer diagnosis and to recall whether or not they: (a) sought information about treatments from their treating physician, (b) sought treatment information from other physicians or health professionals, (c) actively looked for information about their cancer from their treating physician, (d) looked for cancer information from other physicians or health professionals, (e) discussed information from other sources with their treating physician, (f) received suggestions from their treating physician to get information from other sources, (g) actively looked for information about quality of life issues from their treating physician, and (h) looked for quality of life information from other physicians or health professionals. Each item was converted into a Z-score and the average of the eight Z-scores formed the PCIE scale. These standardized items demonstrated reasonable internal consistency in the analyzed sample (Cronbach's α, 0.80).
Control Variables
Demographic variables (age in years, gender, education level, ethnicity, and marital status), respondents' concern about how to reduce their chances of cancer recurrence, and respondents' active seeking of cancer-related information from nonclinical sources (e.g., media and interpersonal sources) were measured in the round 1 questionnaire. Because patient-specific clinical characteristics may confound the relationship between PCIE and adherence to recommended surveillance, we controlled for important factors, including the American Joint Committee on Cancer/International Union Against Cancer tumor–node–metastasis stage (derived from the PCR data and ranging from stage 0 to stage III) [28], self-reported health status (ranging from poor to excellent), treatment received (radiation therapy and systemic therapy with chemotherapy or biologics), and tendency to follow doctors' recommendations for tests to monitor their cancer (ranging from never to always) in round 1.
Statistical Analysis
We conducted the analyses using Stata/SE 11 (StataCorp LP, College Station, TX). Based on initial descriptive analyses, 21.8% of the analyzed sample had missing data for one or more variables. The majority occurred in the outcome measure of adherence to surveillance (16.4%). This was a result of missing data in responses to the frequency of receiving individual surveillance procedures. We performed multiple imputation to address missing data for predictor variables using the Stata MI program according to recommended procedures [29]. Multiple imputation is considered to be superior to ad hoc methods for multivariate regression analyses based on data with missing values (e.g., using listwise deletion, pairwise deletion, or mean imputation) because of less bias and lower sampling variability [30]. Using the imputation procedure, we generated 30 datasets with imputed values of independent variables. Missing values in the outcome measure were not imputed. We next estimated logistic regression coefficients across the imputed datasets, controlling for potential confounders.
Sensitivity Analyses
To examine the potential effect that missing data in the adherence measure has on the results, we assumed that participants who had missing data on this measure (16.4% of the analyzed sample) did not receive the recommended surveillance and repeated the above logistic regression procedure. We noted that ASCO and NCCN recommended annual abdominal CT scans for patients with a higher risk for recurrence (i.e., those with lymphatic or venous invasion or poorly differentiated tumors) in their guidelines for 2005 and that some health care providers were likely to have performed CT imaging as part of routine surveillance of CRC patients [6, 9]. However, annual CT was not uniformly recommended by the different professional societies at the time of round 2 data collection in this study. Nevertheless, we conducted a sensitivity analysis that included annual CT as part of a more stringent measure to rule out the possibility that the proposed relationship would change as a result of this addition. This analysis assumed that the entire analyzed sample was eligible for annual CT because the study questionnaire did not permit assessment of the recurrence risk for individual patients.
Results
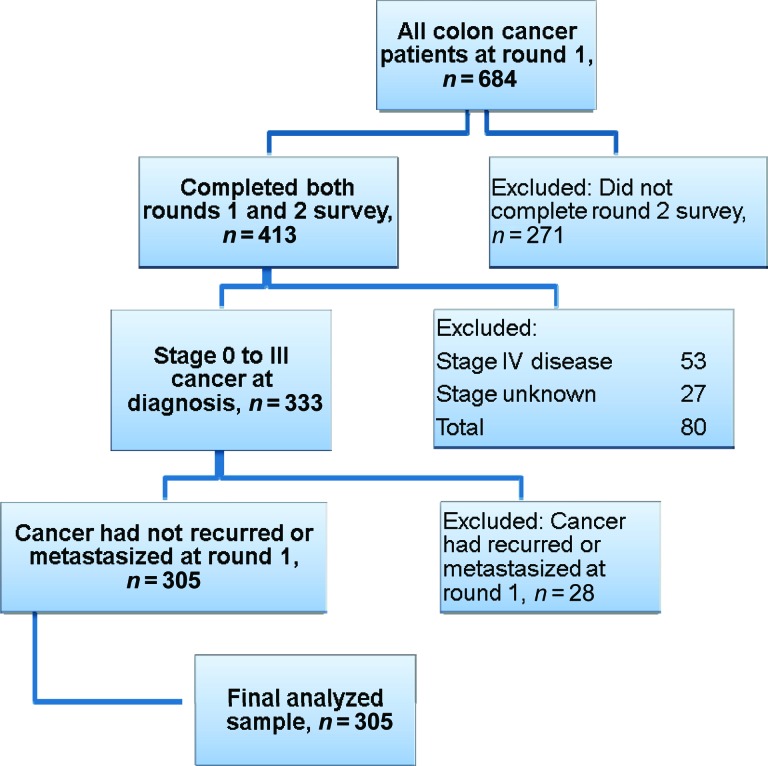
In the fall of 2006, 684 participants with CRC completed the baseline survey (round 1). Of the participants in round 1 who consented to being recontacted, 413 completed the follow-up survey (round 2) in the fall of 2007. The response rate for participants with CRC in round 1 was 61% (American Association for Public Opinion Research response rate, 4) [31], and for round 2, the raw response rate of those who agreed to be contacted was 75%. Figure 1 shows a flow diagram of the selection criteria. CRC patients who completed surveys for round 1 and round 2 were included in the analysis (n = 413). Those who had stage IV disease according to the PCR data were not eligible for surveillance testing because they had metastatic disease (n = 53). We excluded patients if their cancer stage was not known because we could not determine their eligibility for surveillance testing (n = 27). Finally, patients who reported in round 1 that their doctor told them the cancer had spread to other parts of the body (become metastatic) were excluded (n = 28). The final analyzed sample size was 305, or 45% of the initial 684 respondents.
Figure 1.
Selection criteria for analysis.
The mean age of participants at diagnosis was 68 years, 52% were female, 42% had some college education or higher, and 89% were white. Table 1 describes other characteristics of the sample. In round 2, the majority of participants reported undergoing physical examinations, CEA testing, or colonoscopy or sigmoidoscopy at the minimum recommended levels (Table 2). However, less than half of the respondents (41%) reported receiving all three surveillance procedures at the recommended levels over this time period.
Table 1.
Characteristics of analyzed sample (n = 305)
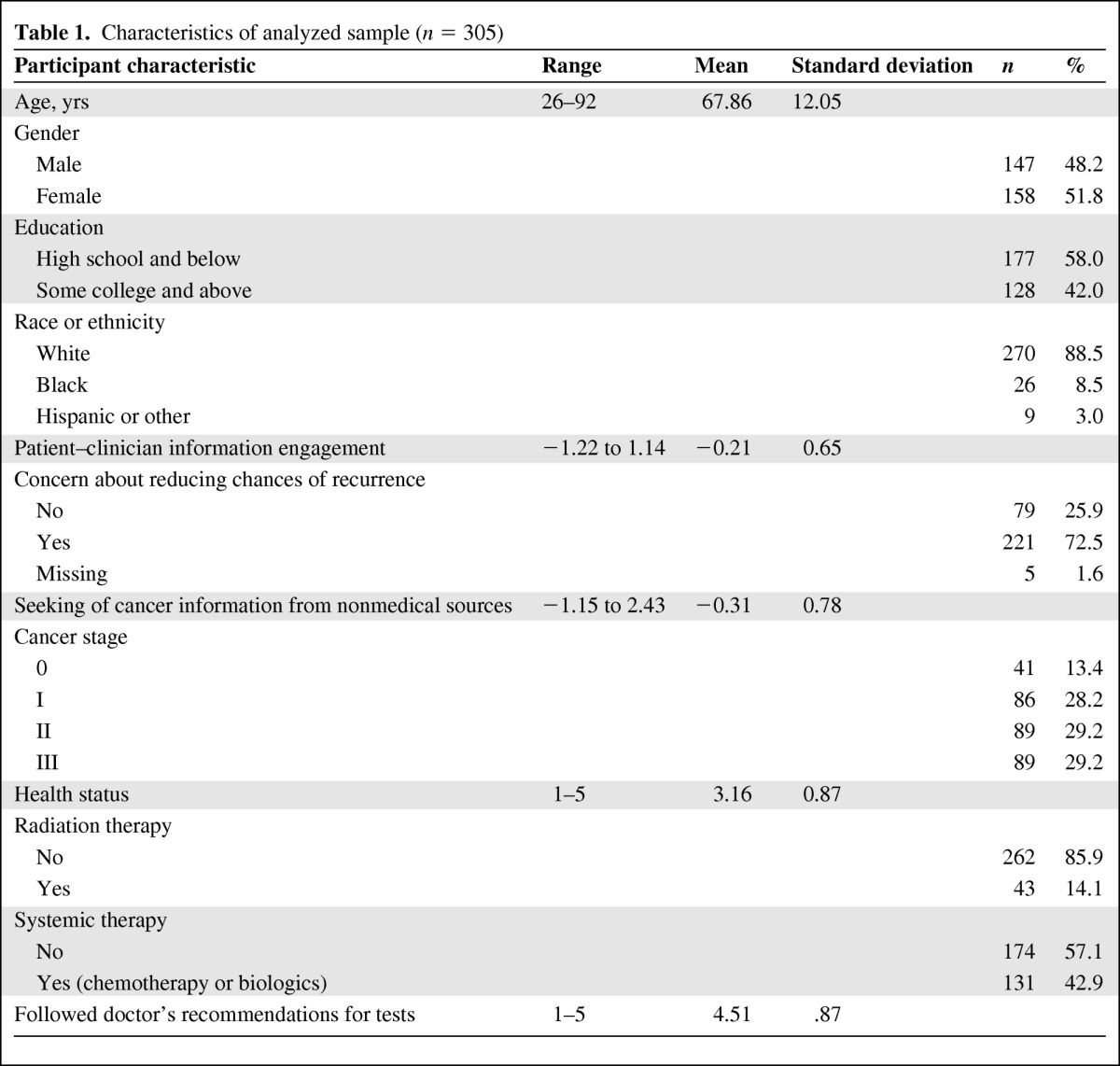
Table 2.
Frequency of undergoing surveillance procedures in the preceding 12 months during round 2 and prevalence of overall adherence to recommended surveillance
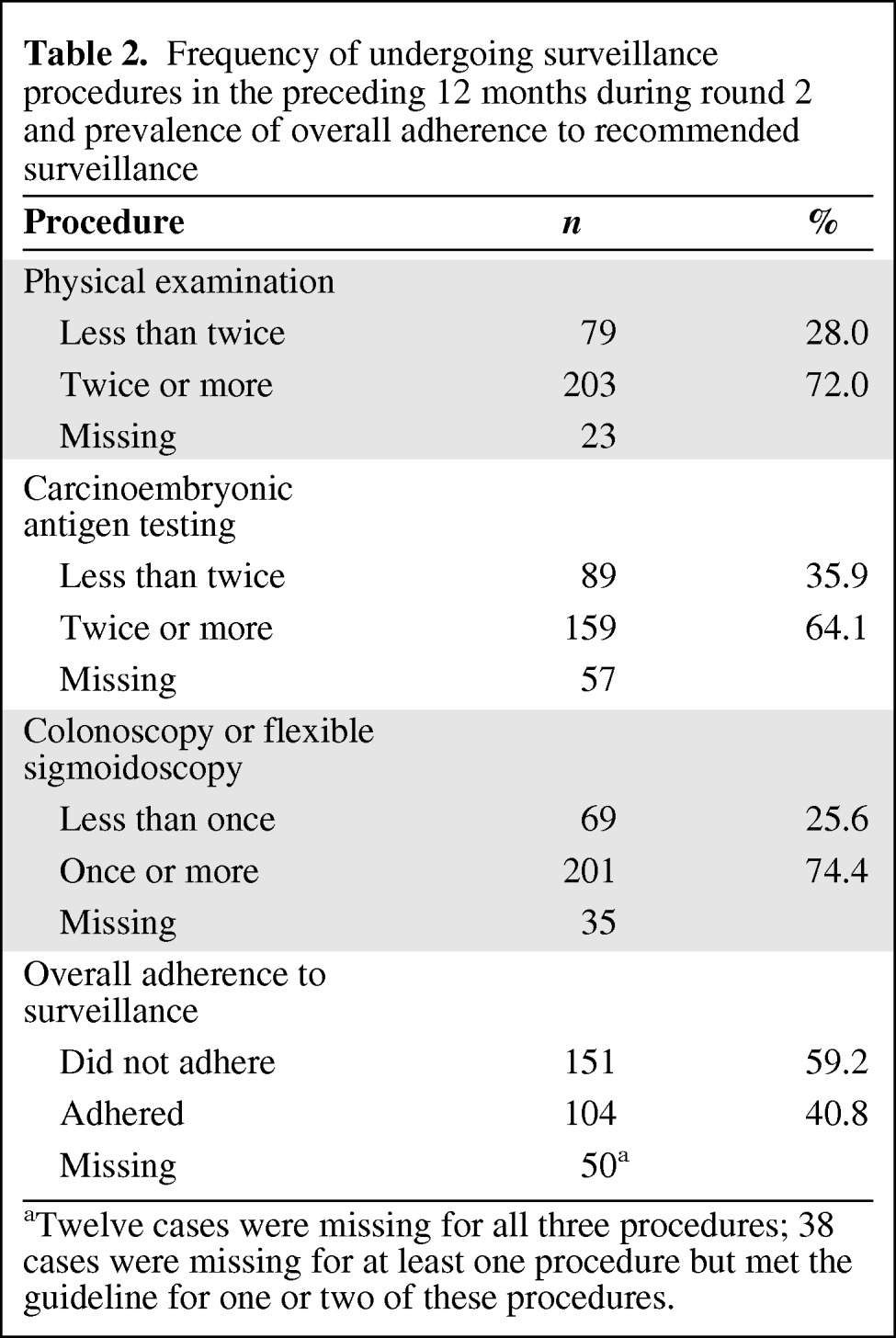
aTwelve cases were missing for all three procedures; 38 cases were missing for at least one procedure but met the guideline for one or two of these procedures.
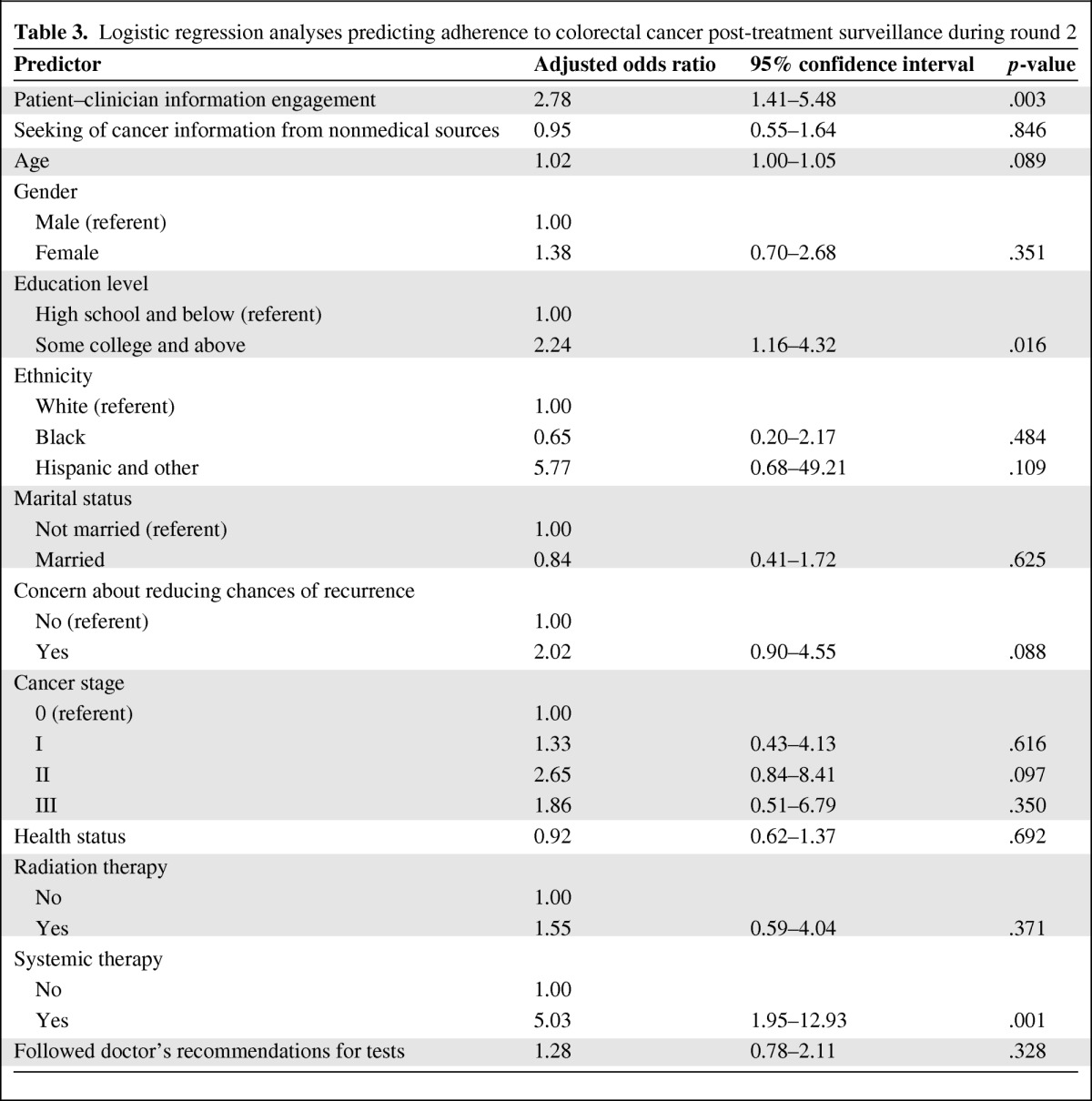
Table 3 shows the results of the logistic regression predicting the odds for adhering to the recommended post-treatment surveillance among CRC patients in round 2, controlling for various confounders. For each unit increase in PCIE in round 1, the odds for participants adhering to recommended surveillance was 2.78 times greater (95% confidence interval, 1.41–5.48; p = .003) in the 12 months preceding the round 2 survey. Other significant predictors of adhering to recommended CRC surveillance included having a higher level of education and having received systemic therapy. To test for the presence of multicollinearity among the independent variables in the model, we further examined the tolerance and variance inflation factor values of each independent variable. All the independent tolerance levels were ≥0.42, whereas the variance inflation factor values were ≤2.40, indicating that multicollinearity was not likely in this analysis.
Table 3.
Logistic regression analyses predicting adherence to colorectal cancer post-treatment surveillance during round 2
The substantive finding that PCIE was a significant predictor of adherence to recommended surveillance was robust to the sensitivity analysis that assumed participants with missing data on the outcome measure did not receive the surveillance procedures at the minimum levels. Additionally, when using a more demanding measure of adherence to cancer surveillance that included annual CT scans as part of the criteria, only 30.9% of respondents were classified as having adhered to surveillance. The sensitivity analysis using this alternative adherence measure showed that PCIE remained a significant predictor of compliance with cancer surveillance recommendations.
Discussion
Despite existing evidence of the long-term survival benefits of routine cancer surveillance among CRC patients after curative treatment [4, 5], this study found that only about two in five patients reported receiving the minimum level of recommended surveillance, including physical examinations, CEA testing, and colonoscopy ∼2 years after diagnosis. The low prevalence of cancer surveillance in this study corroborated findings reported in earlier studies. A study among a large cohort of SEER–Medicare patients found that 39.8% received physical examinations, CEA tests, and colonoscopy at the minimum recommended levels or higher 6–42 months after diagnosis [6]. Other studies reported the prevalence of CRC patients undergoing postoperative colonoscopy, sigmoidoscopy, or barium enema to be in the range of 50%–60% [7–11], whereas estimates of receiving at least one CEA test were in the range of 35%–71% [6–8]. Direct comparisons of these results with those in our study were not possible because of wide variation in designs, patient populations, surveillance procedures, and follow-up periods.
In the context of a low prevalence of cancer surveillance among CRC patients, this study addresses an important issue in cancer survivorship care and advances existing research on the determinants of adherence to post-treatment CRC surveillance in several ways. First, although prior studies have identified various sociodemographic factors, including age, income, and ethnicity, as determinants of receiving CRC surveillance testing [6, 11], the present study examined the predictive influence of PCIE—a form of patient–clinician communication that is potentially modifiable—on adhering to guideline-recommended surveillance. Greater PCIE predicted a higher likelihood that eligible CRC survivors would receive adequate levels of physical examination, CEA testing, and colonoscopy. The practical significance of this finding is underscored by prior interventions targeted at improving patient involvement and communication skills in clinic-based settings, achieving varying levels of success [32]. Some examples of these interventions include providing informational materials to patients in waiting rooms on desirable communication behaviors, role-playing or practice sessions, and formal coaching sessions for patients. Future research should be conducted to examine the feasibility of improving behaviors engendered in PCIE.
Findings from the present study may provide some initial directions for designing and testing pilot interventions that could potentially improve surveillance adherence through active patient engagement with clinicians on cancer-related information. Specifically, CRC survivors who have recently completed treatment may be encouraged to actively engage with their physicians about the importance of routine cancer follow-up and discussing the timing and frequency of different surveillance tests. One suggestion for providing such encouragement could be through face-to-face sessions during which trained patient navigators provide brief counseling and skills training to cancer survivors on talking to their physician about routine follow-up or bringing information from other sources to clarify with their physician. Another consideration could be a pilot program to provide CRC survivors with a checklist of suggested questions about cancer follow-up tests that they should discuss with their physicians prior to each scheduled visit.
Furthermore, PCIE encompasses patients' active seeking of cancer-related information not only from their treating physician but from other doctors and health professionals as well (e.g., primary care physicians, surgeons, medical and radiation oncologists, home nurses, nutritionists, and physical or occupational therapists). We speculate that this constant engagement across a team of providers may be contributing to patients having more opportunities to discuss the role of continued cancer surveillance, leading to their greater awareness and positive attitudes about regular follow-up, and subsequently to their compliance with recommended surveillance [33]. In this hypothesized scenario, patients who engage with a multidisciplinary team of clinicians are less likely to “fall through the cracks” in terms of cancer surveillance.
In this study, we found that the predictive influence of PCIE on receiving recommended surveillance was consistent across various sensitivity analyses. Specifically, one analysis assessed whether or not our decision to limit adherence to surveillance to the three procedures recommended in prevailing guidelines—physical examination, CEA testing, and colonoscopy or sigmoidoscopy—was a reasonable approach. Although this measure was consistent with another study that defined meeting guidelines for CRC surveillance with these three procedures [6], we recognized that annual CT scans were beginning to be part of recommended routine surveillance for higher risk CRC patients around the time of our data collection. Perhaps reflecting that shift, we found that 64% of respondents in this study reported receiving at least one CT scan in the preceding 12 months for routine cancer follow-up. The finding that PCIE significantly predicted a more stringent adherence measure that included an annual CT scan provided reassurance of the robustness of the analysis that defined compliance based on the above three procedures alone. However, we caution against interpreting the findings with annual CT in the outcome measure at face value because surveillance guidelines for CT scans were not yet uniformly recommended by professional societies in 2005. In addition, the study was not able to identify patients who were considered at high recurrence risk, for whom an annual CT is indicated.
An additional strength of this research is the population-based sample from the PCR, which represented CRC patients across different age groups and clinical settings, in comparison with previous studies on cancer surveillance among samples of CRC patients from managed care practices or the reliance on administrative data from the Medicare population [6, 9]. Moreover, studies that examine the effects of patient–clinician communication on health outcomes often rely on cross-sectional data. In contrast, the present study used longitudinal data, enabling more confident interpretations of causality by establishing temporal precedence between PCIE during round 1 and adherence to surveillance during round 2.
There are some limitations to this study. First, we relied on patient self-report for assessing the frequency of cancer surveillance procedures. Although self-reported receipt of surveillance procedures may be subject to recall or social desirability bias, other studies have compared self-reported adherence to CRC screening tests with medical records or with direct observation in various healthy populations and concluded that the self-report approach is an acceptable and valid alternative to more costly methodologies [34–37].
Another limitation is related to the observational design of the study. The observed relationship between PCIE and receipt of cancer surveillance may be a result of the concurrent positive effects of an unmeasured variable on these two measures. Although it is not possible to completely eliminate the threat of spuriousness, we included potential confounders such as demographic and clinical variables shown to be associated with cancer surveillance [9] or with patient–clinician communication [16] to minimize this threat. The third limitation relates to generalizability. Although we have a population-based sample, our study findings might not apply to patients with other cancer types or CRC patients living in other parts of the country. Further research is recommended to assess if the findings from this study can be replicated in other patient populations. This analysis was focused on CRC surveillance specifically because of the differences in eligibility, surveillance procedures, and guidelines across cancer types. We encourage future studies to examine if patient–clinician communication also influences post-treatment surveillance in patients with other types of cancer.
To conclude, based on the analysis of a longitudinal survey among CRC patients in Pennsylvania who were eligible for post-treatment cancer surveillance, this study showed that greater patient engagement with clinicians about cancer-related information improved patients' subsequent adherence to recommended levels of surveillance with physical examinations, CEA testing, and colonoscopy or sigmoidoscopy. We recommend that prospective studies be considered to determine if pilot programs encouraging active patient engagement with clinicians about cancer-related information would be beneficial in terms of increasing the proportion of patients receiving post-treatment surveillance testing, and ultimately in improving patient outcomes and survival.
Acknowledgments
This study was supported by the National Cancer Institute (grants 5P50CA095856-05 and 5P50CA095856-06). Results were presented in part by Dr. Andy S.L. Tan at the 139th American Public Health Association Annual Meeting.
Author Contributions
Conception/Design: Andy S.L. Tan, Stacy W. Gray, Katrina Armstrong, Robert C. Hornik
Collection and/or assembly of data: Andy S.L. Tan, Stacy W. Gray, Katrina Armstrong, Robert C. Hornik
Data analysis and interpretation: Andy S.L. Tan, Mihaela Moldovan-Johnson, Sarah Parvanta, Stacy W. Gray, Katrina Armstrong, Robert C. Hornik
Manuscript writing: Andy S.L. Tan, Mihaela Moldovan-Johnson, Sarah Parvanta, Stacy W. Gray, Katrina Armstrong, Robert C. Hornik
Final approval of manuscript: Andy S.L. Tan, Mihaela Moldovan-Johnson, Sarah Parvanta, Stacy W. Gray, Katrina Armstrong, Robert C. Hornik
References
- 1.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 2.Engstrom PF, Benson AB, 3rd, Chen YJ, et al. Colon cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:468–491. doi: 10.6004/jnccn.2005.0024. [DOI] [PubMed] [Google Scholar]
- 3.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–817. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: A meta-analysis. Dis Colon Rectum. 2007;50:1783–1799. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors: A population-based analysis. Cancer. 2008;113:2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 7.Cooper GS, Payes JD. Temporal trends in colorectal procedure use after colorectal cancer resection. Gastrointest Endosc. 2006;64:933–940. doi: 10.1016/j.gie.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Cooper GS, Yuan Z, Chak A, et al. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer. 1999;85:2124–2131. [PubMed] [Google Scholar]
- 9.Elston Lafata J, Cole Johnson C, Ben-Menachem T, et al. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39:361–372. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 11.Knopf KB, Warren JL, Feuer EJ, et al. Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries. Gastrointest Endosc. 2001;54:563–571. doi: 10.1067/mge.2001.118949. [DOI] [PubMed] [Google Scholar]
- 12.Beckjord EB, Arora NK, McLaughlin W, et al. Health-related information needs in a large and diverse sample of adult cancer survivors: Implications for cancer care. J Cancer Surviv. 2008;2:179–189. doi: 10.1007/s11764-008-0055-0. [DOI] [PubMed] [Google Scholar]
- 13.Rutten LJ, Arora NK, Bakos AD, et al. Information needs and sources of information among cancer patients: A systematic review of research (1980–2003) Patient Educ Couns. 2005;57:250–261. doi: 10.1016/j.pec.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Luker KA, Beaver K, Leinster SJ, et al. Information needs and sources of information for women with breast cancer: A follow-up study. J Adv Nurs. 1996;23:487–495. doi: 10.1111/j.1365-2648.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 15.Landro L. Patient-physician communication: An emerging partnership. The Oncologist. 1999;4:55–58. [PubMed] [Google Scholar]
- 16.Epstein RM, Street RL., Jr Patient-centered communication in cancer care: Promoting healing and reducing suffering. 2007. [accessed March 20, 2012]. Available at http://outcomes.cancer.gov/areas/pcc/communication/pcc_monograph.pdf.
- 17.Fox SA, Heritage J, Stockdale SE, et al. Cancer screening adherence: Does physician-patient communication matter? Patient Educ Couns. 2009;75:178–184. doi: 10.1016/j.pec.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Honda K, Kagawa-Singer M. Cognitive mediators linking social support networks to colorectal cancer screening adherence. J Behav Med. 2006;29:449–460. doi: 10.1007/s10865-006-9068-1. [DOI] [PubMed] [Google Scholar]
- 19.Politi MC, Clark MA, Rogers ML, et al. Patient-provider communication and cancer screening among unmarried women. Patient Educ Couns. 2008;73:251–255. doi: 10.1016/j.pec.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tessaro I, Mangone C, Parkar I, et al. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3:A123. [PMC free article] [PubMed] [Google Scholar]
- 21.Underhill ML, Kiviniemi MT. The impact of provider-patient relationship quality and quality of care on colorectal cancer screening adherence. Presented at the 3rd Health Information National Trends Survey (HINTS) Data Users Meeting; September 24–25, 2009; Washington, DC. [Google Scholar]
- 22.Martinez LS, Schwartz JS, Freres D, et al. Patient–clinician information engagement increases treatment decision satisfaction among cancer patients through feeling of being informed. Patient Educ Couns. 2009;77:384–390. doi: 10.1016/j.pec.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart MA. Effective physician-patient communication and health outcomes: A review. CMAJ. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 25.Tan AS, Bourgoin A, Gray SW, et al. How does patient-clinician information engagement influence self-reported cancer-related problems? Findings from a longitudinal analysis. Cancer. 2011;117:2569–2576. doi: 10.1002/cncr.25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-McLallen A, Fishbein M, Hornik RC. Psychosocial determinants of cancer-related information seeking among cancer patients. J Health Commun. 2011;16:212–225. doi: 10.1080/10810730.2010.522227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillman DA, Dillman DA. Mail and Internet Surveys: The Tailored Design Method. New York: J. Wiley; 2000. p. 464. [Google Scholar]
- 28.Green RJ, Metlay JP, Propert K, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: An analysis of Intergroup 0089. Ann Intern Med. 2002;136:261–269. doi: 10.7326/0003-4819-136-4-200202190-00005. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp. College Station, TX: StataCorp LP; 2009. Stata: Release 11. Statistical Software. [Google Scholar]
- 30.Newman DA. Longitudinal modeling with randomly and systematically missing data: A simulation of ad hoc, maximum likelihood, and multiple imputation techniques. Organ Res Methods. 2003;6:328–362. [Google Scholar]
- 31.The American Association for Public Opinion Research. Lenexa, Kansas: AAPOR; 2006. [accessed March 20, 2012]. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. Available at http://www.aapor.org/uploads/standarddefs_4.pdf. [Google Scholar]
- 32.Rao JK, Anderson LA, Inui TS, et al. Communication interventions make a difference in conversations between physicians and patients: A systematic review of the evidence. Med Care. 2007;45:340–349. doi: 10.1097/01.mlr.0000254516.04961.d5. [DOI] [PubMed] [Google Scholar]
- 33.DiMatteo MR. Future directions in research on consumer-provider communication and adherence to cancer prevention and treatment. Patient Educ Couns. 2003;50:23–26. doi: 10.1016/s0738-3991(03)00075-2. [DOI] [PubMed] [Google Scholar]
- 34.Baier M, Calonge N, Cutter G, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9:229–232. [PubMed] [Google Scholar]
- 35.Hall HI, Van Den Eeden SK, Tolsma DD, et al. Testing for prostate and colorectal cancer: Comparison of self-report and medical record audit. Prev Med. 2004;39:27–35. doi: 10.1016/j.ypmed.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Madlensky L, McLaughlin J, Goel V. A comparison of self-reported colorectal cancer screening with medical records. Cancer Epidemiol Biomarkers Prev. 2003;12:656–659. [PubMed] [Google Scholar]
- 37.DiMatteo MR, Robinson JD, Heritage J, et al. Correspondence among patients' self-reports, chart records, and audio/videotapes of medical visits. Health Commun. 2003;15:393–413. doi: 10.1207/S15327027HC1504_02. [DOI] [PubMed] [Google Scholar]