No standard chemotherapy regimen has been established for unresectable or recurrent small bowel adenocarcinoma (SBA). This article retrospectively reviews the clinical courses of 132 patients with unresectable or recurrent SBA who received chemotherapy at 41 institutions in Japan.
Keywords: Adenocarcinoma, Chemotherapy, Prognostic factor, Small bowel cancer
Abstract
Background.
No standard chemotherapy regimen has been established for unresectable or recurrent small bowel adenocarcinoma (SBA).
Methods.
Clinical courses of 132 patients with unresectable or recurrent SBA who received chemotherapy at 41 institutions in Japan were reviewed retrospectively. Patients were classified into five groups according to first-line chemotherapy regimens: fluoropyrimidine monotherapy (group A), fluoropyrimidine-cisplatin (group B), fluoropyrimidine-oxaliplatin (group C), fluoropyrimidine-irinotecan (group D), and other regimens (group E).
Results.
The number of patients in each group was as follows: groups A, 60 patients; group B, 17 patients; group C, 22 patients; group D, 11 patients; and group E, 22 patients. Median progression-free survival (PFS) times were as follows: group A, 5.4 months; group B, 3.8 months; group C, 8.2 months; group D, 5.6 months; and group E, 3.4 months. Median overall survival (OS) times were as follows: group A, 13.9 months; group B, 12.6 months; group C, 22.2 months; group D, 9.4 months; and group D, 8.1 months. Patients in group C achieved significantly longer PFS times and substantially (but not significantly) longer OS times than patients in group A. After adjusting for clinical background characteristics, fluoropyrimidine-oxaliplatin therapy was a significant positive prognostic factor for PFS and OS times.
Conclusion.
The results suggest that fluoropyrimidine-oxaliplatin combination therapy is the most promising first-line chemotherapy regimen for unresectable or recurrent SBA.
Introduction
Small bowel cancer is rare; it accounts for <3% of all gastrointestinal malignant tumors and <0.5% of all types of cancers [1]. Histologically, adenocarcinoma (25%–40% cases) is the second most common malignant tumor of the small bowel after carcinoid tumor [2–8]. Resection with regional lymph node dissection is considered to be the standard treatment for localized small bowel adenocarcinoma (SBA), and chemotherapy regimens indicated in other gastrointestinal malignancies are generally used for unresectable or recurrent disease. Several retrospective studies suggest that chemotherapy prolongs survival of patients with unresectable or recurrent SBA [9–13]. However, there is no established standard regimen for patients with unresectable or recurrent SBA; their prognosis remains poor, with reported median survival ranging from 8 to 19 months [9–20].
Fluorouracil (5-FU) is the most commonly used agent for the treatment of unresectable or recurrent SBA, and various 5-FU based regimens have been used [11–23]. In a retrospective analysis, Overman et al. reported that combination therapy with 5-FU and platinum compounds showed more favorable results than other regimens [21]. Among the various types of combinations of 5-FU and platinum, oxaliplatin-containing regimens showed favorable efficacy in several studies. A retrospective study carried out by the Association des Gastroentérologues Oncologues (AGEO) showed a median progression-free survival (PFS) times of 6.9 months and a median overall survival (OS) times of 17.8 months with leucovorin + 5-FU + oxaliplatin (FOLFOX) therapy, which exceeded the results achieved with cisplatin + 5-FU combination therapy [22]. A prospective phase II study of capecitabine-oxaliplatin therapy showed a response rate of 50% and median OS time of 15.5 months [23]. Although combination therapy with 5-FU and oxaliplatin appears promising, its superiority to 5-FU monotherapy with regard to OS has not been demonstrated in any study to date.
We conducted this retrospective study to find the most promising regimen for patients with unresectable or recurrent SBA by comparing different regimens with 5-FU monotherapy.
Patients and Methods
This retrospective study was conducted by investigators from 41 institutions in Japan after approval by the respective institutional review boards. Patients with unresectable SBA (metastatic and/or locally advanced so that curative resection was not applicable at initial diagnosis) or recurrent SBA (recurred state after curative resection) who received first-line chemotherapy between April 1999 and March 2009 and met the following selection criteria were enrolled: (a) histologically proven adenocarcinoma of the duodenum, jejunum, or ileum, excluding ampullary carcinoma; (b) no previous chemotherapy or radiotherapy (patients who had completed adjuvant chemotherapy at least 6 months before evidence of recurrence were eligible); (c) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; (d) adequate bone marrow (leukocytes ≥3,000 cells/mm3, hemoglobin 8.0 g/dL, platelets ≥75,000 cells/mm3 in peripheral blood), hepatic function (serum aspartate transaminase ≤100 IU/L, serum alanine aminotransferase ≤100 IU/L), and renal function (serum creatinine ≤1.5 mg/dL); and (e) no concomitant malignancy. The presence of target lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 was not mandatory.
Data Collection
The following data were collected from medical records: patient demographics (age, sex, and ECOG PS); baseline serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) levels; tumor characteristics (primary site, histology, metastatic or locally advanced, metastatic sites, resection of primary tumor, history of adjuvant chemotherapy, and the presence of target lesions). Clinical course was also noted, including chemotherapy regimen, response according to RECIST version 1.0, the date of disease progression, subsequent therapies, and survival status at the most recent follow-up.
Treatment
The patients were divided into five groups according to the first-line regimen used: group A, fluoropyrimidine monotherapy; group B, fluoropyrimidine-cisplatin; group C, fluoropyrimidine-oxaliplatin; group D, fluoropyrimidine-irinotecan; and group E, others. The chemotherapeutic regimens were administered in the five groups as follows. These treatments were generally repeated until detection of disease progression, appearance of unacceptable toxicities, or the patient's refusal to continue treatment.
Group A. The chemotherapy regimens for group A were as follows:
Tegafur, 5-chloro-2,4-dihydropyrimidine, and potassium oxanate (S-1) alone: 80 mg/m2/day orally for 28 days, repeated every 6 weeks.
5-FU + leucovorin: 5-FU 600 mg/m2 bolus plus l-leucovorin 250 mg/m2 once a week for 6 weeks, repeated every 8 weeks.
Continuous infusion of 5-FU: 5-FU 800 mg/m2 for 5 days, repeated every 4 weeks.
Uracil and tegafur (UFT) + leucovorin: UFT 300 mg/m2 per day plus leucovorin 75 mg per day orally for 28 days, repeated every 5 weeks.
Group B. The chemotherapy regimens for group B were as follows:
Combination of 5-FU and cisplatin (FP): Continuous infusion of 5-FU 800 mg/m2 on days 1–5 plus cisplatin 80 mg/m2 on day 1, repeated every 4 weeks.
Combination of S-1 and cisplatin (SP): S-1 80 mg/m2 per day orally on days 1–21 plus cisplatin 60 mg/m2 on day 8, repeated every 5 weeks.
Group C. The chemotherapy regimens for group C were as follows:
Modified FOLFOX6: l-leucovorin 200 mg/m2 plus oxaliplatin 85 mg/m2 plus bolus 5-FU 400 mg/m2, followed by infusion of 5-FU 2,400 mg/m2 for 46 hours, repeated every 2 weeks.
Combination of S-1 and oxaliplatin: S-1 80 mg/m2 per day orally on days 1–14 plus oxaliplatin 130 mg/m2 on day 1, repeated every 3 weeks.
Group D. The chemotherapy regimens for group D were as follows:
Irinotecan + 5-FU + l-leucovorin (IFL): irinotecan 125 mg/m2 plus l-leucovorin 10 mg/m2 plus bolus 5-FU 500 mg/m2 once a week for 4 weeks, then every 6 weeks.
5-FU + l-leucovorin + irinotecan (FOLFIRI): l-leucovorin 200 mg/m2 plus irinotecan 150 or 180 mg/m2 plus bolus 5-FU 400 mg/m2, followed by infusion of 5-FU 2,400 mg/m2 for 46 hours, repeated every 2 weeks.
Combination of S-1 and irinotecan: S-1 80 mg/m2 per day orally on days 1–14 plus irinotecan 125 mg/m2 on days 1 and 15, repeated every 4 weeks.
Group E. Group E included all other regimens.
Statistical Analysis
For continuous variables, the median and interquartile ranges were reported. Categorical variables were summarized as frequencies (percentages). Differences in the distribution of variables were evaluated using the Kruskal-Wallis or χ2 tests, as appropriate. Responses were determined according to RECIST version 1.0. Patients who did not have a target lesion were excluded from the response analysis.
PFS was defined as the time from the initiation of chemotherapy to the confirmation of disease progression or death due to any cause. OS was defined as the time from the initiation of chemotherapy to death due to any cause. Surviving patients were censored on the last follow-up date. PFS and OS were estimated by the Kaplan-Meier method and compared using the log-rank test. We estimated hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) for PFS and OS using multivariate Cox proportional hazards models after stepwise selection of the covariates. Other than treatment groups, these covariates included sex (male/female), age (<65 years/≥65 years), ECOG PS (continuous variable), primary site (jejunum or ileum/duodenum), histological type (undifferentiated/differentiated), resection of primary tumor (yes/no), adjuvant chemotherapy (yes/no), presence of target lesions (positive/negative), number of metastatic sites (continuous variable), baseline CEA level (<5 ng/mL/≥5 ng/mL), and baseline CA19–9 level (<37 ng/mL/≥37 ng/mL).
All reported p values were two sided; p < .05 was considered to be statistically significant. Statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
A total of 132 patients were included in this analysis. Baseline characteristics of the subjects are shown in Table 1. The numbers of patients in each group were as follows: group A, 60 patients; group B, 17 patients; group C, 22 patients; group D, 11 patients; and group E, 22 patients. The median patient age was 60 years; the percentage of men was 65.9%. Primary tumor site was the duodenum in 60.6% of patients and jejunum or ileum in 39.4% of patients. Disease status was unresectable in 92.4% of patients and recurrent in 7.6% of patients. Except for ECOG PS and the presence of lung metastasis, the patients' background characteristics were nearly balanced among the treatment groups.
Table 1.
Baseline characteristics by treatment group
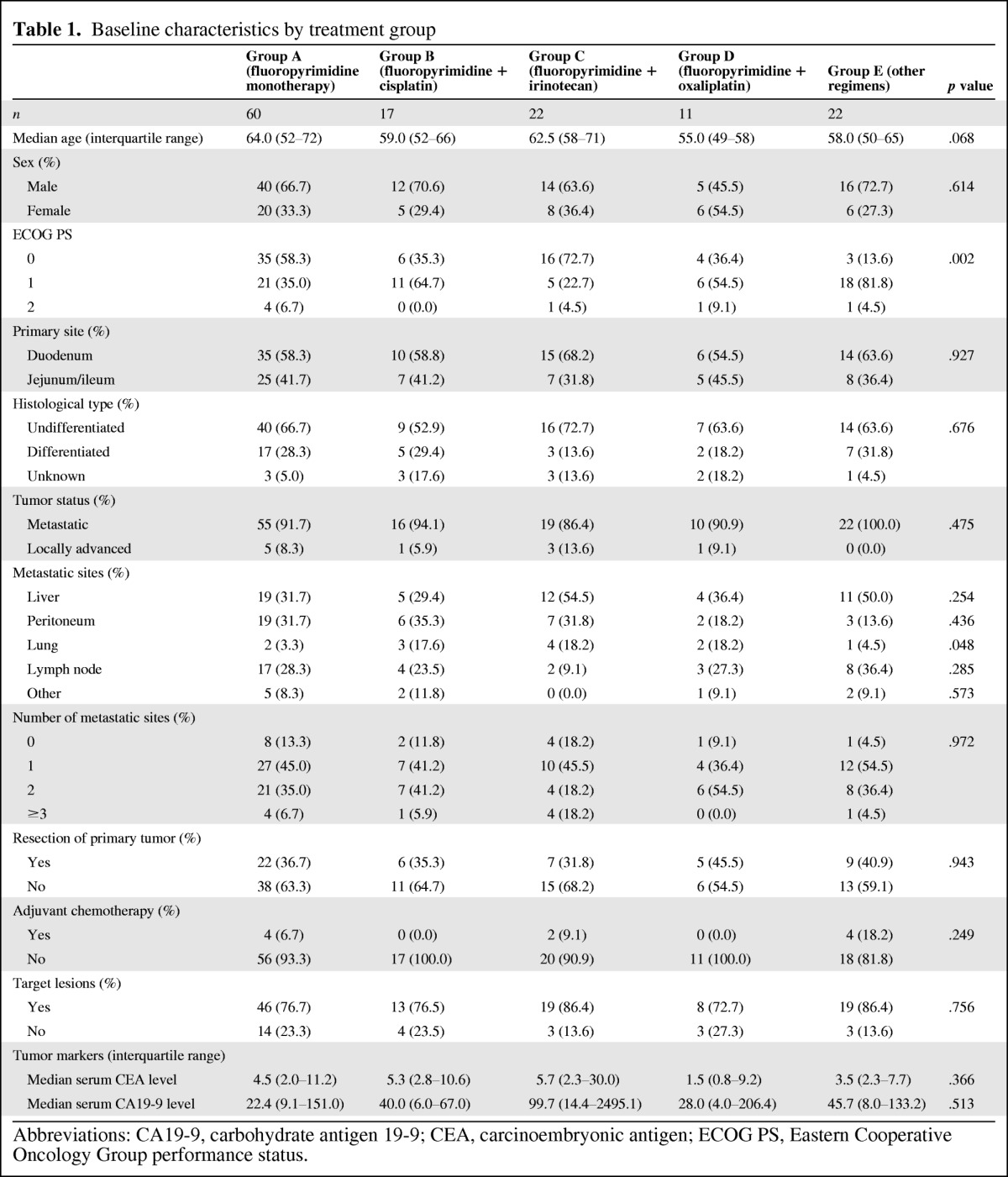
Abbreviations: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 2 shows the regimens used in each group. In group A, the most commonly used regimen was S-1 alone (46 patients) followed by 5-FU + leucovorin (9 patients), UFT + leucovorin (3 patients), and continuous infusion of 5-FU (2 patients). In group B, 10 patients received SP and 7 patients received FP. In group C, 21 patients received modified FOLFOX6 and 1 patient received S-1 + oxaliplatin. In group D, the regimens were distributed as follows: IFL (6 patients), FOLFIRI (4 patients), and S-1 + irinotecan (1 patient). In group E, the most frequently used regimen was irinotecan + cisplatin (13 patients), but various other regimens were also used.
Table 2.
Regimens used in each treatment group
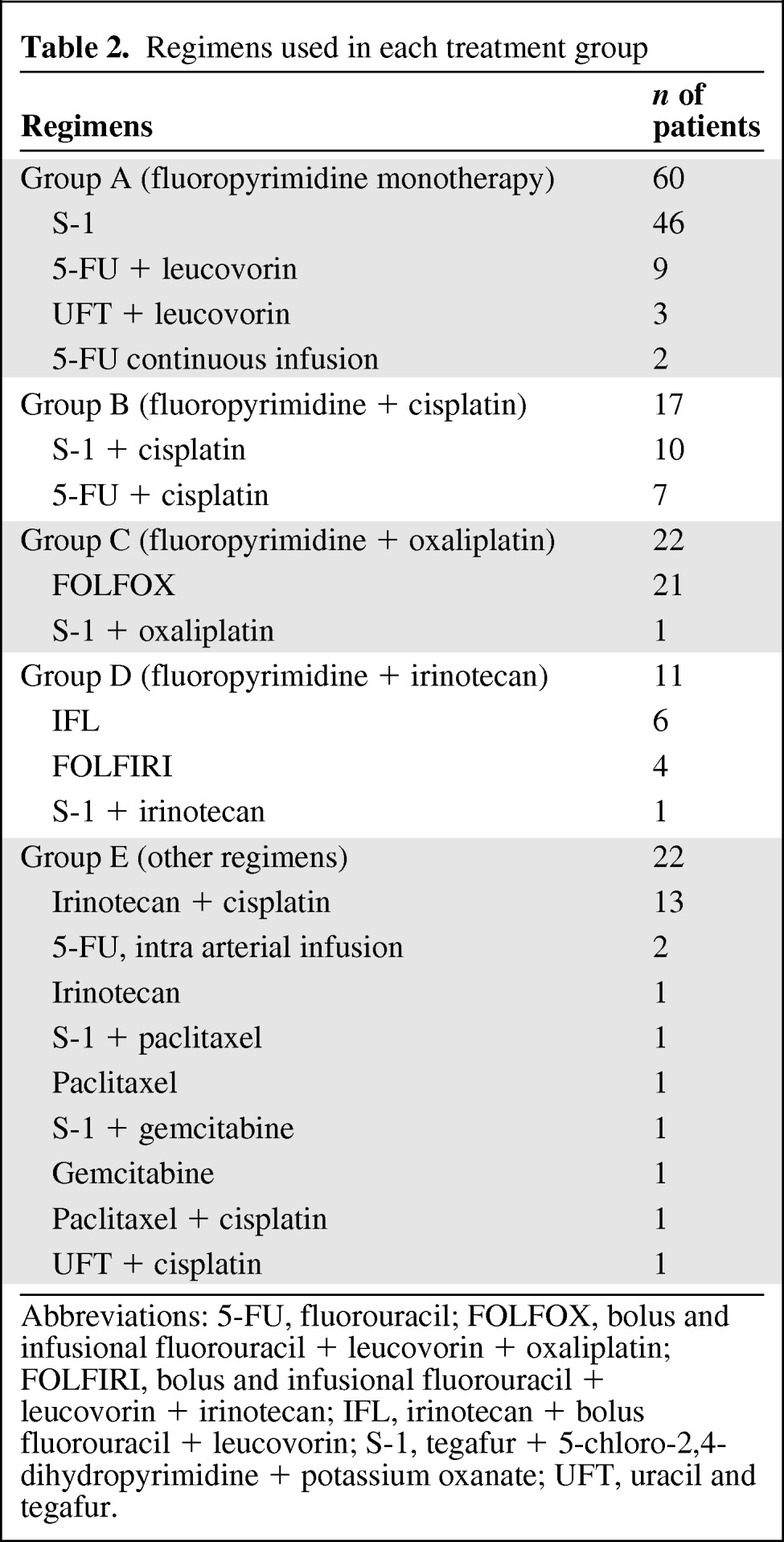
Abbreviations: 5-FU, fluorouracil; FOLFOX, bolus and infusional fluorouracil + leucovorin + oxaliplatin; FOLFIRI, bolus and infusional fluorouracil + leucovorin + irinotecan; IFL, irinotecan + bolus fluorouracil + leucovorin; S-1, tegafur + 5-chloro-2,4-dihydropyrimidine + potassium oxanate; UFT, uracil and tegafur.
Tumor Response
The numbers of patients with target lesions were as follows: group A, 46 patients; group B, 13 patients; group C, 19 patients; group D, 8 patients; and group E, 19 patients. The response rates were as follows: group A, 20% (9 of 46 patients); group B, 38% (5 of 13 patients); group C, 42% (8 of 19 patients); group D, 25% (2 of 8 patients); and group E, 21% (4 of 19 patients). Complete response was obtained in one patient in group A and three patients in group C.
Subsequent Chemotherapy
The proportion of patients who underwent subsequent chemotherapy after failure of first-line chemotherapy was similar between the treatment groups: 60% in group A, 56% in group B, 50% in group C, 73% in group D, and 59% in group E.
Univariate Analysis for Progression-Free Survival and Overall Survival
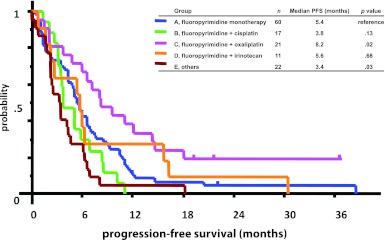
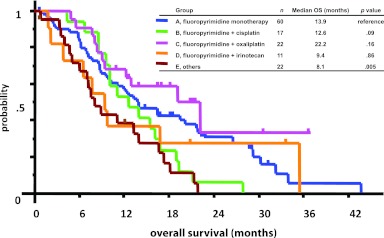
Median PFS times were 5.4 months in group A, 3.8 months in group B, 8.2 months in group C, 5.6 months in group D, and 3.4 months in group E (Fig. 1). Median OS times were 13.9 months in group A, 12.6 months in group B, 22.2 months in group C, 9.4 months in group D, and 8.1 months in group E (Fig. 2). Univariate analysis showed that group C achieved significantly longer PFS times (5.4 vs. 8.2 months, p = .026) and substantially (but not significantly) longer OS times (13.9 vs. 22.2 months, p = .156) than group A. No other treatment group showed longer PFS or OS times than group A.
Figure 1.
Progression-free survival times by treatment group. Group C achieved significantly longer progression-free survival than group A (5.4 vs. 8.2 months, p = .026). One patient in group C was excluded from the analysis because the date of disease progression was not confirmed.
Figure 2.
Overall survival times by treatment groups. Group C achieved substantially, but not significantly, longer overall survival times than group A (13.9 vs. 22.2 months, p = .156).
Multivariate Analysis for Progression-Free Survival and Overall Survival
In the analysis of prognostic factors, we excluded five patients (two patients in group A and three patients in group B) for whom the data regarding tumor markers were not available. Five factors (treatment group, ECOG PS, resection of primary tumor, presence of target lesions, and serum CEA level) were selected by univariate analysis for performing multivariate analysis for PFS. Among these, four factors (group C, enhanced ECOG PS, resection of primary tumor, and presence of target lesions) were independently associated with longer PFS times (Table 3).
Table 3.
Multivariate analysis for progression-free survival
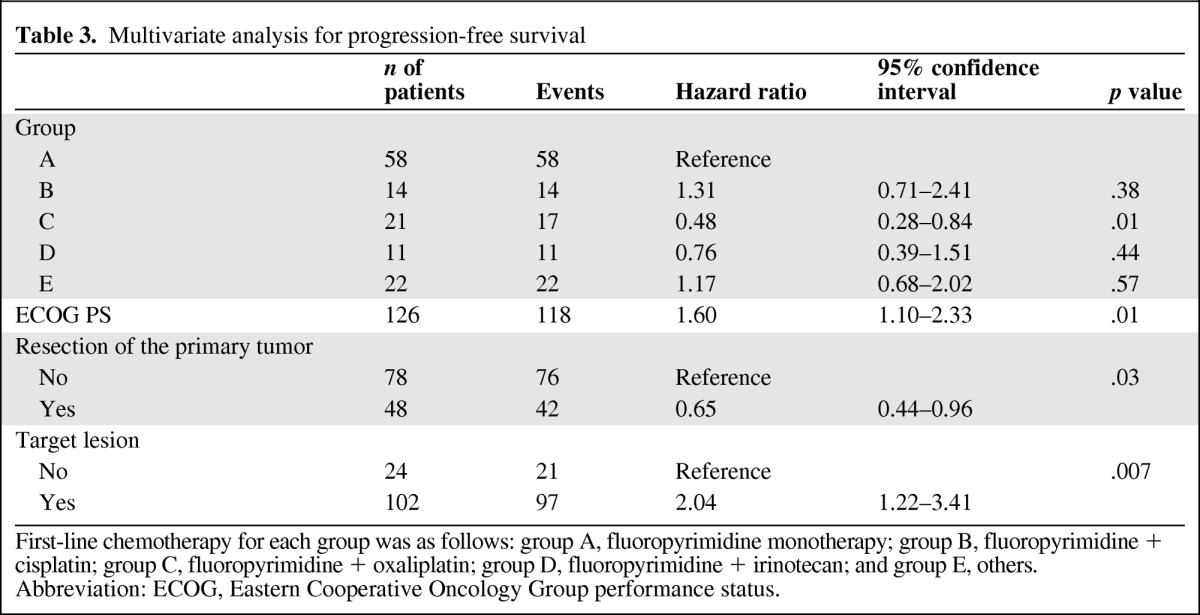
First-line chemotherapy for each group was as follows: group A, fluoropyrimidine monotherapy; group B, fluoropyrimidine + cisplatin; group C, fluoropyrimidine + oxaliplatin; group D, fluoropyrimidine + irinotecan; and group E, others.
Abbreviation: ECOG, Eastern Cooperative Oncology Group performance status.
Seven potentially predictive factors were selected by univariate analysis for performing multivariate analysis for OS (treatment group, ECOG PS, location of primary tumor, histological type, presence of target lesions, serum CEA level, and serum CA 19–9 level). Among these, four factors (treatment group C, enhanced ECOG PS, primary site of jejunum or ileum, and serum CEA level within normal range [<5 ng/mL]) were associated with longer OS times (Table 4). In comparison with fluoropyrimidine monotherapy (group A), after adjusting for these prognostic factors by multivariate analysis, combination regimens including oxaliplatin (treatment group C) were associated with enhanced PFS times (HR = 0.48, 95% CI: 0.28–0.84, p = .01) and OS times (HR = 0.45, 95% CI: 0.23–0.88, p = .02), whereas combination regimens with cisplatin (group B) showed rather poor clinical outcomes (HR = 1.31, 95% CI: 0.71–2.41, p = .38 for PFS; HR = 1.54, 95% CI: 0.83–2.87, p = .17 for OS).
Table 4.
Multivariate analysis for overall survival
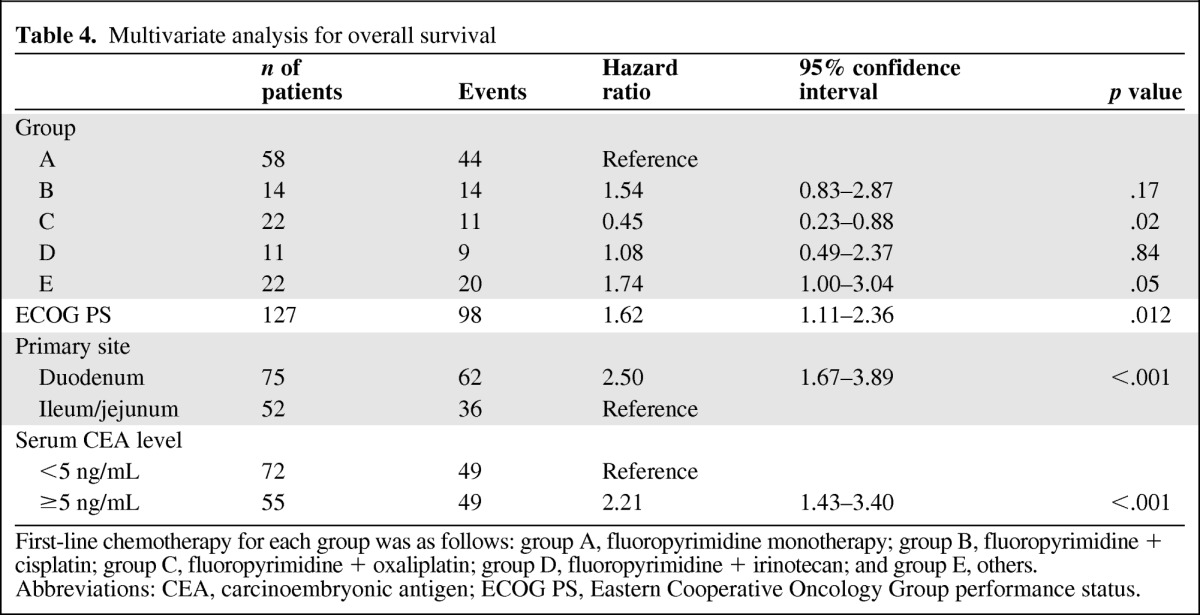
First-line chemotherapy for each group was as follows: group A, fluoropyrimidine monotherapy; group B, fluoropyrimidine + cisplatin; group C, fluoropyrimidine + oxaliplatin; group D, fluoropyrimidine + irinotecan; and group E, others.
Abbreviations: CEA, carcinoembryonic antigen; ECOG PS, Eastern Cooperative Oncology Group performance status.
Discussion
This study demonstrated significantly longer PFS times with oxaliplatin-fluoropyrimidine combination therapy than with fluoropyrimidine monotherapy for patients with unresectable SBA. Fluoropyrimidines are generally considered to be the key drugs for SBA and other gastrointestinal cancers. Most regimens used in previously reported studies of chemotherapy for unresectable SBA included fluoropyrimidines; to date, there has been no active regimen without these drugs [11–23]. However, no prospective or retrospective study has clearly showed advantages of a combination regimen compared with fluoropyrimidine monotherapy for unresectable SBA with regard to PFS or OS times. In the present study, oxaliplatin-fluoropyrimidine combination therapy was revealed to be a good prognostic factor for both PFS and OS times after adjusting for clinical background characteristics by multivariate analysis.
Although Overman et al. reported in a retrospective study that the combination of platinum compounds and 5-FU for unresectable SBA showed improved tumor response and PFS in comparison with other regimens, there was no discrimination in the efficacies of cisplatin and oxaliplatin [21]. A retrospective study conducted by AGEO compared oxaliplatin- and cisplatin-based chemotherapy. Although cisplatin (one of the key drugs for gastric cancer) was shown to be ineffective for colorectal cancer [24–27], FOLFOX therapy (which includes oxaliplatin) was associated with significantly longer PFS and OS times in comparison with the combination of cisplatin and 5-FU for advanced SBA (4.8 vs. 6.9 months, p = .02 for PFS; 17.8 vs. 9.3 months, p = .04 for OS) [22].
In the present study, PFS and OS times for patients in the cisplatin + fluoropyrimidine group (group B) were shorter than those in the fluoropyrimidine monotherapy group (group A), although the former combination achieved a relatively high response rate. Interestingly, the results of our study are very similar to those of the AGEO study, in which it was discussed that SBA behaves more like colorectal cancer than gastric cancer; the study also pointed out that a difference exists between oxaliplatin and cisplatin with regard to their efficacy for unresectable or recurrent SBA. Some retrospective data suggest that the nature of SBA resembles that of colorectal cancer. In an immunophenotypic analysis of SBA, the dominant pattern was CK20 positivity and CK7 negativity, which is seen in 75%–94% of colorectal cancer cases. Caudal-type homeobox transcription factor 2, which is highly expressed in colorectal cancer, was also expressed in most cases of SBA, especially in well-differentiated tumors [28]. In the genome-wide DNA copy number analysis, it was shown that the profiles of SBA overlapped more with colorectal adenocarcinoma than with gastric adenocarcinoma [29]. It is suggested that oxaliplatin is preferable to cisplatin in combination with fluoropyrimidine for unresectable or recurrent SBA.
Irinotecan is another key drug for the treatment of metastatic colorectal cancers. Adding irinotecan to 5-FU and leucovorin has been shown to enhance response rates and prolong survival times for patients with metastatic colorectal cancers [30, 31]. However, the present study could not show additional efficacy for irinotecan administered with fluoropyrimidine monotherapy for SBA, as similarly shown in the AGEO study. Although there is no established predictive factor for irinotecan or oxaliplatin, a future study focusing on the different sensitivities of these chemotherapeutic drugs for SBA and colorectal cancer might help to reveal the nature of these cancers.
Prognostic factors that have been reported for unresectable and recurrent SBA include age, ECOG PS, primary site, resection of primary tumor, histological type, tumor marker (CEA and CA19–9) levels, number of metastatic lymph nodes, and the presence of a metastatic tumor [7–11, 21, 22, 32–36]. In our study, a fluoropyrimidine-oxaliplatin regimen, lower ECOG PS, primary disease of the jejunum or ileum, and serum CEA of normal range were found to be good prognostic factors. With regard to the primary site, it has also been reported in several other studies that duodenal cancer was associated with a poor prognosis, although the reason is unclear. It is speculated that duodenal cancer sometimes causes bile duct obstruction [10], resulting in poor survival times. Embryologically, both the proximal part of the duodenum and the stomach are derived from the foregut and the remaining part of the small intestine is derived from the midgut. Although gastric cancer generally shows shorter survival times than colorectal cancer, it is also speculated that the tumor behavior of duodenal adenocarcinoma, especially that of the proximal part, is different from that of jejunal and ileal adenocarcinoma.
Conclusion
Oxaliplatin-fluoropyrimidine combination therapy, which showed better effects than fluoropyrimidine monotherapy, is the most promising chemotherapy regimen for unresectable or recurrent SBA. We cannot draw definitive conclusions from our study because it is retrospective and each subgroup had only small numbers of patients. However, the results recapitulate the results of a previous study on SBA, which reported better efficacy for a regimen containing oxaliplatin than a regimen containing cisplatin. This study supports the hypothesis that SBA behaves more like colorectal cancers than gastric cancers.
Over the past 10 years, the data of only two prospective studies on chemotherapy for unresectable or recurrent SBA have been published. Prospective studies are needed to establish the standard regimen for unresectable or recurrent SBA. A phase II study of FOLFOX therapy is now in progress in Japan (unique trial number: UMIN 000002797).
See the accompanying commentary on pages 1133–1134 of this issue.
Acknowledgments
We thank Dr. K. Uchino (Kyushu University Hospital); Dr. Y. Hata (Kochi Health Science Center); Dr. T. Horimatsu (Kyoto University Hospital); Dr. K. Kunieda (Saku Central Hospital); Dr. S. Kusida (Hyogo Cancer Center); Dr. M. Goto (Osaka Medical College Hospital); Dr. T. Denda (Chiba Cancer Center); Dr. Y. Kaneko (Ishikawa Prefectural Central Hospital); Dr. S. Kato (Iwate Prefectural Central Hospital); Dr. K. Yamaguchi (Saitama Prefectural Cancer Center); Dr. K. Yamashita (Saitama Medical University International Medical Center); Dr. K. Nakashima (Faculty of Medicine University of Miyazaki); Dr. S. Nakazuru (Osaka National Hospital); Dr. H. Kojima (Aichi Cancer Center Aichi Hospital); Dr. T. Nishi (Tochigi Cancer Center); Dr. M. Oozeki (Ibaraki Prefectural Central Hospital); Dr. Y. Hirashima (Oita University Hospital); Dr. K. Taira (Osaka City General Hospital); Dr. N. Kawai (Osaka Police Hospital); Dr. A. Hosokawa (Toyama University Hospital); Dr. N. Nakayama (Kanagawa Cancer Center); Dr. F. Nagashima (Kyorin University Hospital); Dr. T. Moriwaki (Tsukuba University Hospital); Dr. Y. Tsuji (Tonan Hospital); and Dr. S. Sogabe (Hokkaido University Hospital).
Author Contributions
Conception/Design: Takahiro Tsushima, Narikazu Boku
Provision of study material or patients: Takahiro Tsushima, Yoshitaka Honma, Hideaki Takahashi, Shinya Ueda, Tomohiro Nishina, Hiroki Kawai, Shunsuke Kato, Mitsukuni Suenaga, Fumio Tamura
Collection and/or assembly of data: Takahiro Tsushima
Data analysis and interpretation: Takahiro Tsushima, Masataka Taguri, Satoshi Morita, Narikazu Boku
Manuscript writing: Takahiro Tsushima, Masataka Taguri, Yoshitaka Honma, Hideaki Takahashi, Shinya Ueda, Tomohiro Nishina, Hiroki Kawai, Shunsuke Kato, Mitsukuni Suenaga, Fumio Tamura, Satoshi Morita, Narikazu Boku
Final approval of manuscript: Takahiro Tsushima, Masataka Taguri, Yoshitaka Honma, Hideaki Takahashi, Shinya Ueda, Tomohiro Nishina, Hiroki Kawai, Shunsuke Kato, Mitsukuni Suenaga, Fumio Tamura, Satoshi Morita, Narikazu Boku
References
- 1.Siegel R, Ward E, Bradley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Weiss NS, Yang CP. Incidence of histologic types of cancer of the small intestine. J Natl Cancer Inst. 1987;78:653. [PubMed] [Google Scholar]
- 3.Garcia Marcilla JA, Sanchez Bueno F, Aguilar J, et al. Primary small bowel malignant tumors. Eur J Surg Oncol. 1994;20:630. [PubMed] [Google Scholar]
- 4.DiSario JA, Burt RW, Vargas H, et al. Small bowel cancer: Epidemiological and clinical characteristics from a population-based registry. Am J Gastroenterol. 1994;89:699. [PubMed] [Google Scholar]
- 5.Hatzaras I, Palesty JA, Abir F, et al. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 6.Lepage C, Bouvier A-B, Manfredi S, et al. Incidence and management of primary malignant small bowel cancers: A well-defined French population study. Am J Gastroenterol. 2006;101:2826. doi: 10.1111/j.1572-0241.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 7.Talamoni MS, Goetz LH, Rao S, et al. Primary cancers of the small bowel: Analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137:564–570. doi: 10.1001/archsurg.137.5.564. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 9.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101(3):518–526. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 10.Halfdanarson T, McWilliams RR, Donohue JH, et al. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg. 2010;199:797–803. doi: 10.1016/j.amjsurg.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Fishman PN, Pond GR, Moore MJ, et al. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: A retrospective review of 113 cases. Am J Clin Oncol. 2006;29:225–231. [PubMed] [Google Scholar]
- 12.Czaykowski P, Hui D. Chemotherapy in small bowel adenocarcinoma: 10-year experience of the British Columbia Cancer Agency. Clin Oncol. 2007;19:143–149. doi: 10.1016/j.clon.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Ouriel K, Adams JT. Adenocarcinoma of the small intestine. Am J Surg. 1984;147:66–71. doi: 10.1016/0002-9610(84)90036-9. [DOI] [PubMed] [Google Scholar]
- 14.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. The Oncologist. 2005;10:132–137. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]
- 15.Ono M, Shirao K, Takashima A, et al. Combination chemotherapy with cisplatin and irinotecan in patients with adenocarcinoma of the small intestine. Gastric Cancer. 2008;11:201–205. doi: 10.1007/s10120-008-0484-5. [DOI] [PubMed] [Google Scholar]
- 16.Locher C, Malka D, Boige V, et al. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology. 2005;69:290–294. doi: 10.1159/000089678. [DOI] [PubMed] [Google Scholar]
- 17.Crawley C, Ross P, Hill A, et al. The Royal Marsden experience of small bowel adenocarcinoma treated with protracted venous infusion 5-fluorouracil. British J Cancer. 1998;78:508–510. doi: 10.1038/bjc.1998.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suenaga M, Mizunuma N, Chin K, et al. Chemotherapy for small-bowel adenocarcinoma at a single institution. Surg Today. 2009;39:27–31. doi: 10.1007/s00595-008-3843-2. [DOI] [PubMed] [Google Scholar]
- 19.Jigyasu D, Bedikian AY, Strohlein JR. Chemotherapy for primary adenocarcinoma of the small bowel. Cancer. 1984;53:23–25. doi: 10.1002/1097-0142(19840101)53:1<23::aid-cncr2820530106>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Goetz MP, Erlichman C, Windebank AJ, et al. Phase I and pharmacokinetic study of two different schedules of oxaliplatin, irinotecan, fluorouracil, and leucovorin in patients with solid tumors. J Clin Oncol. 2003;21:3761–3769. doi: 10.1200/JCO.2003.01.238. [DOI] [PubMed] [Google Scholar]
- 21.Overman MJ, Kopetz S, Wen S, et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113:2038–2045. doi: 10.1002/cncr.23822. [DOI] [PubMed] [Google Scholar]
- 22.Zaanan A, Costes L, Gauthier M, et al. Chemotherapy of advanced small-bowel adenocarcinoma: A multicenter AGEO study. Ann Oncol. 2010;21:1786–1793. doi: 10.1093/annonc/mdq038. [DOI] [PubMed] [Google Scholar]
- 23.Overman MJ, Vardhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 24.Kemeny N, Israel K, Neidzwiecki D, et al. Randomized study of continuous infusion fluorouracil versus fluorouracil plus cisplatin in patients with metastatic colorectal cancer. J Clin Oncol. 1990;8:313–318. doi: 10.1200/JCO.1990.8.2.313. [DOI] [PubMed] [Google Scholar]
- 25.Hansen RM, Ryan L, Anderson T, et al. Phase III study of bolus versus infusion fluorouracil with or without cisplatin in advanced colorectal cancer. J Natl Cancer Inst. 1996;88:668–674. doi: 10.1093/jnci/88.10.668. [DOI] [PubMed] [Google Scholar]
- 26.Lokich JJ, Ahlgren JD, Cantrell J, et al. A prospective randomized comparison of protracted infusional 5-fluorouracil with or without weekly bolus cisplatin in metastatic colorectal carcinoma. A Mid-Atrantic Oncology Program study. Cancer. 1991;67:14–19. doi: 10.1002/1097-0142(19910101)67:1<14::aid-cncr2820670104>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Loehrer PJ, Sr, Turner S, Kubilis P, et al. A prospective randomized trial of fluorouracil versus fluorouracil plus cisplatin in the treatment of metastatic colorectal cancer: A Hoosier Oncology Group Trial. J Clin Oncol. 1988;6:642–648. doi: 10.1200/JCO.1988.6.4.642. [DOI] [PubMed] [Google Scholar]
- 28.Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterization of adenocarcinoma of the small intestine. Br J Cancer. 2010;102:144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haan JC, Buffart TE, Eijk PP, et al. Small bowel adenocarcinoma copy number profiles are more closely related to colorectal than to gastric cancers. Ann Oncol. 2012;23:367–374. doi: 10.1093/annonc/mdr122. [DOI] [PubMed] [Google Scholar]
- 30.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 31.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 32.Koo DH, Yun SC, Hong YS, et al. Systemic chemotherapy for treatment of advanced small bowel adenocarcinoma with prognostic factor analysis: Retrospective study. BMC Cancer. 2011;11:205. doi: 10.1186/1471-2407-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speranza G, Doroshow JH, Kummar S. Adenocarcinoma of the small bowel: Changes in the landscape? Curr Opin Oncal. 2010;22:287–393. doi: 10.1097/CCO.0b013e32833a86fe. [DOI] [PubMed] [Google Scholar]
- 34.Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: Review of the National Cancer Data Base, 1985–1995. Cancer. 1999;86:2693–2706. doi: 10.1002/(sici)1097-0142(19991215)86:12<2693::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 35.Wu TJ, Yeh CN, Chao TC, et al. Prognostic factors of primary small bowel adenocarcinoma: Univariate and multivariate analysis. World J Surg. 2000;30:391–398. doi: 10.1007/s00268-005-7898-6. [DOI] [PubMed] [Google Scholar]
- 36.Hong SH, Koh YH, Rho SY, et al. Primary adenocarcinoma of the small intestine: presentation, prognostic factors and clinical outcome. Jpn J Clin Oncol. 2009;39:54–61. doi: 10.1093/jjco/hyn122. [DOI] [PubMed] [Google Scholar]