This study analyzes the severity and timing of sorafenib-induced toxicities in a cohort of unselected consecutive patients with cancer to determine whether the pharmacokinetics of sorafenib influence its toxicity.
Keywords: Angiogenesis inhibitors, VEGF-A, Population pharmacokinetics, Sorafenib, Therapeutic drug monitoring, Toxicity
Learning Objectives
After completing this course, the reader will be able to:
Describe the profile of severe toxicities in patients treated with sorafenib.
Summarize the pharmacokinetics of sorafenib-induced toxicities.
Identify predictive factors for early and delayed toxicities in patients treated with sorafenib.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Sorafenib displays major interpatient pharmacokinetic variability. It is unknown whether the pharmacokinetics of sorafenib influence its toxicity.
Methods.
We analyzed the severity and kinetics of sorafenib-induced toxicities in unselected consecutive patients with cancer, as well as their relationship with biological, clinical, and pharmacokinetic parameters. Toxicity was recorded bimonthly. Sorafenib plasma concentrations were assessed by liquid chromatography.
Results.
For 83 patients (median age, 62 years; range, 21–84 years), median sorafenib 12-hour area under the curve (AUC0–12) was 52.8 mg · h/L (range: 11.8–199.6). A total of 51 patients (61%) experienced grade 3–4 toxicities, including hand-foot skin reactions (23%), asthenia (18%), and diarrhea (11%). Sorafenib AUC0–12 preceding grade 3–4 toxicities was significantly higher than that observed in the remaining population (61.9 mg · h/L vs. 53 mg · h/L). In 25 patients treated with fixed doses of sorafenib for the first 4 months, median dose-normalized AUC0–12 on day 120 was significantly lower than on day 15 (63 vs. 102 mg · h/L). The incidence of hypertension and hand-foot skin reactions significantly decreased over time.
Conclusion.
Sorafenib AUC0–12 decreases over time, similarly to the incidence of hypertension and hand-foot skin reactions. Monitoring of sorafenib plasma concentrations may help to prevent acute severe toxicities and detect patients with suboptimal exposure at disease progression.
Introduction
Sorafenib is an oral multikinase inhibitor that targets the Raf serine/threonine kinases (Raf-1, wild-type B-Raf, and b-raf V600E); vascular endothelial growth factor receptor (VEGFR) 1, VEGFR-2, and VEGFR-3; and the platelet-derived growth factor receptors [1]. Sorafenib is approved for the treatment of patients with advanced renal cell carcinoma and unresectable hepatocellular carcinoma [2, 3]. It has shown clinical activity in patients with a variety of tumors, including differentiated thyroid cancer, melanoma, and soft-tissue sarcoma [4–8]. Sorafenib is highly bound (99%) to plasma proteins, mainly to albumin [9, 10]. Sorafenib is metabolized primarily in the liver undergoing oxidative metabolism, mediated by CYP3A4, as well as glucuronidation mediated by UGT1A9. Sorafenib is mainly eliminated in the feces. Sorafenib undergoes enterohepatic circulation [11]. Its terminal half-life ranges from 25–38 hours [12].
A large interindividual variability of pharmacokinetic parameters was observed during phase I studies of sorafenib [9, 10]. Most sorafenib-induced severe toxicities are nonhematological, including hand-foot skin reactions (HFSRs), diarrhea, and asthenia. These toxicities are difficult to handle and anticipate; they caused treatment reduction in 35% of patients or termination in approximately 10% of patients in an open-label expanded access program [11]. An even higher prevalence of such toxicities may be expected in nonselected patients, as demonstrated in published experiences outside of clinical trials [12, 13]. Furthermore, it is unknown whether sorafenib exposure could influence toxicity prevalence and severity.
In an attempt to answer these questions, we analyzed the severity and timing of sorafenib-induced toxicities in a cohort of unselected consecutive patients with cancer, as well as their relationship with biological, clinical, and pharmacokinetic parameters.
Patients and Methods
All consecutive patients with advanced, metastatic, or recurrent solid tumors who were treated with sorafenib in our institution between April 2008 and October 2010 were eligible for this retrospective analysis. All patients met the following criteria: age ≥18 years; presence of clinically and/or radiologically assessable disease; and adequate bone marrow, liver, and renal function. Patients with brain metastases were included as well. Diets and activity were not restricted. All patients underwent bimonthly monitoring of sorafenib plasma concentrations and provided written informed consent for this procedure.
Treatment Plan, Adverse Events, and Efficacy
Patients were started on sorafenib (200 or 400 mg, based on the treating physician's recommendation) on a twice-daily schedule. Subsequently, doses were adjusted based on adverse events by 200-mg decrements.
During the treatment period, physical examination, complete blood cell count, serum chemistry, and urine analysis were performed twice monthly. Patients received instructions to monitor blood pressure twice daily at home, using a blood pressure monitoring device validated by the French health authorities (AFSSAPS [Agence Française de Sécurité Sanitaire des Produits de Santé]), as recommended by international guidelines. A multidisciplinary team including an oncologist, cardiologist, dermatologist, nephrologist, and pharmacist evaluated prospectively and graded toxicity according to the National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 3.0 [14] at each visit. Hypertension was retrospectively graded according to the NCI-CTCAE version 4.0 [15]. In the case of grade 4 hematological toxicity and grade 3 or 4 nonhematological toxicity, treatment was delayed until side effects had recovered to grades 1–2 or returned to baseline. Subsequent dose reductions were left to the discretion of the treating physician. Dose-limiting toxicity (DLT) was defined as toxicity resulting in dose reduction or permanent discontinuation of sorafenib. Follow-up ended 4 months after the last patient had started treatment.
Pharmacokinetic Assessments
Blood samples (5-mL aliquots) for the determination of plasma concentrations of sorafenib were collected at each follow-up visit. Samples were collected into lithium heparinized Vacutainer tubes (Becton Dickinson, Le Pont-De-Claix, France), and centrifuged at 3,000 rpm for 5 minutes at 4°C. Samples were transferred to polypropylene tubes and kept at −20°C until assay.
Sorafenib concentrations in plasma were determined using high-performance liquid chromatography, with a lower limit of quantitation of 0.5 mg/L [16]. Intra- and interassay precision rates were less than 7% and 10%, respectively, at 0.5, 3, and 20 mg/L.
Plasma pharmacokinetic parameters were calculated, and the projected 12-hour area under the curve (AUC0–12) per 400 mg of sorafenib was estimated using a previously described Bayesian estimator [17]. The potential correlations between AUC0–12 and systemic toxicities associated with the administration of sorafenib were assessed.
Statistical Analysis
Data are expressed as means ± standard deviations or medians (range). To explore the effect of exposure on the occurrence of grade 3–4 adverse events (AEs), sorafenib concentrations measured during the 3 weeks preceding each grade 3–4 AE were pooled.
Associations between biological, pharmacokinetic, and clinical parameters and toxicities were determined by univariate analysis using the Wilcoxon W test or Fisher's exact test, when appropriate. We investigated whether baseline clinical characteristics (age, sex, performance status, body mass index, primary tumor, prior treatment) and biological parameters (baseline and D30 albumin and bilirubin, C-reactive protein) could predict the occurrence of severe toxicities. The worst clinically significant (grade 2–4) treatment-associated toxicities (diarrhea, HFSR, hypertension, and asthenia) were analyzed.
Parameters associated with toxicity with a p-value ≤.10 were entered in a multivariate model. Multivariate analysis was performed using multiple regression with backward and forward procedures and bootstrapping (2,000 iterations).
Areas under the concentration-time curve were compared using the Mann-Whitney U test. All comparison tests were two-sided. A p-value <.05 was considered to be statistically significant. Calculations were performed with NCSS 2007 Software (NCSS, Kaysville, UT).
Results
From April 2008 to October 2010, a total of 86 patients were treated with sorafenib in our institution. Three patients were excluded because of treatment duration <7 days or missing data. The baseline characteristics of the remaining 83 patients are shown in Table 1.
Table 1.
Patient characteristics (n = 83)
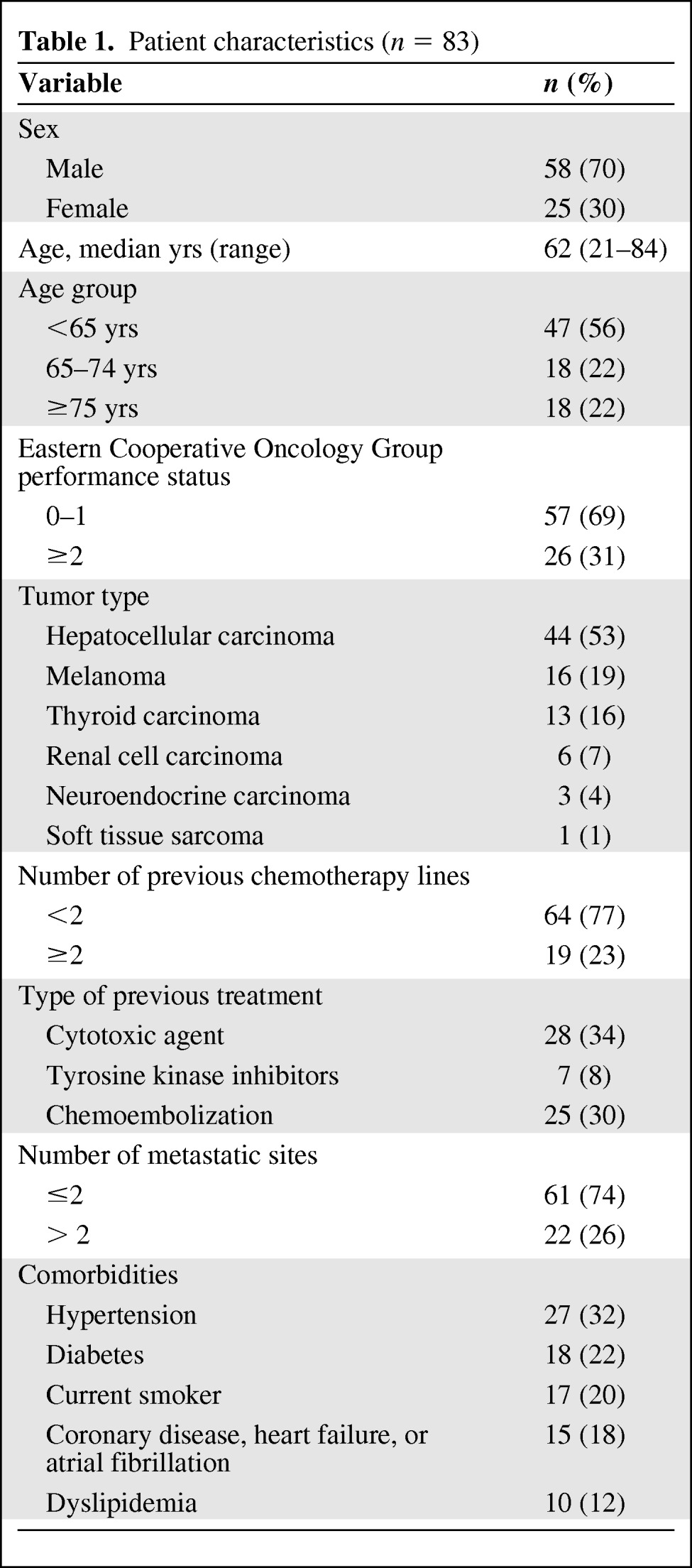
The median patient age was 62 years (range, 21–84 years), with 35% of patients being older than 70 years. Many patients had comorbidities or cardiovascular risk factors, such as hypertension (32%), diabetes (22%), current smoking (20%), and coronary disease, heart failure, or atrial fibrillation (18%). No patient received concomitant treatment with a known CYP3A4 inducer or inhibitor susceptible to interfere with sorafenib metabolism.
The most common primary tumors were hepatocellular carcinoma (HCC, 53%) and melanoma (19%). All patients with HCC had Child-Pugh class A cirrhosis, except for two patients with Child-Pugh class B cirrhosis.
The median duration of treatment was 5.4 months (range, 0.25–43). A majority of patients were started on sorafenib at a dose of 400 mg b.i.d., with 19 patients starting at a dose of 200 mg b.i.d.
Safety and Dose Reductions
Toxicities occurring in >5% of patients and/or reaching grade 3–4 severity are shown in Table 2. The most frequent nonhematological grade 1–2 AEs were asthenia, HFSR, diarrhea, hypertension, rash, anorexia, and alopecia. A total of 51 patients (61%) experienced grade 3 toxicities. The most frequent grade 3 AEs were HFSR (23%), asthenia (18%), and diarrhea (11%). Thrombocytopenia (7%) and anemia (4%) were the most frequently observed hematological grade 1–2 AEs. There were no toxic deaths related to sorafenib. Infrequent toxicities were observed, including pneumatosis intestinalis (n = 1) [18] and spiny follicular hyperkeratosis (n = 9) [19].
Table 2.
Toxicities occurring in >5% of patients and/or reaching grade 3–4 severity
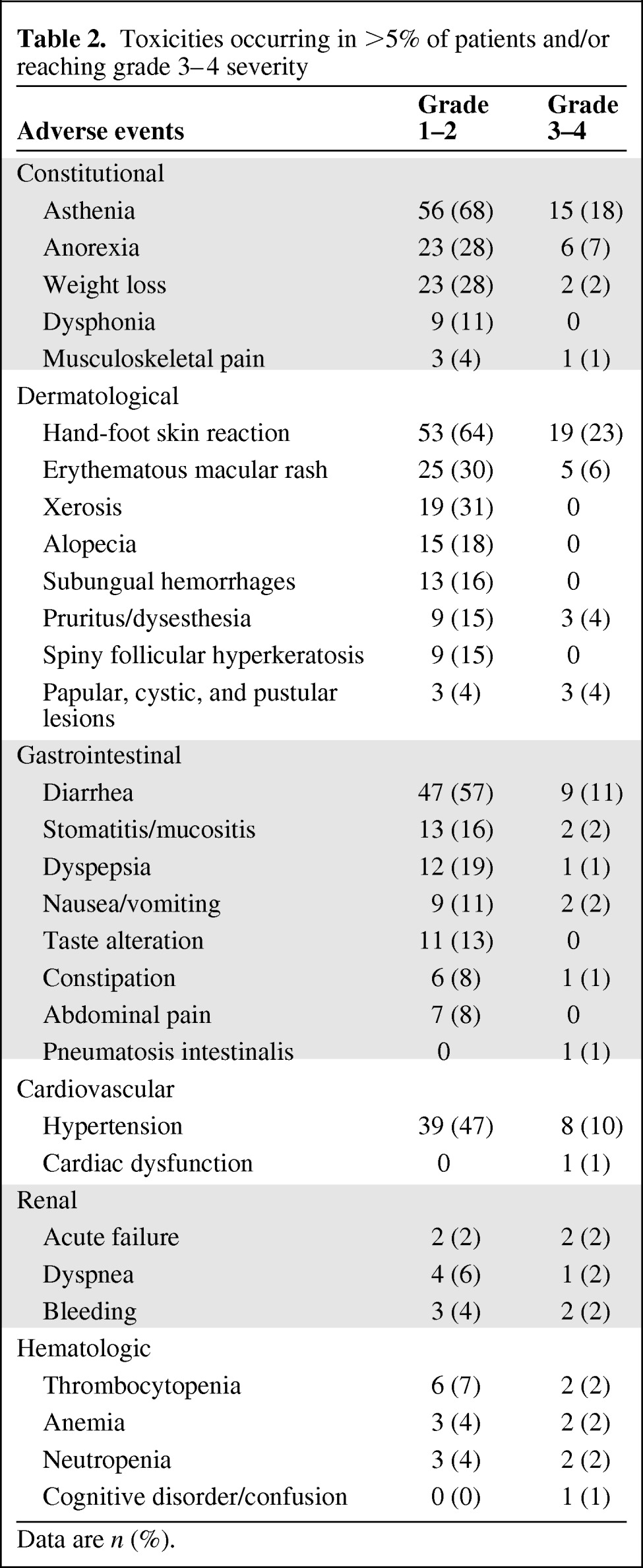
Data are n (%).
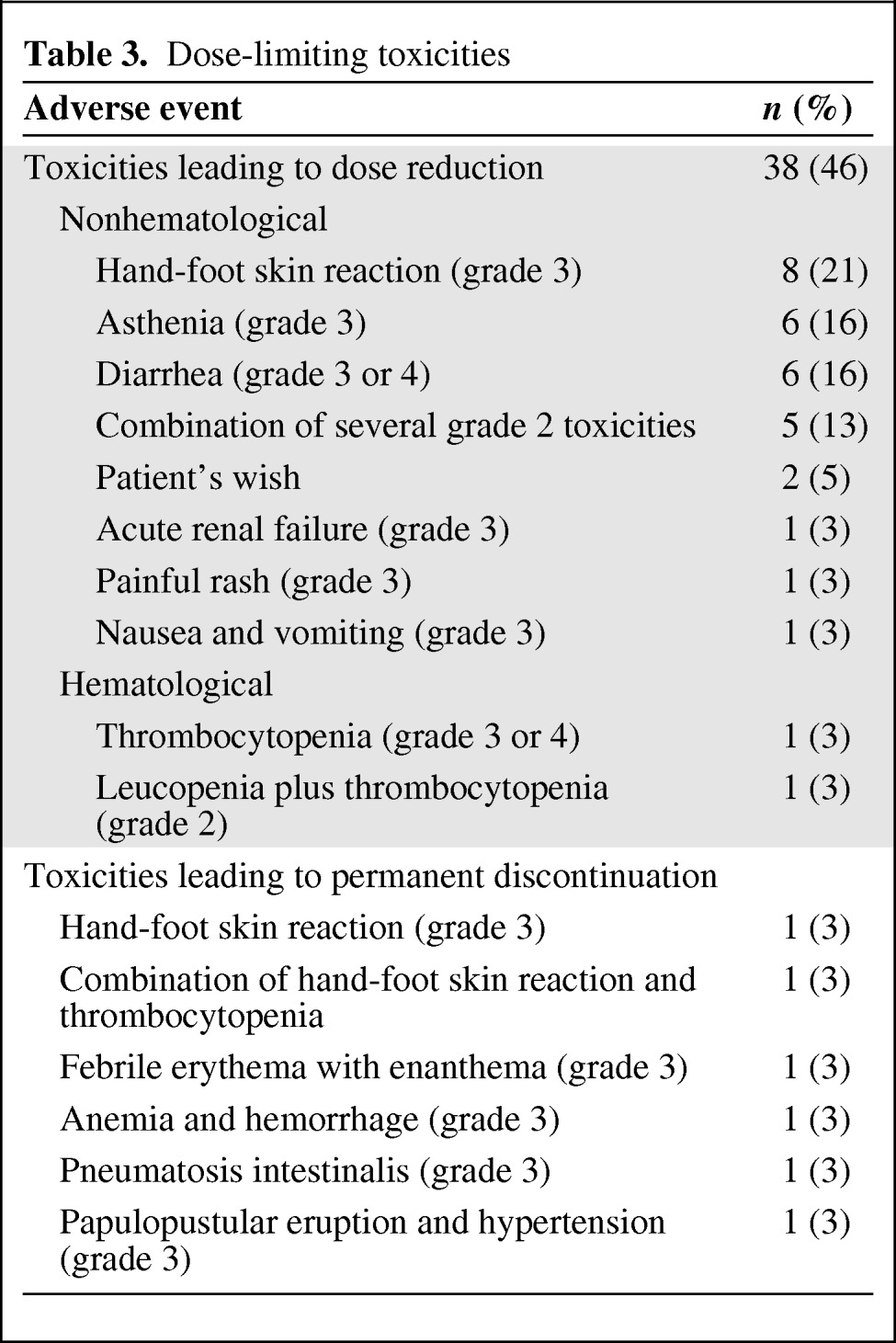
In all, 38 patients (46%) experienced a DLT event, including 32 patients (38.5%) who had a dose reduction and 6 patients (7.2%) for whom treatment was withdrawn (Table 3). During the first 2 months of treatment, a total of 25 patients experienced DLT events, including 21 patients who had a dose reduction and 4 patients who had a permanent discontinuation of treatment. Only three patients experienced more than one DLT event.
Table 3.
Dose-limiting toxicities
Analysis of Common Toxicities over Time, Initial Presentation, Prevalence, and Incidence per Period
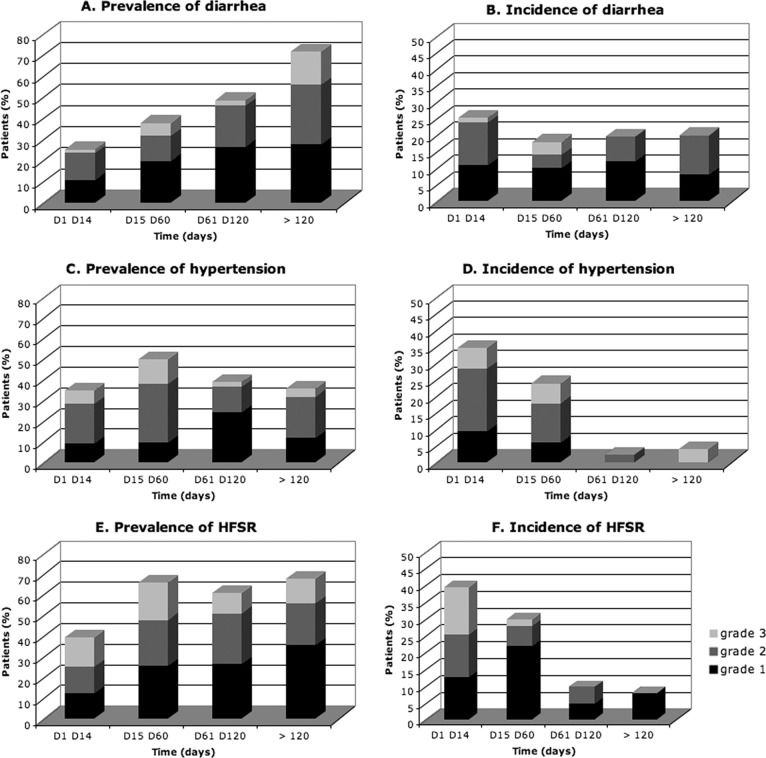
In the first 2 weeks of treatment, HFSR, hypertension, and diarrhea occurred in 36.2%, 31.3%, and 25.2% of patients, respectively. Table 4 indicates the timing of occurrence of these toxicities. The prevalence rates (grades 1–3) and the incidence rates (grades 1–3) are reported per period of treatment in Figure 1.
Table 4.
Kinetics of sorafenib-induced toxicity
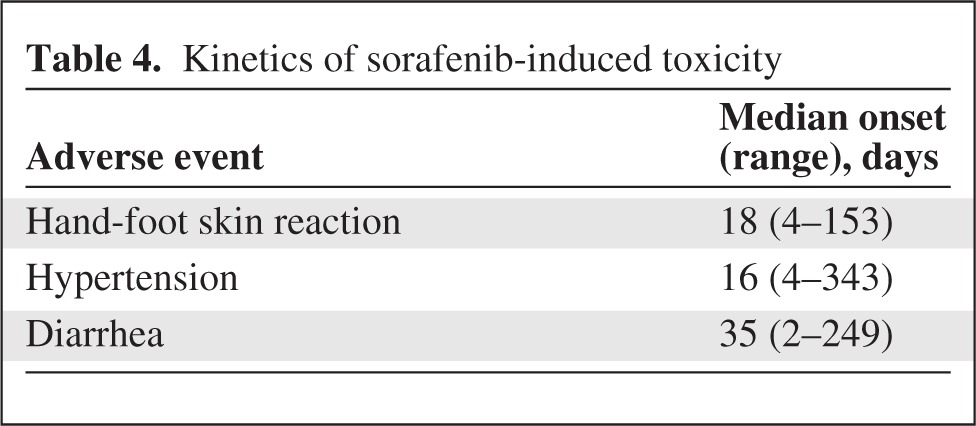
Figure 1.
Prevalence, incidence, and severity of toxicities over time. Abbreviation: HFSR, hand-foot skin reaction.
We analyzed the prevalence, severity, and incidence of diarrhea, hypertension, and HFSR over time: respectively, 63 events occurred between days 1 and 14, 58 events occurred between days 15 and 60, 42 events occurred between days 61 and 120, and 23 events occurred after day 120. Diarrhea was a cumulative toxicity that occurred significantly more frequently over time (p < .001; Fig. 1A). The severity and incidence of diarrhea was not modified over time (Fig. 1B). Hypertension was significantly more prevalent at the end of the first 2 months of treatment (p < .01; Fig. 1C). Hypertension severity was not modified over time. The incidence of hypertension decreased over time (p < .01; Fig. 1D). The prevalence of HFSR significantly increased over time (p = .048; Fig. 1E). HFSR severity was not modified over time. Incidence of HFSR significantly decreased over time (p < .01; Fig. 1F). New cases of HFSR were less severe over time (p = .048).
Pharmacokinetics/Pharmacodynamics
A total of 525 plasma samples from 72 patients were analyzed, including 32 samples that were excluded from the statistical analysis for various reasons (plasma concentration below lower limit of quantitation, sampling time >50 hours after sorafenib intake, iterative drug intake). Median sorafenib AUC0–12 was 52.8 mg · h/L (range, 11.8–199.6), and mean AUC0–12 was 57.7 ± 28.6 mg · h/L.
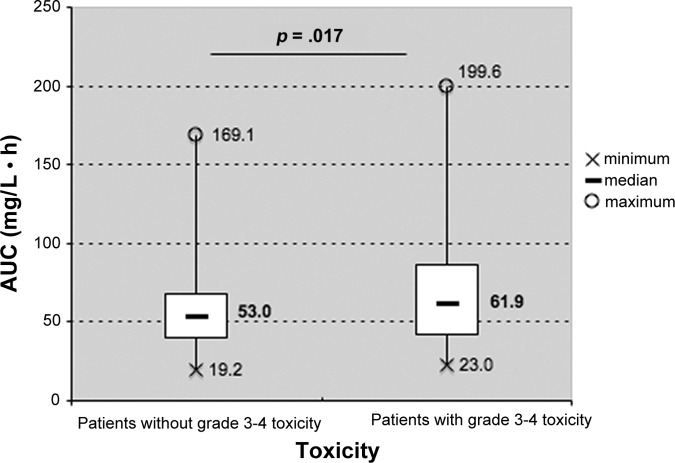
Median sorafenib AUC0–12 preceding severe toxicity in patients experiencing grade 3–4 AE was significantly higher than that observed in the remaining patients (61.9 mg · h/L vs. 53 mg · h/L, p = .017; Fig. 2). Among patients experiencing grade 3–4 AEs, median sorafenib AUC0–12 preceding severe toxicity was significantly higher than that observed at other times during treatment (62.4 vs. 55.7 mg · h/L, p < .001).
Figure 2.
Sorafenib 12-hour area under the curve in patients with and without grade 3–4 toxicity. Abbreviation: AUC, area under the curve.
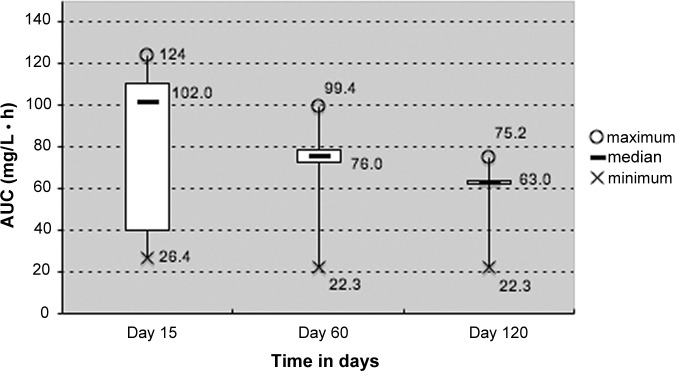
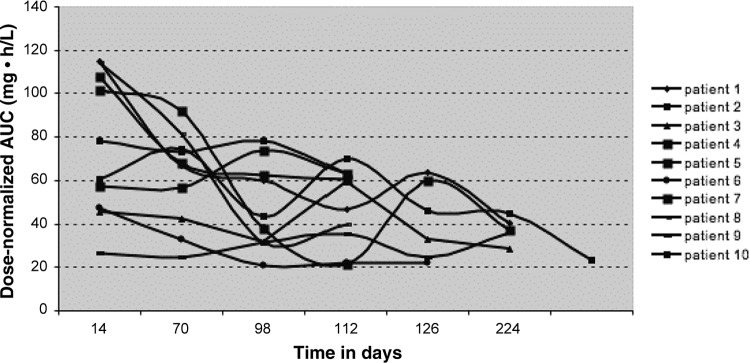
Figure 3 shows the decrease of median sorafenib AUC0–12 over time. In 25 patients treated with a fixed dose of sorafenib for the first 4 months (i.e., without dose adjustment), median dose-normalized AUC0–12 on day 120 was lower than on day 60 (63.0 vs. 76 mg · h/L, respectively), and lower than on day 15 (63 vs. 102 mg · h/L, respectively; p < .01). Interpatient variability in sorafenib AUC was more important on day 15 (interquartile range [IQR], 40–111 mg · h/L) and less pronounced on day 60 (IQR, 73–79 mg · h/L) and on day 120 (IQR, 63–63 mg · h/L). Sorafenib AUC0–12 decreased over time, similarly to the incidence of hypertension and HFSR (Fig. 1C, 1D). Regarding intrapatient variability, we observed a decrease in sorafenib dose-normalized AUC0–12 over time in 10 consecutive patients treated at fixed dose, as illustrated in Figure 4.
Figure 3.
Decreasing sorafenib dose-normalized 12-hour area under the curve over time in 25 patients treated at fixed doses. Boxes indicate median values and the ends of the vertical lines show the minimum and maximum values. The bottoms and tops of the boxes represent the first and third quartiles, respectively. Abbreviation: AUC, area under the curve.
Figure 4.
Evolution of exposition (area under the curve) over time in 10 consecutive patients treated at fixed dose (200 or 400 mg bid).
Predictive Factors of Early and Delayed Toxicity
No factor predicting early acute diarrhoea or hypertension was identified. Conversely, high baseline albumin and high AUC0–12 on day 30 were associated with the occurrence of HFSR (p = .009 and .03, respectively). Low baseline albumin, high baseline C-reactive protein, and prior treatment by sunitinib were associated with the occurrence of asthenia of grade 2 or higher (p < .001, .046, and .04, respectively). In multivariate analysis, no independent variable was identified.
Young age, female sex, treatment duration, high baseline and day 30 albumin, and high baseline bilirubin were associated with the occurrence of HFSR (p = .04, .01, .01, .02, .02, and .01, respectively). In multivariate analysis, no independent variable was identified. Young age and treatment duration were correlated with the occurrence of diarrhea (p = .006 and .03, respectively). In multivariate analysis, both variables remained significant (p = .02 and .047, respectively).
No variable was correlated with the occurrence of delayed asthenia. The occurrence of delayed hypertension was associated with treatment duration and female gender (p = .001 and .10, respectively). In multivariate analysis, only treatment duration significantly predicted delayed hypertension (p = .001).
Discussion
To the best of our knowledge, this is the first analysis of sorafenib toxicity including a pharmacokinetic/pharmacodynamic approach in unselected patients with cancer. In all, 35% of patients were aged 70 years or older. In large randomized clinical trials, elderly patients are underrepresented, as previously discussed [20]. Our results suggest that sorafenib is feasible in elderly patients. Age older than 62 years—the median age of our population—was not predictive of more frequent or severe acute or delayed toxicities. The management of cardiovascular toxicity, asthenia, and digestive toxicities is a major issue in daily practice, particularly in elderly patients. A subset analysis of the Treatment Approaches in Renal cancer Global Evaluation Trial (TARGET) study suggested a similar benefit in terms of progression-free survival in patients aged over 65 years compared with their younger counterparts, without greater apparent toxicity [21].
Sorafenib side effects are systemic and may concern any tissue. However, side effects are generally limited to cutaneous toxicity, diarrhea, and asthenia. The timing of the occurrence of the side effects is poorly described, contrasting with the predictable kinetics of neutropenia for a given protocol of intravenous conventional chemotherapy.
The kinetics of sorafenib-induced toxicities are demonstrated by Table 2, which shows a high prevalence of toxicities increasing over time (Fig. 1). No toxic deaths occurred, but the incidence of grade 3–4 toxicities was higher than that reported in phase III trials [2, 3]. Several hypotheses can be raised to explain the high prevalence of toxicities in our cohort of patients. First, patients were prospectively and frequently evaluated for toxicity by a multidisciplinary team. Second, most of our patients had significant comorbidities and multiple sites of metastasis, including brain metastases; in addition, our population included vulnerable patients (19% of patients had heavily pretreated melanoma). Patients enrolled in pivotal studies are often not typical of the wider population with the disease. Exclusion of common comorbidities is frequent. Symptoms caused by comorbidities, such as fatigue, may be exacerbated by sorafenib treatment, and drug interactions with ongoing therapies can cause additional toxicities.
The most common DLTs were diarrhea, HFSR, asthenia, rash, and anorexia. However, dose reductions were due not only to grade 3 toxicities, but also to the sum of various grade 2 toxicities interfering excessively with daily life, such as HFSR that limits walking, stomatitis, or taste alteration.
The present analysis provides insights on long-term administration of sorafenib that may help guide clinical decision making. Indeed, we described the kinetics of common toxicities (HFSR, hypertension, and diarrhea) in a daily practice population. We found that HFSR frequently occurred early (within 2 weeks of treatment). These findings are consistent with a recent meta-analysis showing that 21%–93% of patients experienced HFSR, usually within 6 weeks of starting sorafenib [22]. In the present analysis, patients who did not experience severe HFSR early in the course of the treatment were unlikely to develop HFSR later, in line with data from the TARGET study [23]. Furthermore, we found that the occurrence of acute HFSR was associated with high AUC0–12 on day 30 and high albuminemia. Although an increased incidence of HSFR with higher starting doses of sorafenib was seen in phase I studies [24], a pooled analysis of three phase II studies failed to demonstrate association between HFSR and sorafenib AUC [25]. Univariate analysis of our cohort showed that the occurrence of delayed HFSR was associated with female sex, young age, prolonged treatment duration, and high albuminemia (on baseline and day 30). Lijima et al. observed that women have a greater tendency to experience grade 3 HFSR (21% vs. 6%) [26]. Azad et al. found that sorafenib-induced HFSR was related to cumulative sorafenib dose [25].
The ability to identify patients at risk of developing severe toxicities would be invaluable for optimal management. Several biomarkers and predictors have been proposed to predict the treatment response [27, 28] and drug toxicity [29–31]. However, by multivariate analysis, we were not able to identify any baseline clinical or biological independent predictor of severe early toxicities.
Regarding sorafenib exposure, the mean AUC0–12 observed in our patients treated at the recommended dose was in the same range than that previously reported in phase I trials (57.7 ± 28.6 mg · h/L vs. 71.7 ± 30.8 mg · h/L, respectively) [10]. Our data suggest a relationship between drug-related grade 3–4 AEs and exposure to sorafenib. Indeed, sorafenib AUC0–12 prior to the occurrence of grade 3–4 toxicity was significantly greater than that observed in the remaining patients. However, these results were obtained in a small cohort of patients and deserve validation in larger populations of patients.
Most importantly, we also observed that sorafenib AUC0–12 decreased progressively over time in 25 patients treated with a fixed dose of sorafenib. We illustrate this fact with an additional figure (Figure 4).
Our data show that diarrhea was a cumulative toxicity, significantly more frequent over time. This suggests that the intestinal absorption of sorafenib might decrease over time and, therefore, also sorafenib exposure.
Such a decrease in exposure over time has already been reported with imatinib [32]. It might be related to the induced expression of efflux pumps in the gut wall, such as ABCG2 [33] and/or an auto-induction of sorafenib metabolism.
Monitoring of sorafenib plasma concentrations may help to prevent severe toxicities by adjusting dose and schedule. Our findings indicate that fixed dosing schedules for sorafenib result in significant interpatient pharmacokinetic variability, with dose-limiting toxicities occurring in a sizeable population of patients. Besides, in the present series, we observed a decrease of sorafenib exposure over time. These findings might have relevant clinical implications in patients with initial clinical benefit and subsequent progression. As suggested by others [36], a logical approach would consist in a dose-escalation at the time of progression, aiming to restore an adequate exposure in patients in whom AUC0–12 markedly decreased over time and, therefore, supporting therapeutic drug monitoring.
For instance, we have shown that dose escalation is an effective strategy in patients with progressive metastatic melanoma [35], and sorafenib dose escalation is under investigation in other malignancies [36, 37]. Dedicated pharmacoclinical studies are required to determine the threshold drug exposure that might condition antitumor efficacy in different tumor types.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Pascaline Boudou-Rouquette, Stanislas Ropert, Olivier Mir, François Goldwasser
Provision of study material or patients: Pascaline Boudou-Rouquette, Stanislas Ropert, Olivier Mir, Bertrand Billemont, Laure Cabanes, Nathalie Franck, Benoit Blanchet, François Goldwasser
Collection and/or assembly of data: Pascaline Boudou-Rouquette
Data analysis and interpretation: Pascaline Boudou-Rouquette, Stanislas Ropert, Olivier Mir, Romain Coriat, Bertrand Billemont, Michel Tod, Laure Cabanes, Nathalie Franck, Benoit Blanchet, François Goldwasser
Manuscript writing: Pascaline Boudou-Rouquette, Olivier Mir, Romain Coriat, Michel Tod, Benoit Blanchet, François Goldwasser
Final approval of manuscript: Pascaline Boudou-Rouquette, Stanislas Ropert, Olivier Mir, Romain Coriat, Bertrand Billemont, Michel Tod, Laure Cabanes, Nathalie Franck, Benoit Blanchet, François Goldwasser
References
- 1.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;11:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;24:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Gupta-Abramson VTA, Nellore A, Puttaswamy K, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;10:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: A report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 7.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 8.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 10.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 11.Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 116:1272–1280. doi: 10.1002/cncr.24864. [DOI] [PubMed] [Google Scholar]
- 12.La Vine DB, Coleman TA, Davis CH, et al. Frequent dose interruptions are required for patients receiving oral kinase inhibitor therapy for advanced renal cell carcinoma. Am J Clin Oncol. 2010;33:217–220. doi: 10.1097/COC.0b013e3181a650a6. [DOI] [PubMed] [Google Scholar]
- 13.Riechelmann RP, Chin S, Wang L, et al. Sorafenib for metastatic renal cancer: The Princess Margaret experience. Am J Clin Oncol. 2008;31:182–187. doi: 10.1097/COC.0b013e3181574084. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common terminology criteria for adverse events. Bethesda, MD: National Institutes of Health; 2006. [Google Scholar]
- 15.National Cancer Institute. Common terminology criteria for adverse events. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 16.Blanchet B, Billemont B, Cramard J, et al. Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J Pharm Biomed Anal. 2009;49:1109–1114. doi: 10.1016/j.jpba.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Hornecker M, Blanchet B, Billemont B, et al. Saturable absorption of sorafenib in patients with solid tumors: A population model. Invest New Drugs. 2011 Oct 18; doi: 10.1007/s10637-011-9760-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Coriat R, Ropert S, Mir O, et al. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs. 2011;29:1090–1093. doi: 10.1007/s10637-010-9458-7. [DOI] [PubMed] [Google Scholar]
- 19.Franck N, Barete S, Moguelet P, et al. Spiny follicular hyperkeratosis eruption: A new cutaneous adverse effect of sorafenib. J Clin Oncol. 28:e640–642. doi: 10.1200/JCO.2010.31.3783. [DOI] [PubMed] [Google Scholar]
- 20.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 21.Eisen T, Oudard S, Szczylik C, et al. Sorafenib for older patients with renal cell carcinoma: Subset analysis from a randomized trial. J Natl Cancer Inst. 2008;100:1454–1463. doi: 10.1093/jnci/djn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Zhou Q, Ma L, et al. Meta-analysis of dermatological toxicities associated with sorafenib. Clin Exp Dermatol. 2011;36:344–350. doi: 10.1111/j.1365-2230.2011.04060.x. [DOI] [PubMed] [Google Scholar]
- 23.Hutson TE, Bellmunt J, Porta C, et al. Long-term safety of sorafenib in advanced renal cell carcinoma: Follow-up of patients from phase III TARGET. Eur J Cancer. 2010;46:2432–2440. doi: 10.1016/j.ejca.2010.06.121. [DOI] [PubMed] [Google Scholar]
- 24.Strumberg D, Awada A, Hirte H, et al. Pooled safety analysis of BAY 43–9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome? Eur J Cancer. 2006;42:548–556. doi: 10.1016/j.ejca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Azad NS, Aragon-Ching JB, Dahut WL, et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–1416. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima M, Fukino K, Adachi M, et al. Sorafenib-associated hand-foot syndrome in Japanese patients. J Dermatol. 2011;38:261–266. doi: 10.1111/j.1346-8138.2010.01059.x. [DOI] [PubMed] [Google Scholar]
- 27.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: A review. Eur J Cancer. 2006;42:3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Bellmunt J, Gonzalez-Larriba JL, Prior C, et al. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: Baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol. 2011;22:2646–2653. doi: 10.1093/annonc/mdr023. [DOI] [PubMed] [Google Scholar]
- 29.van Erp NP, Eechoute K, van der Veldt AA, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol. 2009;27:4406–4412. doi: 10.1200/JCO.2008.21.7679. [DOI] [PubMed] [Google Scholar]
- 30.van der Veldt AA, Boven E, Helgason HH, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer. 2008;99:259–265. doi: 10.1038/sj.bjc.6604456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalantari HR. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer. 2009;101:1222–1223. doi: 10.1038/sj.bjc.6605303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judson I, Ma P, Peng B, et al. Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: A retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 2005;55:379–386. doi: 10.1007/s00280-004-0876-0. [DOI] [PubMed] [Google Scholar]
- 33.Burger H, van Tol H, Brok M, et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4:747–752. doi: 10.4161/cbt.4.7.1826. [DOI] [PubMed] [Google Scholar]
- 34.Semrad TJ, Gandara DR, Lara PN., Jr Enhancing the clinical activity of sorafenib through dose escalation: Rationale and current experience. Ther Adv Med Oncol. 2011;3:95–100. doi: 10.1177/1758834010396117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pécuchet N, Kerob D, Billemont B, et al. Relationship between dose, exposure, and antitumoral activity of sorafenib in melanoma. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 36.Pressiani LR, Boni C, Labianca R, et al. Phase II randomized trial on dose-escalated sorafenib (S) versus best supportive care (BSC) in patients with advanced hepatocellular carcinoma (HCC) with disease progression on prior S treatment. J Clin Oncol. 2011:29. [Google Scholar]
- 37.Gore RJJ, Ravaud A, Kuczyk M, et al. Efficacy and safety of intrapatient dose escalation of sorafenib as first-line treatment for metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2011;29(Suppl) Abstract 4609. [Google Scholar]