This randomized multicenter phase II study aimed to evaluate the efficacy and toxicity of single-agent gemcitabine versus gemcitabine plus docetaxel as second-line therapy for patients with metastatic or relapsed leiomyosarcoma. Patients were stratified by the leiomyosarcoma's site of origin (uterine versus nonuterine).
Keywords: Leiomyosarcoma, Chemotherapy, Docetaxel, Gemcitabine
Abstract
Background.
This study aimed to evaluate the efficacy and toxicity of single-agent gemcitabine versus gemcitabine plus docetaxel as second-line therapy in patients with uterine and nonuterine leiomyosarcoma (LMS).
Patients and Methods.
Patients had metastatic or unresectable LMS and had received one prior anthracycline-based regimen. A total of 90 patients received either single-agent gemcitabine (arm A; gemcitabine, 1,000 mg/m2 i.v. for 100 minutes on days 1, 8, and 15 of a 28-day cycle) or a combination of gemcitabine and docetaxel (arm B; gemcitabine, 900 mg/m2 i.v. for 90 minutes on days 1 and 8, plus docetaxel, 100 mg/m2 i.v. for 1 hour on day 8 of a 21-day cycle with lenograstim). The primary endpoint was the objective response rate.
Results.
The objective response rates were 19% and 24% in arm A (gemcitabine) and arm B (gemcitabine plus docetaxel), respectively, for patients with uterine LMS. For patients with nonuterine LMS, the objective response rates were 14% and 5% for arms A and B, respectively. The median progression-free survival times for arms A and B were 5.5 months and 4.7 months, respectively, for patients with uterine LMS. For patients with nonuterine LMS, the median progression-free survival times were 6.3 months and 3.8 months for arms A and B, respectively. One toxic death occurred in arm B.
Conclusions.
Both single-agent gemcitabine and gemcitabine plus docetaxel were found to be effective second-line therapies for leiomyosarcomas, with a 3-month progression-free survival rate of 40% for LMS with both uterine and nonuterine sites of origin. Single-agent gemcitabine yielded results similar to those of gemcitabine plus docetaxel in this trial, but patients using single-agent gemcitabine experienced less toxicity.
Introduction
Leiomyosarcoma (LMS) is the most common form of uterine sarcoma, comprising 25% of soft-tissue sarcomas [1]. Patients with locally advanced or metastatic LMS have a poor prognosis [2–6], with a median overall survival (OS) time of ∼17 months after first-line treatment with a doxorubicin-based regimen [7]. LMS has a wide anatomical distribution and exhibits complex nonrecurring genomic alterations; no specific molecular targets are known to serve as drivers for these sarcomas.
Some phase II studies indicated that LMS patients may exhibit higher rates of objective response than patients with other histological subtypes of soft-tissue sarcoma. In addition, LMS that originates in the uterus may be more chemosensitive than LMS in other sites [8]. Given the known heterogeneity of soft-tissue sarcomas (except for gastrointestinal stromal tumors), studies exploring therapeutic agents in sarcomas should be stratified or specifically designed for particular histological subtypes and sites of disease origin.
In the metastatic setting, the highest response rates reported to be associated with single-agent chemotherapies or combination regimens (i.e., doxorubicin, ifosfamide, cisplatin, gemcitabine, trabectedin, doxorubicin plus ifosfamide, gemcitabine plus docetaxel) are in the range of 15%–53% for uterine LMS [3, 9–15]. Note that response rates vary in the range of 10%–50% for soft-tissue sarcomas in trials without stratification by histological subtype [16–21]. Several phase II studies have investigated gemcitabine alone in a 30-minute infusion and reported low response rates in the range of 3%–18% [22–27].
A fixed-dose-rate infusion of gemcitabine at 10 mg/m2 per minute may offer a pharmacologic advantage and has also been explored [25, 28, 29]. The activity of single-agent docetaxel has been reported to be quite limited, with response rates of 0%–18% [30–32]. The combination of fixed-dose-rate infusion gemcitabine plus docetaxel has documented activity against metastatic LMS, particularly uterine LMS [29, 33–36]. However, it is unclear whether this effect results from the prolonged infusion of gemcitabine or the synergy between the two drugs. Maki et al. [35] demonstrated that the combination of gemcitabine and docetaxel may be superior to a higher dose of gemcitabine; the adaptively randomized phase II study included all sarcoma subtypes with broad pretreatment characteristics ranging from zero to three prior chemotherapy regimen(s).
To expand on these investigations, the French Sarcoma Group designed the TAXOGEM study in 2004. This multicentric, open-label, phase II study included patients with metastatic or relapsed LMS after failure of one prior anthracycline-based regimen. Patients were randomized to receive either a fixed-dose-rate infusion of gemcitabine alone or a combination of gemcitabine and docetaxel. Stratification of LMS by uterine and nonuterine sites of origin was planned so that two distinct phase II studies could be efficiently conducted in one trial, taking into account possible differences in clinical behavior for the two sites of origin.
Patients and Methods
Eligibility
Patients were eligible if they had metastatic or unresectable LMS that was histologically confirmed by an expert sarcoma pathologist at the local center and originated in either the uterus or another site (nonuterine LMS). Patients had measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 and had previously received only one doxorubicin-containing chemotherapy regimen. Patients who had received adjuvant therapy <1 year before a relapse were considered to have received first-line therapy for metastatic disease.
Inclusion criteria included the following: at least one progressive target lesion outside the radiation field based on computed tomography or magnetic resonance imaging; adequate organ function, defined as an absolute neutrophil count (ANC) ≥1,000/μL, platelet count ≥100,000/μL, total bilirubin ≤1.5-fold the institutional upper limit of normal (ULN), alanine transaminase, aspartate aminotransferase, and alkaline phosphatase ≤2.5-fold the institutional ULN; serum creatinine ≤1.5-fold the institutional ULN; and an Eastern Cooperative Oncology Group performance status score ≤2. Patients had to have completed any previous chemotherapy, radiotherapy, or hormonotherapy at least 4 weeks before enrollment. Pregnant or lactating women and patients with a history of malignancy, a history of grade 3 or 4 neuropathy, or known central nervous system metastases were excluded. All patients provided written informed consent. Histological diagnoses were centrally reviewed by a pathologist from the French Sarcoma Group after inclusion.
Study Design
This randomized phase II study (ClinicalTrials.gov identifier, EU 20518, NCT00227669) was stratified according to the primary tumor location (uterine LMS versus nonuterine LMS). Each stratum of the study was considered to be an independent randomized phase II study. Treatments were administered in the outpatient setting and planned for eight cycles, unless there was evidence of disease progression or unacceptable toxicity.
In the gemcitabine-only arm, 1,000 mg/m2 of gemcitabine was administered at a fixed-dose rate of 10 mg/m2 per minute via a 100-minute i.v. infusion on days 1, 8, and 15 every 28 days. In the gemcitabine plus docetaxel arm, gemcitabine was administered at a fixed-dose rate of 900 mg/m2 in a 90-minute infusion on days 1 and 8, with docetaxel at 100 mg/m2 in a 60-minute infusion on day 8 after gemcitabine, every 21 days with lenograstim, a recombinant human G-CSF, in a daily injection of 150 μg/m2 from day 9 to day 15. Patients who had previously received pelvic radiation therapy received gemcitabine at a dose of 675 mg/m2 infused over 68 minutes on days 1 and 8, followed by docetaxel, 75 mg/m2, on day 8 infused over 1 hour. The recommended premedication for docetaxel was 8 mg of dexamethasone administered orally twice daily for 4 days starting the day before docetaxel. Treatment was planned to be stopped after eight cycles, unless there was evidence of disease progression or unacceptable toxicity.
Dose Adjustments
Treatment toxicities were graded by National Cancer Institute Common Toxicity Criteria. In the gemcitabine-only arm, treatment was cancelled on day 8 or day 15 for grade >2 ANC or grade >1 platelet count. A 25% reduction in the previous dose was permitted for persistent ANC of grade >2 or platelet count of grade ≥1. Two reductions of the previous dose were permitted. Recombinant G-CSF could be used in all subsequent cycles.
For grade 3–4 hepatic toxicity, treatment was delayed up to a maximum of 14 days until toxicity dropped to grade ≤2. In the gemcitabine plus docetaxel arm, the doses of both gemcitabine and docetaxel were reduced to 75% if patients experienced febrile neutropenia or thrombopenia of grade ≥3 for >5 days, neutropenia of grade 3, or platelet count of grade 2 at day 8. If the patient experienced grade 3–4 neurotoxicity, treatment was delayed for 1 week, with a 25% dose reduction of docetaxel (if neurotoxicity had resolved to grade ≤2). If the patient's bilirubin level was >1.5 mg/dL, docetaxel was withheld during that cycle.
Evaluation During Treatment
Pretreatment evaluations included a medical history, a physical examination, laboratory tests, an electrocardiogram, computed tomography scans of the pelvis and abdomen, and disease evaluation. During treatment, CBCs were performed weekly. Biochemical profiles were obtained every 3 weeks. The tumor status was assessed every two cycles (every 8 weeks in the gemcitabine-only arm and every 6 weeks in the gemcitabine plus docetaxel arm). To be considered assessable for response, patients had to receive at least one injection of the treatment regimen.
Statistical Analysis
The primary endpoint of the study was the objective response rate. Secondary endpoints were the progression-free survival (PFS) interval, the duration of response, toxicity, and the OS duration.
A Simon phase II study design was used to calculate the number of patients, with stratification according to the primary tumor location in two parallel phase II studies (uterine and nonuterine LMS). Each stratified substudy (uterine or nonuterine LMS) planned to include 20 evaluable patients per arm (gemcitabine versus gemcitabine plus docetaxel), for a total of 80 patients in the study overall. The probability of selecting the best treatment for uterine LMS (response rate of 50%) was 74%, assuming an underlying response rate of 40%. The probability of selecting the best treatment for nonuterine LMS (response rate of 40%) was 91.8%, assuming an underlying response rate of 20%.
Rates of response and 95% confidence intervals (CIs) were determined. The survival analysis was performed on the intent-to-treat population and on the population evaluable for therapy. OS and PFS times were calculated using the Kaplan–Meier method. The PFS interval was calculated from the date of randomization until the date of the first progression, date of death, or last documented contact. The OS times was calculated from the date of randomization until the date of death or the date of the last known contact.
Results
Patient Characteristics
Patients were enrolled from February 2006 to December 2008 in 17 institutions across France. In all, 90 patients were included: 46 in the uterine LMS group (Table 1) and 44 in the nonuterine LMS group (Table 2). In total, seven patients were excluded from the efficacy analysis because of major protocol violations: three patients in the nonuterine group (i.e., no prior anthracycline-based regimen, a single target lesion in a previously irradiated field, and three prior chemotherapy regimens for metastases) and four patients in the uterine group (i.e., no measurable target lesion, progression before the first course of treatment [two patients], and an interval >12 months since the end of adjuvant chemotherapy). In all, 73 tumors (81%) were histologically reviewed and confirmed in 95% of cases: 35 (76%) in the uterine group and 38 (86%) in the nonuterine group.
Table 1.
Patient characteristics in the uterine group
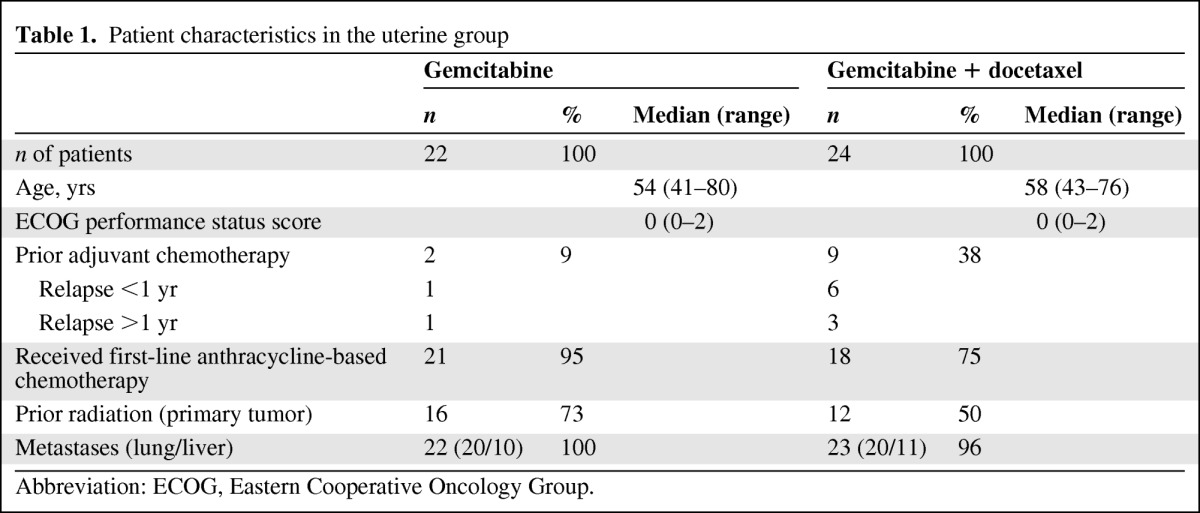
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 2.
Patient characteristics in the nonuterine group
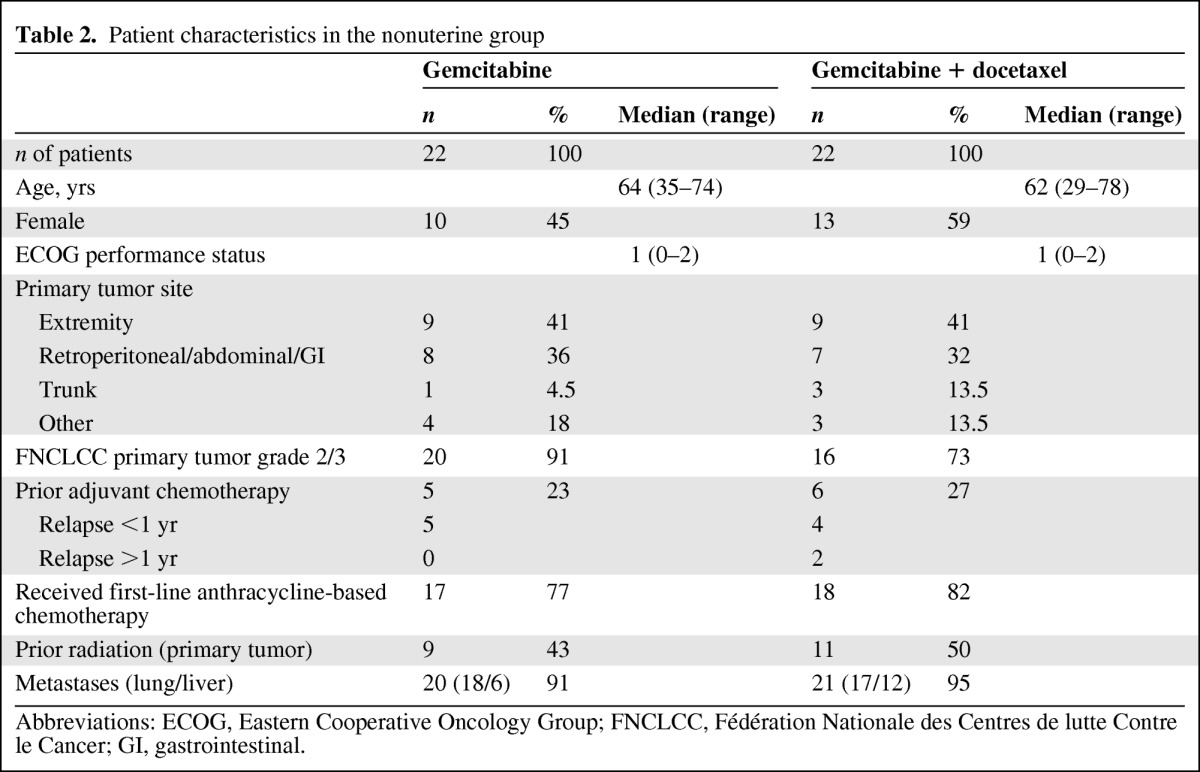
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FNCLCC, Fédération Nationale des Centres de lutte Contre le Cancer; GI, gastrointestinal.
A total of 11 patients (24%) in the uterine LMS group and 11 patients (25%) in the nonuterine LMS group had received prior adjuvant chemotherapy (seven and nine patients, respectively, had received it within 1 year). The median patient age was 63 years (range, 29–78 years) in the nonuterine group and 57 years (range, 41–80 years) in the uterine group. The median Eastern Cooperative Oncology Group performance status score was 1 in the nonuterine group and 0 in the uterine group. In total, 83 patients were evaluated for efficacy: 41 in the nonuterine group and 42 in the uterine group.
In the nonuterine group, most cases of LMS were high-grade tumors. All patients had received first-line anthracycline-based chemotherapy for metastatic disease, except for five patients in the gemcitabine-only arm and four patients in the gemcitabine plus docetaxel arm. For seven patients, the interval between the end of adjuvant anthracycline-based chemotherapy and first-line treatment of metastases was <1 year. Two patients were ineligible.
In the uterine LMS group, all but one patient in the gemcitabine arm and all but seven patients in the gemcitabine plus docetaxel arm had received anthracycline-based chemotherapy for metastatic disease. One patient was ineligible because the interval between the end of adjuvant anthracycline-based chemotherapy and first-line treatment for metastatic disease was >1 year.
In the nonuterine LMS group, postoperative radiation had been delivered for nine and 11 patients in the gemcitabine-only and gemcitabine plus docetaxel arms, respectively. In the uterine group, 16 and 12 patients in the gemcitabine-only and gemcitabine plus docetaxel arms, respectively, had received postoperative radiation. More than 90% of the patients had distant metastases at inclusion in both groups. The lung and liver were the two most common metastatic sites.
Treatment Delivery and Toxicity
The median numbers of administered cycles were four in the nonuterine LMS group and five in the uterine group (Table 3). The rate of the dose received compared with the dose planned in the nonuterine group was 85% in the gemcitabine-only arm; in the gemcitabine plus docetaxel arm, the rates were 95% for gemcitabine and 86% for docetaxel. In the uterine group, the rate of the dose received compared with the dose planned was 96% in the gemcitabine-only arm; in the gemcitabine plus docetaxel arm, the rates were 89% for gemcitabine and 82% for docetaxel.
Table 3.
Toxicity for the uterine group, nonuterine group, and all sarcomas
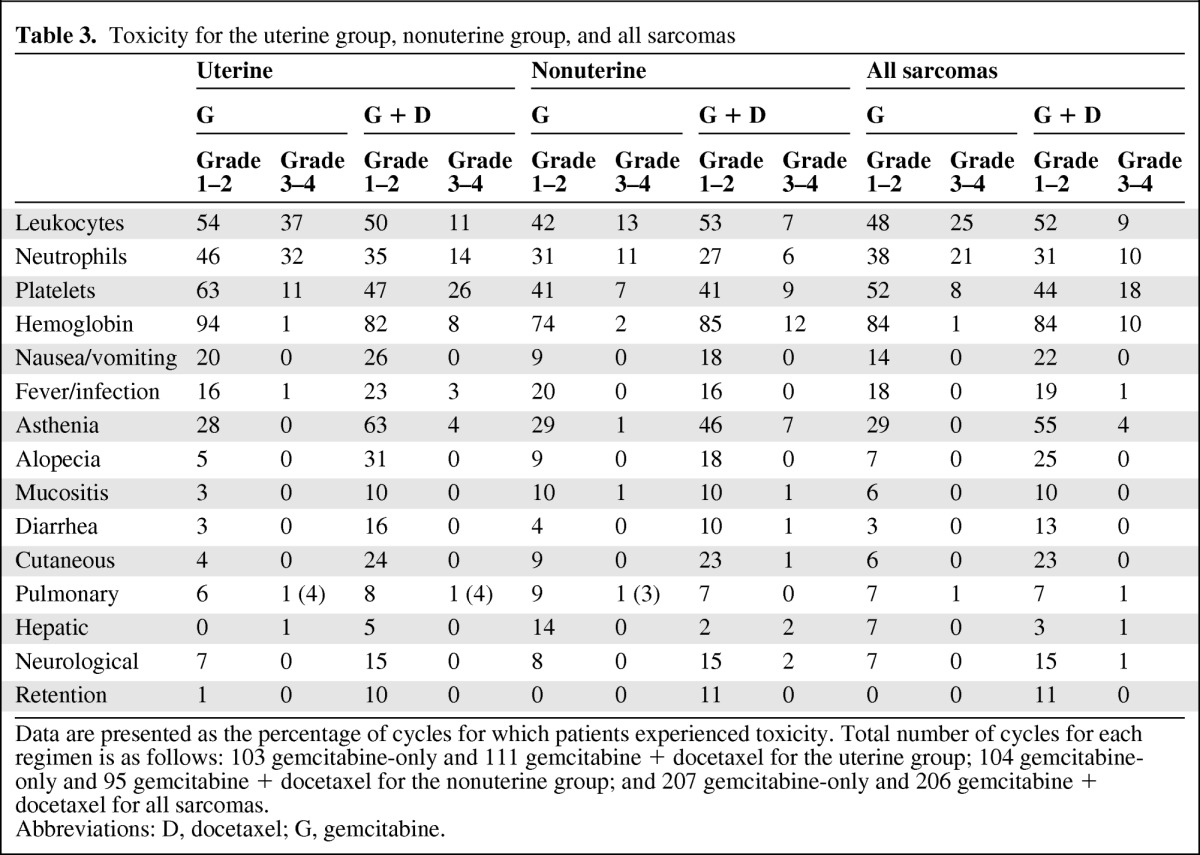
Data are presented as the percentage of cycles for which patients experienced toxicity. Total number of cycles for each regimen is as follows: 103 gemcitabine-only and 111 gemcitabine + docetaxel for the uterine group; 104 gemcitabine-only and 95 gemcitabine + docetaxel for the nonuterine group; and 207 gemcitabine-only and 206 gemcitabine + docetaxel for all sarcomas.
Abbreviations: D, docetaxel; G, gemcitabine.
A small percentage of cycles were delayed in both arms; the median lengths of delay were 7 days and 9 days for the nonuterine group and 11 days and 7 days for the uterine group in the gemcitabine-only and gemcitabine plus docetaxel arms, respectively. The main reasons for these delays were personal convenience in the gemcitabine-only arm and grade 2–3 toxicities in the gemcitabine plus docetaxel arm. The main reasons for the interruption of treatment were progression and completion of therapy after eight cycles (in both arms) and toxicity (mainly in the combination arm). No differences in toxicity were observed between the two LMS locations (uterine versus nonuterine). One toxic death occurred from a hemorrhage related to grade 4 thrombocytopenia.
Treatment Responses and Survival
In the uterine LMS group, one complete response and three partial responses were observed in the gemcitabine-only arm (19%; 95% CI, 5%–42%). Five partial responses were observed in the gemcitabine plus docetaxel arm (24%; 95% CI, 8%–47%) (Table 4).
Table 4.
Responses to treatment
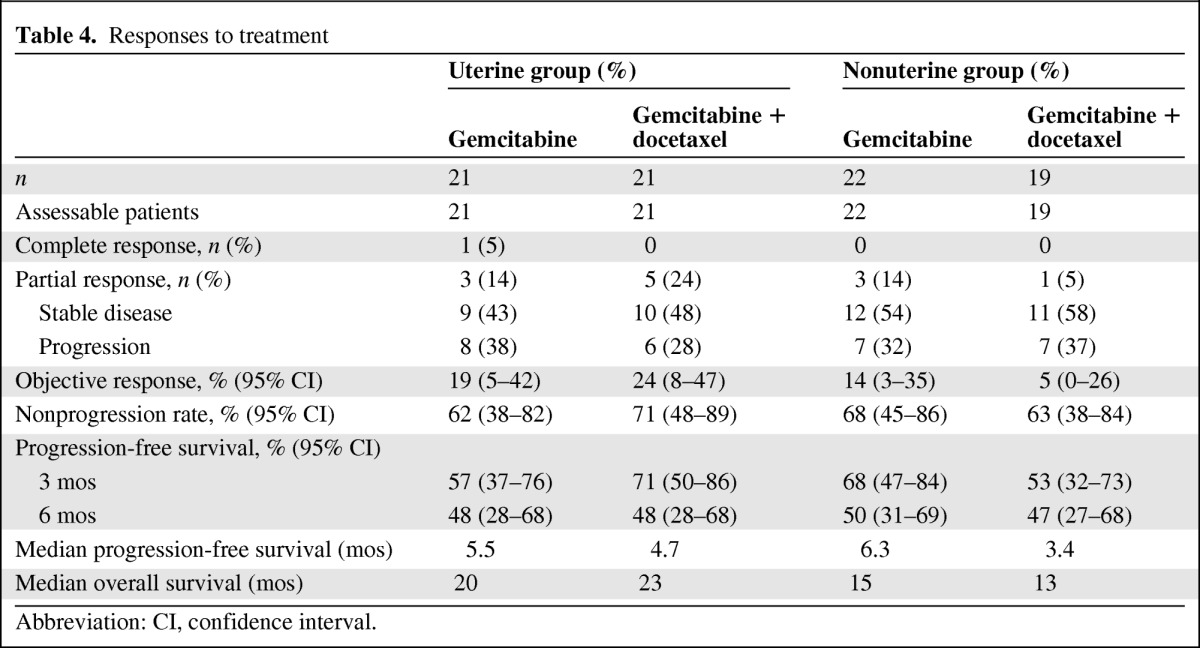
Abbreviation: CI, confidence interval.
In the nonuterine LMS group, three partial responses were observed (14%; 95% CI, 3%–35%) in the gemcitabine-only arm. Only one partial response was observed in the gemcitabine plus docetaxel arm (5%; 95% CI, 0%–26%).
The nonprogression rates (responses and stable disease) were 68% and 63%, respectively, in the gemcitabine and gemcitabine plus docetaxel arms for the nonuterine group, respectively. For the uterine group, nonprogression rates were 62% and 71% in the gemcitabine and gemcitabine plus docetaxel arms, respectively.
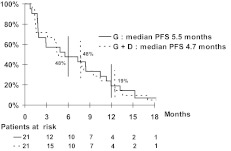
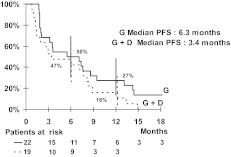
For the uterine group, the median PFS times were 5.5 months in the gemcitabine arm and 4.7 months in the gemcitabine plus docetaxel arm (Fig. 1). For the nonuterine group, the median PFS times were 6.3 months in the gemcitabine arm and 3.4 months in the gemcitabine plus docetaxel arm (Fig. 2). PFS rates at 3 months and 6 months were not statistically different for either the two drug regimens or the two sites of origin (Table 4). Moreover, the median OS times were 15 months and 13 months in the gemcitabine and gemcitabine plus docetaxel arms, respectively, for the nonuterine group. The median OS times seemed to be better in the uterine group: 20 months and 23 months in the gemcitabine and gemcitabine plus docetaxel arms, respectively.
Figure 1.
Kaplan–Meier curve of progression-free survival for the uterine leiomyosarcoma group.
Figure 2.
Kaplan–Meier curve of progression-free survival for the nonuterine leiomyosarcoma group.
Discussion
Clinical management of soft-tissue sarcomas has changed considerably since the advent of molecular-targeted therapies (e.g., imatinib and sunitinib) for specific sarcoma subtypes, such as gastrointestinal stromal tumors and dermatofibrosarcoma protuberans. It is increasingly recognized that different sarcoma subtypes may exhibit unique clinical behaviors that may justify different forms of therapy [37]. Doxorubicin and ifosfamide, either alone or in combination, have served as the chemotherapy backbone for metastatic sarcoma therapy for >15 years. With the limited number of drugs available for sarcoma, there has been an ongoing search for new agents for specific sarcoma subtypes, both cytotoxic and cytostatic.
Great enthusiasm has been generated by the clinical activity reported in a single-institution phase II study of gemcitabine in combination with docetaxel in patients with metastatic and/or unresectable LMS (originating in either the uterus or other soft-tissue sites) [33]. That study enrolled 34 patients, 84% of whom had uterine LMS, and demonstrated that the combination was tolerable and highly active in treated and untreated patients with LMS. A fixed-dose-rate infusion of gemcitabine seemed to result in a higher response rate.
Our TAGOXEM study focused only on LMS patients to test the activity of a true second-line treatment, following failure of a first-line anthracycline-based regimen, in a multi-institutional setting. This trial documented a modest (<25%) objective response rate per the RECIST for both uterine and nonuterine LMS using either gemcitabine alone or a gemcitabine plus docetaxel combination regimen as second-line therapy for metastatic disease. Interestingly, these results are relatively consistent with a retrospective study by the French Sarcoma Group, which reported an objective response rate of 24% with gemcitabine plus docetaxel in LMS patients [36]. Although this study was not stratified, the results are similar to some phase II studies, indicating that LMS originating in the uterus may be more chemosensitive than other sites of LMS [8], with a higher response rate and better OS outcome than in the nonuterine group.
The relative benefits of the gemcitabine plus docetaxel combination versus gemcitabine alone are not so clear in our conventionally randomized phase II trial, in contrast with the Bayesian randomized phase II trial performed by the SARC (Sarcoma Alliance for Research through Collaboration) group [35]. There are many differences between these trials; for example, the TAXOGEM study was performed in 17 centers in France, whereas the SARC study was performed in eight U.S. centers. Perhaps more importantly, the TAXOGEM study enrolled purely second-line patients after only one prior chemotherapy regimen, whereas the SARC trial enrolled patients who received a variety of prior chemotherapy regimens, ranging from chemotherapy-naïve patients to patients with up to three prior failed chemotherapy regimens. In addition, LMS histology was restricted in the TAXOGEM study, whereas all histologies were present in the SARC study. Moreover, the SARC study included only nine cases (18%) of LMS in the gemcitabine arm, versus 29 cases (40%) in the gemcitabine plus docetaxel arm. Results could have been influenced if gemcitabine is more effective for LMS. More liver localizations were included in the gemcitabine plus docetaxel arm of the nonuterine group in the TAXOGEM study. No significant differences in activity were described for the metastatic localizations.
Additionally, there are differences in drug delivery and dose intensity between these studies. In the gemcitabine arm of the SARC trial, the dose intensity of gemcitabine was somewhat higher than in our TAXOGEM study (800 mg/m2 per week in the SARC study versus 750 mg/m2 per week in the TAXOGEM study) but remained the same in the gemcitabine plus docetaxel arm. However, the dose intensity of gemcitabine was lower in the combination arm for the SARC study but the same in the TAXOGEM study (750 mg/m2). The possible impact is the same in the SARC study.
It should also be noted that the schedules of drug administration were different between the studies. Gemcitabine was administered at 1,200 mg/m2 per week for two of 3 weeks in the SARC study and 1,000 mg/m2 per week for three of 4 weeks in the TAXOGEM study, with differences in staging intervals. Moreover, TAXOGEM had a different staging interval between the two arms (“every two cycles” equates to every 8 weeks in the gemcitabine arm versus every 6 weeks in the gemcitabine plus docetaxel arm). The TAXOGEM study was designed to evaluate the response rate as the primary endpoint; these staging intervals may have inflated time to progression in the gemcitabine arm but had no impact on the response rate and OS time.
Despite these differences, the classical randomization design of TAXOGEM allows important comparative insights about the relative safety and efficacy of gemcitabine plus docetaxel versus single-agent gemcitabine without the possible confounding variables associated with changes in patient-related variables that occurred over the duration of the SARC study. Results may be disproportionally influenced by a study with a Bayesian design [38].
The results from the TAXOGEM study confirm prior observations that the objective response rate is higher for uterine LMS (24%) than for nonuterine LMS using a combination of gemcitabine and docetaxel [33–35]. The statistical design of the TAXOGEM study took this into account prospectively, using statistical hypotheses based on the anatomic origin of primary tumors (uterine versus nonuterine LMS).
Interestingly, PFS rates were high in the TAXOGEM study, with 3-month PFS rates in the gemcitabine and gemcitabine plus docetaxel arms of 57% and 71% in the uterine group and 68% and 53% in the nonuterine group, respectively. PFS rates were ∼50% at 6 months in both arms and both groups. These results support the hypothesis that both regimens are active as second-line therapy for LMS and match the criteria defining active agents proposed by Van Glabekke et al. [39]. The EORTC STBSG group (EORTC and Soft Tissue and Bone Sarcoma Group) reported that agents used in second-line therapy for metastatic soft-tissue sarcomas should demonstrate a 3-month PFS rate > 40% and a 6-month PFS rate of 14% to be considered active.
Moreover, the TAXOGEM study reported median PFS durations in the gemcitabine and gemcitabine plus docetaxel arms, respectively, of 6.3 months and 3.4 months for nonuterine LMS and 5.5 months and 4.7 months for uterine LMS. To put this in the context of prior studies, Hensley et al. [33] in 2002 reported a median PFS time of 5.6 months with a gemcitabine plus docetaxel combination in metastatic LMS only, with a 47% 6-month PFS rate. In 2007, the SARC study reported median PFS intervals of 3 months and 6.2 months in the gemcitabine and gemcitabine plus docetaxel arms, respectively, for all sarcoma subtypes with a Bayesian study design and heterogeneity in inclusion criteria.
Furthermore, despite the fact that the RECIST-defined response rates were low for both the gemcitabine-only and gemcitabine plus docetaxel treatments in the TAXOGEM study, a significant number of patients experienced the clinical benefit of prolonged stable disease. These findings confirm the hypothesis that durable stable disease in response to systemic therapy is an important clinical endpoint for patients with metastatic LMS. Moreover, efforts should be made to enhance results with other new drugs or new associations, such as gemcitabine plus dacarbazine (already tested in phase II studies [40]) or gemcitabine plus pazopanib (an association tested on second-line LMS in the new LMS03 French Sarcoma Group study).
See the accompanying commentary on pages 1129–1132 of this issue.
Acknowledgments
This work was supported by Chugai Pharma France and Sanofi-Aventis France. Investigators included Christine Chevreau, Cécile Guillemet, Antoine Thyss, Olivier Collard, Pierre Kerbrat, Jacques-Olivier Bay, Guilhem Bousquet. We thank Lorna Saint Ange for editing the manuscript, the patients and families involved, and participating centers and teams.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Patricia Pautier, Annie Rey, Florence Duffaud
Provision of study material or patients: Patricia Pautier, Anne Floquet, Nicolas Penel, Sophie Piperno-Neumann, Nicolas Isambert, Emmanuelle Bompas, Angela Cioffi, Corinne Delcambre, Didier Cupissol, Jean-Yves Blay, Florence Duffaud
Collection and/or assembly of data: Patricia Pautier, Annie Rey, Françoise Collin, Marta Jimenez
Data analysis and interpretation: Patricia Pautier, Annie Rey, Françoise Collin, Florence Duffaud
Manuscript writing: Patricia Pautier
Final approval of manuscript: Patricia Pautier, Anne Floquet, Nicolas Penel, Sophie Piperno-Neumann, Nicolas Isambert, Annie Rey, Emmanuelle Bompas, Angela Cioffi, Corinne Delcambre, Didier Cupissol, Françoise Collin, Jean-Yves Blay, Marta Jimenez, Florence Duffaud
References
- 1.Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 2.Pautier P, Genestie C, Rey A, et al. Analysis of clinicopathologic prognostic factors for 157 uterine sarcomas and evaluation of a grading score validated for soft tissue sarcoma. Cancer. 2000;88:1425–1431. [PubMed] [Google Scholar]
- 3.O'Cearbhaill R, Hensley ML. Optimal management of uterine leiomyosarcoma. Expert Rev Anticancer Ther. 2010;10:153–169. doi: 10.1586/era.09.187. [DOI] [PubMed] [Google Scholar]
- 4.Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 5.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline- containing first-line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen OS, Blay J-Y, Judson IR, et al. Metastatic soft tissue sarcoma: Prognosis and treatment options. Am J Cancer. 2003;2:211–221. [Google Scholar]
- 7.Penel N, Italiano A, Isambert N, et al. Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Ann Oncol. 2010;21:1361–1365. doi: 10.1093/annonc/mdp485. [DOI] [PubMed] [Google Scholar]
- 8.Oosten AW, Seynaeve C, Schmitz PI, et al. Outcomes of first-line chemotherapy in patients with advanced or metastatic leiomyosarcoma of uterine and non-uterine origin. Sarcoma. 2009;2009 doi: 10.1155/2009/348910. 348910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazinoimidazole carboxamide in advanced uterine sarcomas. Cancer. 1983;52:626–632. doi: 10.1002/1097-0142(19830815)52:4<626::aid-cncr2820520409>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Sutton GP, Blessing J, Barrett R, et al. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: A Gynecologic Oncologic Group study. Am J Obstet Gynecol. 1992;166:556–559. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 11.Thigpen JT, Blessing Beecham J, et al. Phase II trial of cisplatin as first line chemotherapy in patients with advanced or recurrent uterine sarcomas (a Gynecologic Oncologic Group study) J Clin Oncol. 1991;9:1962–1966. doi: 10.1200/JCO.1991.9.11.1962. [DOI] [PubMed] [Google Scholar]
- 12.Sutton GP, Blessing JA, Ball HG. A phase II study of paclitaxel in patients with advanced or recurrent uterine leiomyosarcomas unexposed to other chemotherapy: A Gynecologic Oncology Group study. Proc Am Soc Clin Oncol. 1997;16:1302. [Google Scholar]
- 13.Pautier P, Genestie C, Fizazi K, et al. Cisplatin-based chemotherapy regimen (DECAV) for uterine sarcomas. Int J Gynecol Cancer. 2002;12:749–754. doi: 10.1046/j.1525-1438.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 14.Sutton GP, Blessing JA, Malfetano JH. Ifosfamide and doxorubicine in the treatment of advanced leiomyosarcomas of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol. 1996;62:226–229. doi: 10.1006/gyno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 15.Muss HB, Bundy B, DiSaia PJ, et al. Treatment of recurrent or advanced uterine sarcoma: A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group) Cancer. 1985;55:1648–1653. doi: 10.1002/1097-0142(19850415)55:8<1648::aid-cncr2820550806>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010 doi: 10.1155/2010/506182. 506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann JT, Patel S. Recent developments in salvage chemotherapy for patients with metastatic soft tissue sarcoma. Drugs. 2005;65:167–178. doi: 10.2165/00003495-200565020-00002. [DOI] [PubMed] [Google Scholar]
- 18.Worden FP, Taylor JM, Biermann JS, et al. Randomized phase II evaluation of 6 g/m2 of ifosfamide plus doxorubicin and granulocyte colony-stimulating factor (G-CSF) compared with 12 g/m2 of ifosfamide plus doxorubicin and G-CSF in the treatment of poor-prognosis soft tissue sarcoma. J Clin Oncol. 2005;23:105–112. doi: 10.1200/JCO.2005.05.108. [DOI] [PubMed] [Google Scholar]
- 19.Zucali PA, Bertuzzi A, Parra HJ, et al. The “old drug” dacarbazine as second/third-line chemotherapy in advanced soft tissue sarcomas. Invest New Drugs. 2008;26:175–181. doi: 10.1007/s10637-007-9086-z. [DOI] [PubMed] [Google Scholar]
- 20.Lecesne A, Blay JY, Judson, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 21.Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–899. doi: 10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- 22.Spath-Schwalbe E, Genvresse I, Koschuth A, et al. Phase II trial of gemcitabine in patients with pretreated advanced soft tissue sarcomas. Anticancer Drugs. 2000;11:325–329. doi: 10.1097/00001813-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Svancarova L, Blay JY, Judson IR, et al. Gemcitabine in advanced adult soft-tissue sarcomas. A phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2002;38:556–559. doi: 10.1016/s0959-8049(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 24.Merimsky O, Meller I, Flusser G, et al. Gemcitabine in soft tissue or bone sarcoma resistant to standard chemotherapy: A phase II study. Cancer Chemother Pharmacol. 2000;45:177–181. doi: 10.1007/s002800050027. [DOI] [PubMed] [Google Scholar]
- 25.Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483–3489. doi: 10.1200/JCO.2001.19.15.3483. [DOI] [PubMed] [Google Scholar]
- 26.Okuno S, Edmonson J, Mahoney M, et al. Phase II trial of gemcitabine in advanced sarcomas. Cancer. 2002;94:3225–3229. doi: 10.1002/cncr.10602. [DOI] [PubMed] [Google Scholar]
- 27.Look KY, Sandler A, Blessing JA, et al. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: A Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2004;92:644–647. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixe dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 29.Hensley ML, Blessing JA, Degeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Hoesel QG, Verweij J, Catimel G, et al. Phase II study with docetaxel (Taxotere) in advanced soft tissue sarcomas of the adult. EORTC Soft Tissue and Bone Sarcoma Group. Ann Oncol. 1994;5:539–542. doi: 10.1093/oxfordjournals.annonc.a058909. [DOI] [PubMed] [Google Scholar]
- 31.Edmonson JH, Ebbert LP, Nascimento AG, et al. Phase II study of docetaxel in advanced soft tissue sarcomas. Am J Clin Oncol. 1996;19:574–576. doi: 10.1097/00000421-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Verweij J, Lee SM, Wlodzimir R, et al. Randomized phase II study of docetaxel versus doxorubicin in first- and second line chemotherapy for locally advanced of metastatic soft tissue sarcoma in adults. A study of the EORTC and Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2081–2086. doi: 10.1200/JCO.2000.18.10.2081. [DOI] [PubMed] [Google Scholar]
- 33.Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J Clin Oncol. 2002;20:2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 34.Leu KM, Ostruszka LJ, Shewach D, et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma. J Clin Oncol. 2004;22:1706–1712. doi: 10.1200/JCO.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 35.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002. J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 36.Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: A retrospective analysis. Int J Cancer. 2006;119:706–711. doi: 10.1002/ijc.21867. [DOI] [PubMed] [Google Scholar]
- 37.Maki RG. Gemcitabine and docetaxel in metastatic sarcoma: Past, present, and future. The Oncologist. 2007;12:999–1006. doi: 10.1634/theoncologist.12-8-999. [DOI] [PubMed] [Google Scholar]
- 38.Byar DP, Simon RM, Friedewald WT, et al. Randomized clinical trials. Perspectives on some recent ideas. N Engl J Med. 1976;8:74–80. doi: 10.1056/NEJM197607082950204. [DOI] [PubMed] [Google Scholar]
- 39.Van Glabbeke M, Verweij J, Judson I, et al. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 40.García-Del-Muro X, López-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J Clin Oncol. 2011;20:2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]