Abstract
Background and Aims
Studies on the effects of sub- and/or supraoptimal temperatures on growth and phosphorus (P) nutrition of perennial herbaceous species at growth-limiting P availability are few, and the impacts of temperature on rhizosphere carboxylate dynamics are not known for any species.
Methods
The effect of three day/night temperature regimes (low, 20/13 °C; medium, 27/20 °C; and high, 32/25 °C) on growth and P nutrition of Cullen cinereum, Kennedia nigricans and Lotus australis was determined.
Key Results
The highest temperature was optimal for growth of C. cinereum, while the lowest temperature was optimal for K. nigricans and L. australis. At optimum temperatures, the relative growth rate (RGR), root length, root length per leaf area, total P content, P productivity and water-use efficiency were higher for all species, and rhizosphere carboxylate content was higher for K. nigricans and L. australis. Cullen cinereum, with a slower RGR, had long (higher root length per leaf area) and thin roots to enhance P uptake by exploring a greater volume of soil at its optimum temperature, while K. nigricans and L. australis, with faster RGRs, had only long roots (higher root length per leaf area) as a morphological adaptation, but had a higher content of carboxylates in their rhizospheres at the optimum temperature. Irrespective of the species, the amount of P taken up by a plant was mainly determined by root length, rather than by P uptake rate per unit root surface area. Phosphorus productivity was correlated with RGR and plant biomass.
Conclusions
All three species exhibited adaptive shoot and root traits to enhance growth at their optimum temperatures at growth-limiting P supply. The species with a slower RGR (i.e. C. cinereum) showed only morphological root adaptations, while K. nigricans and L. australis, with faster RGRs, had both morphological and physiological (i.e. root carboxylate dynamics) root adaptations.
Keywords: Australian native legumes, carboxylates, climate change, growth, perennial pastures, phosphorus, photosynthesis, root morphology, temperature, water-use efficiency
INTRODUCTION
Phosphorus (P) is frequently a limiting nutrient for crop production (Vance et al., 2003; Simpson et al., 2011). Demand for P in agriculture is increasing, and global P reserves are expected to be depleted in 50–100 years (Cordell et al., 2009). Even though soil can hold large amounts of P, the plant-available fraction of P in soil is very low. The concentration of P in soil solution is partly determined by the solubility of soil phosphates (e.g. Ca-P, Al-P and Fe-P) (Elrashidi and Larsen, 1978). Calcium phosphates dominate in neutral–alkaline soils, and increase in solubility with decreasing pH. Conversely, P is bound to Fe and Al oxides in acid soils, and becomes increasingly soluble with increasing pH (Lindsay, 1979; Bolan et al., 1987). One approach to address the limited P supply in natural and agricultural soils is to identify plants that can access sparingly soluble soil P efficiently (Lambers et al., 2006), but ultimately all P that is removed through cropping will have to be replaced (Simpson et al., 2011).
Plants have evolved numerous mechanisms to utilize relatively immobile, and often poorly available, P (e.g. FePO4 in acidic soils) which allows them to acclimate to P deficiency (Vance et al., 2003; Raghothama and Karthikeyan, 2005). Typical mechanisms by which they enhance P acquisition under low P availability include altered root physiology (Neumann and Martinoia, 2002), such as by enhanced exudation of carboxylates (e.g. malate and citrate) and phosphohydrolases (Richardson et al., 2000; Wouterlood et al., 2005), and altered root morphology, such as by increased root development, higher root:shoot ratios, finer roots, longer and more root hairs and the formation of arbuscular mycorrhizas, all of which facilitate exploration of a greater soil volume (Raghothama, 1999; Smith and Smith, 2011). Depending on the extent of these mechanisms, nutrient availability in the rhizosphere may change and affect plant nutrient uptake (Raynaud et al., 2008). Apart from these mechanisms that enhance P uptake, plants also have adaptations efficiently to use P which is taken up. Phosphorus productivity (Pprod), i.e. the rate of biomass production per unit of P in the plant, is a more important determinant of plant P use efficiency than the total amount of P taken up by a plant (Ericsson and Ingestad, 1988; Ryser et al., 1997).
Temperature is a major factor determining the length of the growing season, net primary productivity and plant community composition and/or structure (Van Cleve et al., 1981; Harte and Shaw, 1995; Thompson and Naeem, 1996). Moreover, under predicted climate change scenarios, such as increasing temperature, dramatic changes in plant growth and nutrient use responses are expected (Sinclair, 1992). Our knowledge of the impact of temperature, above the optimum range, on root growth and P dynamics is very limited, and contradictory results are reported in the literature. For example, for ponderosa pine seedlings (Pinus ponderosa Laws.), high temperature did not increase P uptake (Delucia et al., 1997), while an increase in P uptake was found for muskmelons (Cucumis melo L.) (Stoltzfus et al., 1998). Temperatures below the plant's optimum range usually result in reduced overall growth and increased relative investment of biomass in roots (Clarkson et al., 1988). At low soil temperature, P uptake usually decreases more than the uptake of other nutrients (Bravo and Uribe, 1981; Engels et al., 1992). This may be mainly due to reduced root growth, because at low temperatures P uptake is dominated by root production, rather than by chemical availability in soil or physiological uptake capacity (Mackay and Barber, 1984). To date, the way that temperatures either below or above the optimum range affect rhizosphere carboxylate concentration and composition, and thus impact on plant P uptake and growth, is not known for any plant species. When considering shoot responses, photosynthetic rate (A), stomatal conductance (gs) and water use-efficiency (WUE) are affected by temperature (Comstock and Ehleringer, 1992; Lambers et al., 2008; Wertin et al., 2011). Moreover, a plant's WUE depends on both A and gs, and therefore WUE (i.e. A/gs) is a better indicator of a plant's physiological adaptability to change in response to changes in environmental temperature than A or gs alone.
Australian native perennial legumes are likely to have evolved in P-impoverished environments (Beadle, 1966; Handreck, 1997), and are now considered to have potential for development as pasture legumes (Dear et al., 2007; Pang et al., 2010; Suriyagoda et al., 2010b, 2011). Incorporation of native species into Australian agricultural systems may result in more P-efficient agricultural systems, greater plant diversity in agroecosystems and, presumably, wider adaptation to diverse climatic and soil conditions (Cocks, 2001; Suriyagoda et al., 2010a, 2011; Bennett et al., 2011; Lambers et al., 2011). However, the responses of these species to the range of temperatures found under Australian farming conditions are not known. Therefore, the objective of the present study was to investigate the relative change in root morphology, growth and rhizosphere carboxylate dynamics (both concentration and composition) of Australian native perennial legumes with pasture potential when grown below or above their optimal temperature at a low soil P supply. We used relatively insoluble (‘plant-unavailable’) FePO4 as the source of P; therefore, plants would need to respond morphologically and/or physiologically to take up this sparingly soluble source of P. Moreover, under predicted climate change scenarios, an increase in temperature during the growing season is likely. However, the ability of relatively insoluble P sources such as FePO4 to supply P for plant uptake at elevated temperatures is largely unknown. We hypothesized that (1) at sub- or supraoptimal temperatures, plants would produce relatively less root biomass (dry weight), have shorter and thicker roots (i.e. low root surface area per unit root biomass), and have a lower root mass ratio (RMR; i.e. the ratio of root dry weight to total dry weight) than at optimal temperature; (2) tissue P concentration, Pprod and amount of rhizosphere carboxylates would be higher at optimal temperatures than at sub- or supraoptimal temperatures; (3) a reduction in total plant P content (mg P per plant) at sub- or supraoptimal temperatures would be mainly due to a reduced uptake rate (mg P cm−2 root d−1), rather than a reduced root length (i.e. morphological adaptation), because physiological adaptations are less costly than morphological adaptations; and (4) WUE would be lower at sub- or supraoptimal temperatures than that at optimal temperatures, and this would be partly due to a low photosynthetic rate (A).
MATERIALS AND METHODS
Selection of species and accessions
Cullen cinereum (Lindl.) J.W.Grimes accession Fortescue was collected from the Fortescue River Valley, Western Australia (21°29′S, 116°14′E) where the mean monthly minimum and maximum temperatures are 20 and 32 °C, respectively. Moreover, in an ecogeographical survey to find the adaptability of a range of Cullen spp., Bennett et al. (2011) found that C. cinereum is highly adapted to warmer regions of Australia. In contrast, Kennedia nigricans Lindl. accession NS19026 was collected from the Fitzgerald River National Park, Western Australia (33°75'S, 119°75′E), where the mean monthly minimum and maximum temperatures are 11 and 20 °C, respectively. Lotus australis accession SA33610 was collected from Sellicks Beach, South Australia (35°55'S, 138°62′E), where the mean monthly minimum and maximum temperatures are 12 and 21 °C, respectively. Thus, both K. nigricans accession NS19026 and Lotus australis Andrews accession SA33610 naturally grow in cooler habitats than does C. cinereum accession Fortescue.
Growth conditions
Cullen cinereum accession Fortescue, Kennedia nigricans accession NS19026 and Lotus australis accession SA33610 were grown in 45 cm tall, 10 cm diameter vertically split pots in a growth room. There were three temperature treatments: 20/13, 27/20 and 32/25 °C (day/night). Daylength was 12 h with 240 µmol m−2 s−1 intensity. Daily average relative humidity was 20–30 %. Eight replicate pots of each species and four replicate control pots without plants were established with 5·8 kg per pot of thoroughly washed, steam-sterilized dried river sand. Basic nutrient concentrations in sand are given in Table 1. Ammonium and nitrate-N concentrations were determined as described by Searle (1984), available P and potassium (K) were measured using the Colwell test (Colwell, 1965; Rayment and Higginson, 1992), available sulfur (S) according to Blair et al. (1991), organic carbon according to Walkley and Black (1934), with the remainder of the analyses using protocols outlined in Rayment and Higginson (1992). All essential nutrients, excluding P, were provided by amending the sand with 126·6 mg kg−1 Ca(NO3)2·4H2O, 42·8 mg kg−1 NH4NO3, 178 mg kg−1 K2SO4, 101 mg kg−1 MgSO4·7H2O, 11 mg kg−1 CaCl2·2H2O, 12 mg kg−1 MnSO4·H2O, 8·8 mg kg−1 ZnSO4·7H2O, 1·96 mg kg−1 CuSO4·5H2O, 0·68 mg kg−1 H3BO3, 1·01 mg kg−1 NaMoO4·2H2O and 32·9 mg kg−1 FeNaEDTA. P was supplied with 60 mg P kg−1 dry soil using FePO4. At 32/25 °C only, an additional four pots for each species were established with 60 mg P kg−1 dry soil of KH2PO4 as the P source while other nutrients and management were unchanged. This allowed us to confirm that the plants supplied with FePO4 were P limited at the higher temperatures; this has already been established at lower temperatures (Lambers et al., 2002; Pearse et al., 2007; Shane et al., 2008). Seeds of each species were mechanically scarified and allowed to imbibe, before being sown in seedling trays at staggered times according to their pre-determined germination times. The experiment was carried out in controlled-environment chambers at the University of Western Australia, Perth. Pots were re-randomized every day. Three seedlings were planted in each pot and thinned to one plant at 1 week. An additional 100 mL of a 2 mm NH4NO3 solution was twice added to each pot; when plants had produced five and eight fully expanded main stem leaves. Four replicates of each species were harvested twice; when plants had produced three and ten fully expanded leaves on their main stem. KH2PO4-treated plants in the 32/25 °C treatment were harvested when plants had produced ten fully expanded leaves on their main stem. Therefore, days to harvest differed among species and temperature treatments (Table 2). Control (i.e. plant-free) pots were harvested when the second harvest was made for each temperature treatment. Pots were maintained at field capacity. Any drained water from the bottom of each pot was collected into buckets and topped up regularly.
Table 1.
Chemical characteristics of the river sand used in the experiment
| Mean ± s.e. | |
|---|---|
| NH4-N (mg kg−1) | 1 ± 0·01 |
| NO3-N (mg kg−1) | 1 ± 0·01 |
| Bicarbonate-extractable K (mg kg−1) | 17 ± 1·2 |
| Bicarbonate-extractable P (mg kg−1) | 2 ± 0·01 |
| Available S (mg kg−1) | 4·6 ± 0·34 |
| Organic carbon (g kg−1) | 0·006 ± 0·001 |
| Conductivity (dS m−1) | 0·018 ± 0·003 |
| pH (CaCl2) | 6·1 ± 0·12 |
| pH (H2O) | 6·7 ± 0·09 |
Table 2.
Number of days required for plants of each species to reach the first and second harvest (i.e. when they had produced three and ten fully expanded leaves on the main stem, respectively) at low, medium and high temperatures
| Low (20/13 °C) |
Medium (27/20 °C) |
High (32/25 °C) |
||||
|---|---|---|---|---|---|---|
| Species | First harvest | Second harvest | First harvest | Second harvest | First harvest | Second harvest |
| Cullen cinereum | 58 | 142 | 24 | 110 | 23 | 92 |
| Kennedia nigricans | 38 | 82 | 24 | 86 | 43 | 98 |
| Lotus australis | 48 | 80 | 36 | 86 | 33 | 87 |
Physiological measurements
The photosynthetic rate (A) and gs were measured on the youngest fully expanded leaf of all plants, using a portable gas-exchange system (LI6400 portable, LiCor Inc., Lincoln, NE, USA) equipped with a light source (6400-02B LED, LiCor). Measurements were taken 1 week before the final harvest. Photosynthetic photon flux density at the leaf surface was maintained at 1500 µmol m−2 s−1 during the measurement of A. Leaf temperature and ambient humidity of the incoming air to the leaf chamber was left at that of the growth room environment. A was measured when the ambient CO2 concentration of the incoming gas stream was 380 µmol mol−1, a value close to that during plant growth.
Plant analyses
Due to the fact that leaf appearance and expansion rates of the three species under the three temperature treatments differed, time between the start of the experiment and harvest varied. At each harvest, shoots were severed at the base of the stem, and leaf area (both green and dead leaves) of each plant determined with a LiCor LI-3000 Portable Area Meter, which was equipped with a LI-3050A Transparent Belt Conveyer Accessory (LiCor). Root systems were gently removed from the bulk soil, shaken slightly to remove the excess soil and the remaining soil was defined as rhizosphere soil (Veneklaas et al., 2003). Root systems were gently dipped in a 200 mL vial of 50 mL of a 0·2 mm CaCl2 solution and carefully washed until as much rhizosphere soil as possible was removed. Care was taken to minimize root damage. A sub-sample of the rhizosphere extract was then filtered using a 0·2 µm syringe filter into a 1 mL HPLC vial. The vial was acidified with one drop of concentrated phosphoric acid, placed on dry ice and transferred to a –20 °C freezer until HPLC analysis. Detailed methodology of the carboxylate analysis is given in Suriyagoda et al. (2010b). The root systems were then washed more thoroughly to remove any residual soil. The length, diameter and area of the collected roots were measured using the commercial software package WinRHIZO 4·1 (Regent Instruments Inc., Quebec, Canada, 2000). Root, stem and leaf samples of each plant were then dried at 60 °C for 1 week and weighed. The RMR was calculated as the ratio between root dry weight and total dry weight of a plant. As the root, stem and leaf dry weight of each plant was very small, those components were combined and ground together in a steel ball mill. For analysis of tissue P concentration, approx. 100 mg of sub-sample was taken and digested in nitric/perchloric acid and analysed using the molybdate and malachite green method (Motomizu et al., 1983). Data from the first and second harvest were used to calculate the mean P uptake rate (PUR) and mean relative growth rate (RGR) for each treatment combination. The mean PUR (mg P cm−2 root d−1) for each treatment combination was calculated by taking the difference between the total amounts of P present in plant tissues (i.e. leaves, stems and roots) at the second and first harvests and dividing that value by the difference between the average root areas at the two harvests and the time gap between the two harvests. The mean RGR (mg g−1 d−1) of each treatment combination during the harvest period was calculated as the slope of the least squares linear regression fit of the log-transformed dry weight data against time (Hoffmann and Poorter, 2002). The ability of plants to produce dry matter per unit of time and unit of P is called the phosphorus productivity (Pprod) (Ericsson and Ingestad, 1988). It can be calculated using the formula
where Pfinal is the final P concentration (mg P g−1 d. wt). Phosphorus productivity is then expressed as g d. wt g−1 P in plants h−1.
Statistical analyses
Data were subjected to two-way analyses of variance (ANOVA) in SAS/STAT software Version 9·1 (SAS Institute Inc., Cary, NC, USA) to examine the impact of species and temperature and their interactions on response variables at the second harvest. No transformations were needed to meet ANOVA assumptions. Comparisons between means were made using Tukey's Honest Significant Difference procedure. Means are presented with standard errors (s.e.) and significance is expressed at P < 0·05. When estimating net PUR, RGR and Pprod, means of each treatment combination were used for each harvest and, therefore, standard errors could not be calculated.
RESULTS
Irrespective of the temperature treatment, C. cinereum had the lowest root, stem and leaf dry weight (i.e. total dry weight) among the three species. Total dry weight of C. cinereum increased from low to medium growth temperature, while that of K. nigricans and L. australis was reduced from low to high temperature, with the greatest reduction being for L. australis (Fig. 1A, Table 3). The dry weight of leaves was the greatest and that of stems was the lowest, across all species and temperatures (Fig. 1A). Total dry weight of KH2PO4-treated plants (high temperature only) was more than twice that of the FePO4-treated plants for C. cinereum and L. australis, but was only 40 % higher for K. nigricans, the species with the highest dry weight (Fig. 1A). The RMR (the ratio between root dry weight and total plant dry weight) of C. cinereum and L. australis was 0·35 and 0·34, respectively, and it was not affected by the temperature (Fig. 1B, Table 3). However, for K. nigricans, the RMR at low temperature was 0·33 and it decreased to 0·25 at medium and high temperatures. The RGR showed a similar response to dry weight, i.e. the RGR of C. cinereum increased from low to high temperature, while that of K. nigricans and L. australis decreased (Fig. 1C).
Fig. 1.
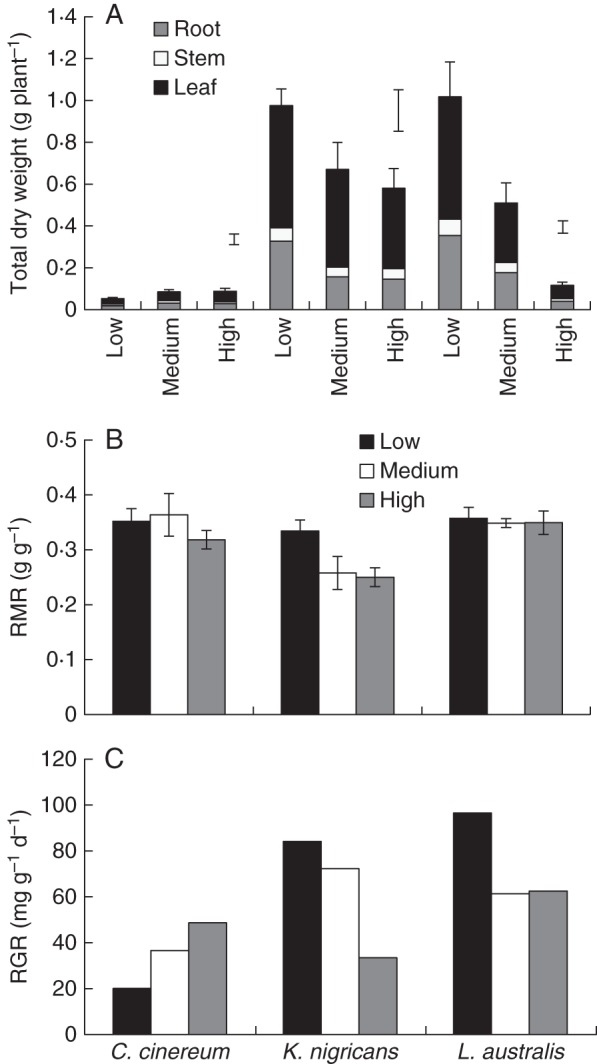
(A) Total dry weight (partitioned into root, stem and leaf) at the second harvest, (B) root mass ratio (RMR) at the second harvest (i.e. with ten fully expanded main stem leaves) and (C) relative growth rate (RGR) between the first and second harvests of Cullen cinereum, Kennedia nigricans and Lotus australis when grown at low, medium or high temperature with addition of 60 mg kg−1 of P as FePO4 (mean ± s.e., n = 4). Th error bars above the high temperature treatment in (A) are for the dry weight of plants that received 60 mg kg−1 of P as KH2PO4; in (C), RGR was calculated using the log-transformed treatment means at the first and second harvest and therefore the s.e. could not be calculated.
Table 3.
Significance of different sources of variability [species (S), temperature (T) and their interaction effects] for the FePO4-treated plants
| Source of variability |
||||
|---|---|---|---|---|
| Variable | S | T | S × T | R2 |
| Total dry weight | *** | *** | * | 0·84 |
| Leaf dry weight | *** | ** | NS | 0·78 |
| Stem dry weight | *** | *** | NS | 0·77 |
| Root dry weight | *** | *** | *** | 0·91 |
| Root mass ratio | *** | * | * | 0·60 |
| Root length | *** | *** | ** | 0·85 |
| Root diameter | *** | NS | * | 0·75 |
| Specific root length | ** | NS | * | 0·66 |
| Root length to leaf area ratio | * | NS | ** | 0·59 |
| Plant P concentration | ** | NS | * | 0·78 |
| Total P (plant−1) | *** | *** | * | 0·84 |
| Carboxylate content | *** | *** | *** | 0·78 |
| A | *** | * | *** | 0·74 |
| gs | ** | *** | * | 0·47 |
| WUE | *** | *** | *** | 0·75 |
NS, no significant difference; *P < 0·05; **P < 0·01; ***P < 0·001.
R2 for the full model is given.
Similar to the dry weight response, root length of C. cinereum increased from low to medium temperature, while that of K. nigricans and L. australis decreased from low to high temperature, with the greatest reduction being for L. australis (Fig. 2A, Table 3). Cullen cinereum had the lowest root length among the three species, especially at the low and medium temperatures. The root diameter of C. cinereum and L. australis was reduced from low to high temperature, while that of K. nigricans was unaffected (Fig. 2B, Table 3). Comparing species, the root diameter of C. cinereum was lower than that of K. nigricans and L. australis, especially at the medium and high temperatures. Specific root length (SRL, cm g−1 root d. wt) of C. cinereum increased from low to high temperature, while that of K. nigricans and L. australis was not responsive (Fig. 2C, Table 3). The root length to leaf area ratio of C. cinereum increased from low to high temperature, while that of K. nigricans and L. australis decreased (Fig. 2D, Table 3).
Fig. 2.
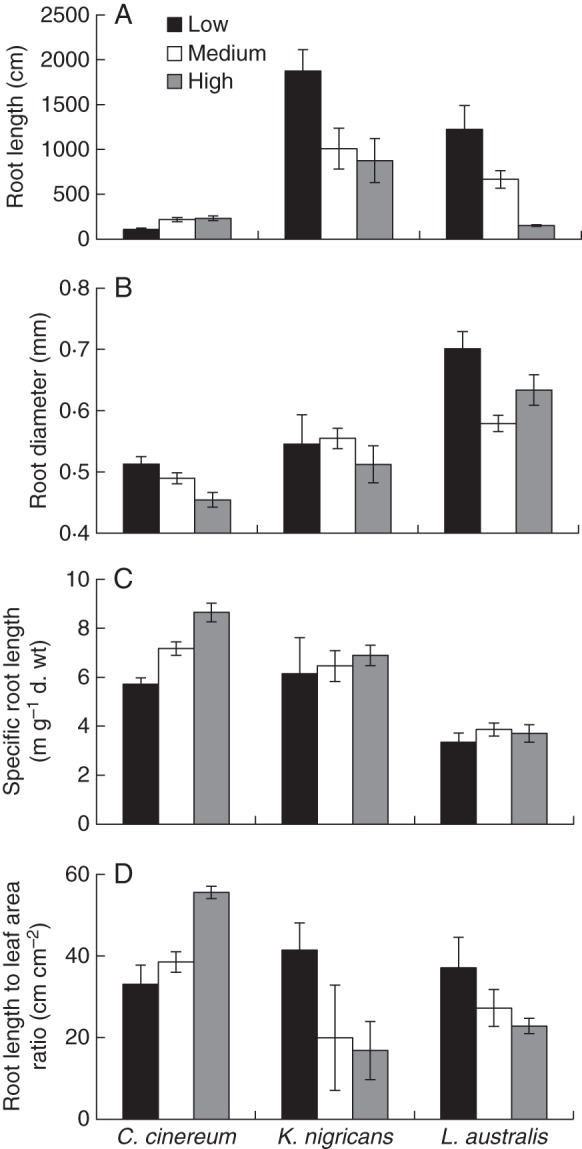
(A) Root length, (B) root diameter, (C) specific root length and (D) root length to leaf area ratio of Cullen cinereum, Kennedia nigricans and Lotus australis at the second harvest (i.e. with ten fully expanded main stem leaves) when grown at low, medium or high temperature with addition of 60 mg kg−1 of P as FePO4 (mean ± s.e., n = 4).
For L. australis, tissue P concentration increased from low to high temperature, while that of C. cinereum and K. nigricans was unchanged (Fig. 3A, Table 3). When comparing the P concentration of plants treated with KH2PO4 with that of plants treated with FePO4 at high temperature, the P concentration of plants treated with KH2PO4 was 60, 90 and 25 % higher for C. cinereum, K. nigricans and L. australis, respectively (Fig. 3A). Similar to the dry weight and root length response, plant total P for C. cinereum increased from low to high temperature, while that of K. nigricans and L. australis decreased, with the greatest reduction for L. australis (Fig. 3B, Table 3). When comparing species, total P taken up was lower for C. cinereum than for K. nigricans and L. australis. For C. cinereum, Pprod increased from low to high temperature, while that of K. nigricans and L. australis decreased (Fig. 3C). The change in Pprod with temperature was greatest for L. australis. The PUR of C. cinereum increased and that of K. nigricans decreased from low to high temperature (Fig. 3D). However, the PUR of L. australis increased only up to the medium temperature.
Fig. 3.
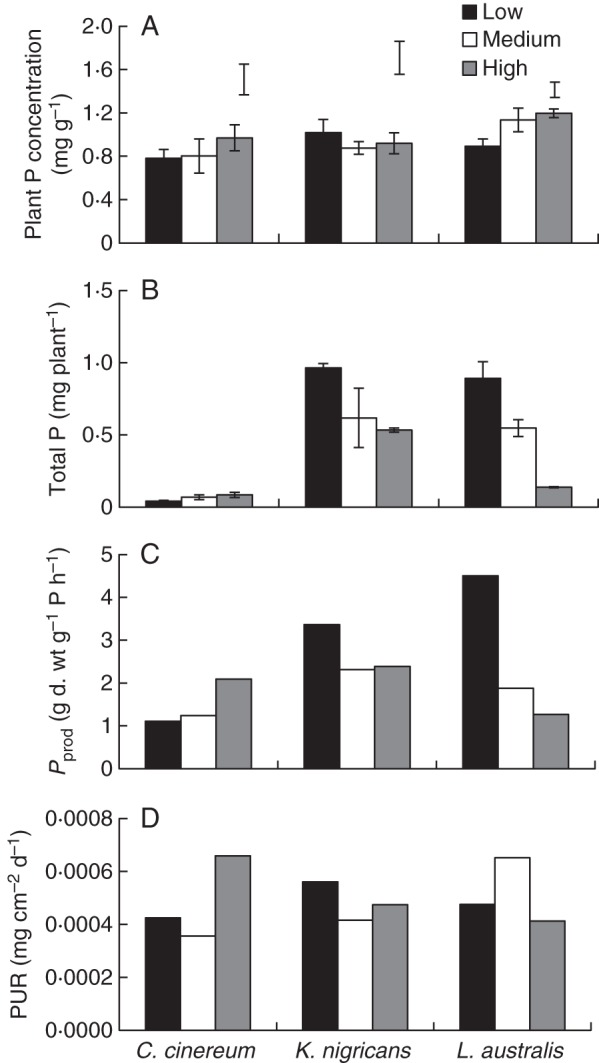
(A) Plant P concentration at the second harvest, (B) total plant P at the second harvest (i.e. with ten fully expanded main stem leaves), (C) phosphorus productivity (i.e. Pprod), and (D) phosphorus uptake rate (PUR) of Cullen cinereum, Kennedia nigricans and Lotus australis when grown at low, medium or high temperature with addition of 60 mg kg−1 of P as FePO4 (mean ± s.e., n = 4). The error bars above the high temperature treatment in (A) are for P concentration of plants that received 60 mg kg−1 of P as KH2PO4; Pprod (C) and PUR (D) were calculated using treatment means at the first and second harvest and therefore the s.e. could not be calculated.
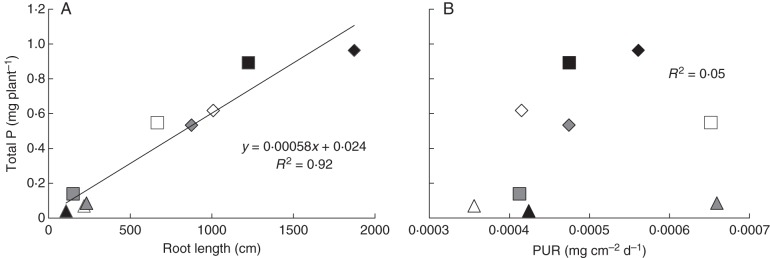
Across temperature treatments and species, root length was strongly correlated with total P content in a plant (r = 0·96, P < 0·0001; Fig. 4A) while PUR (mg P cm−2 root area d−1) was not correlated (r = 0·23, P > 0·05; Fig. 4B).
Fig. 4.
(A) Relationship between total P content per plant and root length, and (B) total P content per plant and P uptake rate (PUR) for plants grown with addition of 60 mg kg−1 of P as FePO4 at the second harvest. Black, white and grey symbols are for low, medium and high temperature treatments, and triangles, diamonds and squares are for Cullen cinereum, Kennedia nigricans and Lotus australis, respectively.
Rhizosphere carboxylates (μmol g−1 root d. wt) and composition varied depending on species and temperature (Fig. 5, Table 3). Cullen cinereum had the lowest amount of carboxylates, irrespective of the temperature treatment. For all the species, rhizosphere carboxylates decreased greatly at high temperature (i.e. approx. 0, 23 and 32 % of the low temperature treatment for C. cinereum, K. nigricans and L. australis, respectively). Citrate was the main constituent in K. nigricans and L. australis. At the high temperature for K. nigricans and L. australis, citrate made up >90 % of carboxylates, while at the medium and low temperatures it made up only 67–80 % of total carboxylates. Very low concentrations (i.e. <0·2 µmol g−1 root d. wt) of fumaric, maleic, cis-aconitic and trans-aconitic acids were also detected. Similar results were found at the first harvest (data not shown). Carboxylates in the bulk soil were also measured, where very low concentrations of maleic and fumaric acids (i.e. <0·0002 µmol g−1 soil d. wt) were present at medium and high temperature treatments, but they were not detected at low temperature treatment.
Fig. 5.

Amount of rhizosphere carboxylates for Cullen cinereum, Kennedia nigricans and Lotus australis when grown at low, medium or high temperature at the second harvest (i.e. with ten fully expanded main stem leaves) with addition of 60 mg kg−1 of P as FePO4 (mean ± s.e., n = 4).
For all species, A increased from low to medium temperature, and at high temperature A was either similar to or lower than that at medium temperature (Fig. 6A, Table 3). The gs of C. cinereum was reduced with increasing temperature, while for K. nigricans and L. australis it increased (Fig. 6B, Table 3). The WUE of C. cinereum increased, while that of K. nigricans and L. australis decreased, as temperature increased from low to medium (Fig. 6C).
Fig. 6.
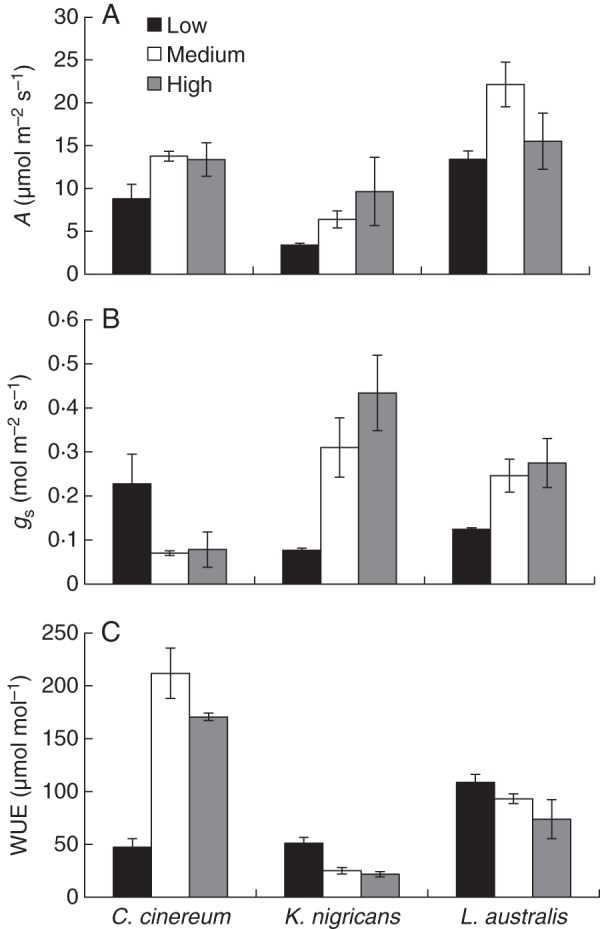
(A) Photosynthetic rate (A), (B) stomatal conductance (gs) and (C) photosynthetic water use efficiency (WUE; A/gs) of Cullen cinereum, Kennedia nigricans and Lotus australis 1 week before the second harvest when grown at low, medium or high temperature with addition of 60 mg kg−1 of P as FePO4 (i.e. with ten fully expanded main stem leaves) (mean ± s.e., n = 4).
DISCUSSION
Cullen cinereum plants increased dry weight, root length and RGR with increasing temperature. Therefore, the low temperature treatment (i.e. 20/13 °C) was sub-optimal for growth of C. cinereum. In contrast, dry weight, root length and RGR of K. nigricans and L. australis decreased from low to high temperature, indicating that the low temperature (i.e. 20/13 °C) was in the optimum range for their growth, and both medium and high temperatures (i.e. 27/20 °C and 32/25 °C) were supraoptimal. These findings are consistent with the mean environmental temperatures of the collection locations of the three species. Therefore, we would expect P nutrition and related processes such as root morphology and physiology of these species to vary among low, medium and high temperature treatments.
Cullen cinereum, with a lower RGR, had a higher degree of root morphological adaptation than K. nigricans and L. australis
The first hypothesis that plants would produce less root dry weight and have shorter and thicker roots (i.e. low root surface area per root dry weight) with a lower RMR at sub- or supraoptimal temperatures compared with optimal temperatures was partially supported. As hypothesized, for all species, root dry weight, root length and root length to leaf area ratio were higher at optimum temperatures [i.e. medium to high temperatures (27/20 and 32/25 °C, respectively) for C. cinereum and low temperature (20/13 °C) for K. nigricans and L. australis] than at sub- or supraoptimal temperatures. Similar results have been reported for root growth in deciduous and evergreen temperate tree species (Alvarez-Uria and Körner, 2007). As for other adaptations, root diameter was lower and SRL (cm g−1 root d. wt) was higher at optimum temperature only for C. cinereum, thus only partially supporting our first hypothesis. These responses can be considered to reflect a more efficient investment of resources for greater soil exploration which would contribute to faster nutrient and water absorption at optimum temperatures. This is particularly important when mycorrhization is very poor (Körner, 1998), as well as when rhizosphere carboxylate content is very low, as we discuss below. Kennedia nigricans responded differently to C. cinereum, as its RMR was reduced at supraoptimal temperatures, thus supporting the first hypothesis. Therefore, the three species exhibited different root morphological responses to temperature treatments (i.e. C. cinereum was the most responsive while L. australis was the least responsive).
Pprod for all species, and carboxylate content for K. nigricans and L. australis was highest at optimal temperatures
In addition to morphological root responses, plants may have physiological adaptations to enable greater P uptake at optimum temperatures. Therefore, the second hypothesis that tissue P concentration, P productivity (Pprod) and rhizosphere carboxylate content would be higher at optimal growth temperatures than at sub- or supraoptimal temperatures was tested. Increased tissue P concentration for L. australis in the high temperature treatment (32/25 °C) (i.e. supraoptimal temperature) and unchanged tissue P concentration for C. cinereum and K. nigricans with temperature treatment are not in agreement with hypothesis 2. In the present experiment, tissue P concentration of FePO4-treated plants ranged from 0·8 to 1·2 mg P g−1 d. wt, while tissue P concentration of plants treated with 60 mg kg−1 of KH2PO4 ranged from 1·5 to 1·7 mg P g−1 d. wt. Thus the generally low growth and low tissue P concentration across all the species, even after a supply of 60 mg kg−1 Fe(PO4), is likely to be due to the limitation of P supply through FePO4. However, the Pprod was higher at optimal temperatures for all the species, supporting the second hypothesis, and a similar response was observed for dry weight and RGR (Fig. 1). Therefore, across species, Pprod was highly correlated with dry weight (r > 0·90) and RGR (r > 0·97), indicating that P was efficiently used for growth at optimal temperatures by all the species, more so than at sub- and supraoptimal temperatures. Ryser et al. (1997) also reported a similar correlation between Pprod and RGR for several other plant species in a single temperature treatment.
Carboxylate content was higher at the optimal, low temperature, and reduced at the supraoptimal, high temperature, for K. nigricans and L. australis, supporting hypothesis 2. Increased plant growth and P demand at optimal temperatures might have enhanced the exudation of more carboxylates. On the other hand, increased rhizosphere carboxylate content at optimal temperatures might have favoured a higher PUR and Pprod for L. australis and K. nigricans. This relationship warrants further study. Carboxylate composition also changed at supraoptimal temperatures for K. nigricans and L. australis, with the contribution of malic acid reduced. However, results for C. cinereum were not in agreement with our hypothesis, although the amount of carboxylates present was very much lower than for K. nigricans and L. australis.
Therefore, the three species exhibited different responses when acquiring P from the P-limited soil: C. cinereum had extensive root morphological adaptations [i.e. long (higher root length per leaf area), thin roots with higher SRL], while physiological adaptation (i.e. high content of carboxylates in the rhizosphere) was prominent in K. nigricans and L. australis, at their optimum range of temperatures. Lack of root physiological adaptations for C. cinereum might be due to its lower RGR and thus lower P demand than that of K. nigricans and L. australis. Moreover, Clarkson et al. (1988) suggested that the degree to which soil temperature might alter nutrient uptake capacity is largely determined by the degree to which growth and RMR are affected (i.e. if changes in soil temperature lead to an increased nutrient-absorbing root surface relative to shoot size, then there will be a downregulation of ion influx, and vice versa). Since root morphological and physiological characteristics are inter-related, they must be studied in conjunction with other compensatory factors, whose responses might counterbalance each other.
The amount of carboxylates in the bulk soil, as expected, was very low compared with that in the rhizosphere, across all temperatures and species. The amount present was higher at the high temperature treatment. However, overall, it seems that the impact of soil biota on increasing soil P availability in bulk soil via carboxylate exudation was negligible under our experimental conditions.
Plant P status was highly correlated with root length, rather than with PUR or root diameter
Hypothesis 3, that the reduction in the total amount of P taken up at sub- or supraoptimal temperature compared with that at optimal temperature was mainly due to reduced uptake rate per unit root surface area, rather than reduced root length (i.e. morphological adaptation), was not supported by our results. In this study, P supply through FePO4 was very low and thus P availability was limited for plants without a capacity to mobilize P. For all species, across temperature treatments, root length and total plant P were highly correlated. However, root diameter showed no correlation with the total amount of P in plant tissues (r = 0·13, P > 0·05).
Only for C. cinereum and K. nigricans was PUR (mg P cm−2 root area d−1) higher at optimal temperatures, but it had a weak correlation with the total amount of P in plant tissues. Therefore, for all species across temperature treatments, plant P status was highly correlated with root length, rather than with PUR or root diameter. This result is in agreement with the results of Mackay and Barber (1984), who found that plant P uptake is mainly determined by root production, rather than by physiological uptake capacity. Our results further validate this across a range of temperatures from optimal to sub- or supraoptimal. Moreover, Ercoli et al. (1996) highlighted that temperature and P supply independently weaken growth at sub-optimal temperatures. This is supported by our results as tissue P concentration was not reduced at sub- or supraoptimal temperatures, even though the growth and amount of P in plant tissues were reduced.
Hypothesis 4, that WUE would be lower at sub- or supraoptimal temperatures than that at optimal temperatures and reduced WUE would be partly due to a low photosynthetic rate (A), was only partly supported. As hypothesized, A (per unit leaf area) was higher at optimal temperature only for C. cinereum. A similar response was observed for Quercus rubra L. seedlings (Wertin et al., 2011). However, gs was lower and, therefore, WUE was higher at optimal temperatures for all the species in the present experiment. In a comparison of Triticum aestivum L. cultivars at a single temperature, Boogaard et al. (1997) found that differences in WUE at the leaf level were related to variation in gs, rather than in the A, which supports our findings. Therefore, WUE is a better indicator of a plant's physiological adaptability to environmental temperature than A or gs alone (Comstock and Ehleringer, 1992; Lambers et al., 2008). This was achieved by a reduction in gs for all the species and, for C. cinereum only, an increase in A, at optimal temperatures.
In the high temperature treatment, the dry weight of KH2PO4-treated plants was more than twice that of FePO4-treated plants for C. cinereum and L. australis, but was only 40 % higher for K. nigricans. The high temperature treatment was the optimal temperature for C. cinereum and, therefore, a faster growth rate would be expected in the presence of an abundant readily available source of P from KH2PO4. For K. nigricans and L. australis, the high temperature treatment was supraoptimal, so for these two species we would expect a smaller growth increase due to KH2PO4. This was indeed observed for K. nigricans, but for L. australis the increase in growth was still higher than we would expect. This might be due to a species-specific characteristic where L. australis has fewer adaptations to allow it to utilize poorly soluble P from FePO4 and thus showed a greater response to addition of KH2PO4. Moreover, the differential growth responses can be explained using RGR values of the three species in the high temperature treatment, where C. cinereum and L. australis showed a higher RGR and K. nigricans a lower RGR. Therefore, for species with a higher RGR a higher increase in growth rate in the presence of KH2PO4 can be expected.
Concluding remarks
The highest temperature in the present experiment (i.e. 32/25 °C) was the optimum for growth of C. cinereum, while the low temperature (i.e. 20/13 °C) was the optimum for K. nigricans and L. australis. At optimum temperatures, when RGR was highest, Pprod was also the highest for all the species. RGR and Pprod were highly correlated across temperature treatments. All species exhibited multiple adaptive shoot and root responses to achieve a greater dry weight at their growth-limiting P supply and optimum temperatures. For shoots, all the species responded similarly by reducing gs and increasing WUE at optimum temperatures. However, root responses differed among species. Cullen cinereum had several morphological adaptations, including long, thin roots to explore a greater volume of soil, at optimum temperatures. Kennedia nigricans and L. australis only exhibited long roots as morphological adaptations, but had a higher content of carboxylates in the rhizosphere as an additional adaptation, which would have enhanced the mobilization of P from FePO4 at optimum temperatures. The amount of P taken up by a plant was mainly determined by root length, rather than by PUR. The present results aid understanding of plant P dynamics and root adaptations when plants are grown in different regions with contrasting temperatures, seasons and under predicted climate change scenarios.
ACKNOWLEDGEMENTS
This study was supported by the School of Plant Biology and the Future Farm Industries Cooperative Research Centre, The University of Western Australia. L.D.B.S. also appreciates the SIRF/UIS Scholarship awarded by the University of Western Australia and further scholarship support from the late Frank Ford. Kevin Foster and Basu Dev Regmi provided valuable comments on an earlier version of this manuscript.
LITERATURE CITED
- Alvarez-Uria P, Körner C. Low temperature limits of root growth in deciduous and evergreen temperate tree species. Functional Ecology. 2007;21:211–218. [Google Scholar]
- Beadle NCW. Soil phosphate and its role in molding segments of the Australian flora and vegetation, with special reference to xeromorphy and sclerophylly. Ecology. 1966;47:992–1007. [Google Scholar]
- Bennett R, Ryan MH, Colmer T, Real D. Prioritisation of novel pasture species for use in water-limited agriculture: a case study of Cullen in the Western Australian wheatbelt. Genetic Resources and Crop Evolution. 2011;58:83–100. [Google Scholar]
- Blair GJ, Chinoim N, Lefroy RDB, Anderson GC, Crocker GJ. A soil sulphur test for pastures and crops. Australian Journal of Soil Research. 1991;29:619–626. [Google Scholar]
- Bolan NS, Robson AD, Barrow NJ. Effects of phosphorus application and mycorrhizal inoculation on root characteristics of subclover and ryegrass in relation to phosphorus uptake. Plant and Soil. 1987;104:294–298. [Google Scholar]
- Boogaard RVD, Alewijnse D, Veneklaas EJ, Lambers H. Growth and water-use efficiency of 10 Triticum aestivum cultivars at different water availability in relation to allocation of biomass. Plant, Cell and Environment. 1997;20:200–210. [Google Scholar]
- Bravo FP, Uribe EG. Temperature dependence of the concentration kinetics of absorption of phosphate and potassium in corn roots. Plant Physiology. 1981;67:815–819. doi: 10.1104/pp.67.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Earnshaw MJ, White PJ, Cooper HD. Temperature dependent factors influencing nutrient uptake: an analysis of responses at different levels of organization. Symposia of the Society for Experimental Biology. 1988;42:281–230. [PubMed] [Google Scholar]
- Cocks PS. Ecology of herbaceous perennial legumes: a review of characteristics that may provide management options for the control of salinity and water logging in dryland cropping systems. Australian Journal of Agricultural Research. 2001;52:137–151. [Google Scholar]
- Colwell JD. An automatic procedure for the determination of phosphorus in sodium hydrogen carbonate extract of soil. Chemistry and Industry. 1965;10:893–895. [Google Scholar]
- Comstock JP, Ehleringer JR. Correlating genetic variation in carbon isotopic composition with complex climatic gradients. Proceedings of the National Academy of Sciences, USA. 1992;89:7747–7751. doi: 10.1073/pnas.89.16.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell D, Drangert J, White S. The story of phosphorus: global food security and food for thought. Global Environmental Change. 2009;19:292–305. [Google Scholar]
- Dear BS, Li GD, Hayes RC, Hughes SJ, Charman N, Ballard RA. Cullen australasicum (syn. Psoralea australasica): a review and some preliminary studies related to its potential as a low rainfall perennial pasture legume. Rangeland Journal. 2007;29:121–132. [Google Scholar]
- Delucia EH, Callaway RM, Thomas EM, Schlesinger WH. Mechanisms of phosphorus acquisition for ponderosa pine seedlings under high CO2 and temperature. Annals of Botany. 1997;79:111–120. [Google Scholar]
- Engles C, Munkle L, Marschner H. Effect of root zone temperature and shoot demand on uptake and xylem transport of macronutrients in maize (Zea mays L.) Journal of Experimental Botany. 1992;43:537–547. [Google Scholar]
- Elrashidi MA, Larsen S. The effect of phosphate addition on the solubility of phosphate in soil. Plant and Soil. 1978;50:585–596. [Google Scholar]
- Ercoli L, Mariotti M, Masoni A, Massantini F. Effect of temperature and phosphorus fertilization on phosphorus and nitrogen uptake by sorghum. Crop Science. 1996;36:348–354. [Google Scholar]
- Ericsson T, Ingestad T. Nutrition and growth of birch seedlings at varied relative phosphorus addition rates. Physiologia Plantarum. 1988;72:227–235. [Google Scholar]
- Handreck KA. Phosphorus requirements of Australian native plants. Australian Journal of Soil Research. 1997;35:241–289. [Google Scholar]
- Harte J, Shaw R. Shifting dominance within a montane vegetation community: results of a climate-warming experiment. Science. 1995;267:876–880. doi: 10.1126/science.267.5199.876. [DOI] [PubMed] [Google Scholar]
- Hoffmann WA, Poorter H. Avoiding bias in calculations of relative growth rate. Annals of Botany. 2002;90:37–42. doi: 10.1093/aob/mcf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martínez-Ferri E. The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant and Soil. 2002;238:111–122. [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany. 2006;98:693–713. doi: 10.1093/aob/mcl114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, Pons TL. Plant physiological ecology, 2nd edn. New York: Springer; 2008. [Google Scholar]
- Lambers H, Finnegan PM, Laliberté E, et al. Phosphorus nutrition of Proteaceae in severely phosphorus-impoverished soils: are there lessons to be learned for future crops? Plant Physiology. 2011;156:1058–1066. doi: 10.1104/pp.111.174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay WL. Chemical equilibria in soils. New York: John Wiley & Sons; 1979. [Google Scholar]
- Mackay AD, Barber SA. Soil temperature effects on root growth and phosphorus uptake by corn. Soil Science Society of America Journal. 1984;48:818–823. [Google Scholar]
- Motomizu S, Wakimoto T, Toei K. Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. The Analyst. 1983;108:361–367. doi: 10.1016/0039-9140(84)80269-6. [DOI] [PubMed] [Google Scholar]
- Neumann G, Martinoia E. Cluster roots: an underground adaptation for survival in extreme environments. Trends in Plant Science. 2002;7:162–167. doi: 10.1016/s1360-1385(02)02241-0. [DOI] [PubMed] [Google Scholar]
- Pang J, Tibbett M, Denton MD, et al. Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant and Soil. 2010;328:133–143. [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H. Carboxylate composition of root exudates does not relate consistently to a crop species' ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytologist. 2007;173:181–190. doi: 10.1111/j.1469-8137.2006.01897.x. [DOI] [PubMed] [Google Scholar]
- Raghothama KG. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS. Phosphate acquisition. Plant and Soil. 2005;274:37–49. [Google Scholar]
- Rayment GE, Higginson FR. Australian laboratory handbook of soil and water chemical methods. Melbourne: Inkata Press; 1992. [Google Scholar]
- Raynaud X, Jaillard B, Leadley PW. Plants may alter competition by modifying nutrient bioavailability in rhizosphere: a modeling approach. American Naturalist. 2008;171:44–58. doi: 10.1086/523951. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Hadobas PA, Hayes JE. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant, Cell and Environment. 2000;23:397–405. [Google Scholar]
- Ryser P, Verduyn B, Lambers H. Phosphorus allocation and utilization in three grass species with contrasting response to N and N supply. New Phytologist. 1997;137:293–302. doi: 10.1046/j.1469-8137.1997.00807.x. [DOI] [PubMed] [Google Scholar]
- SAS. SAS/STAT User's Guide. Cary, NC: SAS Institute Inc; 2003. Version 9·1. [Google Scholar]
- Searle PL. Berthelot of indophenol reaction and its use in the analytical chemistry of nitrogen. A review. Analyst. 1984;109:549–568. [Google Scholar]
- Shane MW, Lambers H, Cawthray GR, Kuhn AJ, Schurr U. Impact of phosphorus mineral source (Al-P or Fe-P) and pH on cluster-root formation and carboxylate exudation in Lupinus albus L. Plant and Soil. 2008;304:169–178. [Google Scholar]
- Simpson R, Oberson A, Culvenor R, et al. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant and Soil. 2011;349:89–120. [Google Scholar]
- Sinclair TR. Mineral nutrition and plant growth response to climate change. Journal of Experimental Botany. 1992;43:1141–1146. [Google Scholar]
- Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual Review of Plant Biology. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RMB, Taber HG, Aiello AS. Effect of increasing root-zone temperature on growth and nutrient uptake by ‘Gold Star’ muskmelon plants. Journal of Plant Nutrition. 1998;21:321–328. [Google Scholar]
- Suriyagoda LDB, Lambers H, Ryan MH, Renton M. From controlled environments to field simulations: developing a growth model for the novel perennial pasture legume Cullen australasicum. Agricultural and Forest Meteorology. 2010a;150:1373–1382. [Google Scholar]
- Suriyagoda LDB, Ryan MH, Renton M, Lambers H. Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Annals of Botany. 2010b;105:755–767. doi: 10.1093/aob/mcq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyagoda LDB, Ryan MH, Renton M, Lambers H. Above- and below-ground interactions of grass and pasture legume species when grown together under drought and low phosphorus availability. Plant and Soil. 2011;348:281–297. [Google Scholar]
- Thompson LJ, Naeem S. The effects of soil warming on plant recruitment. Plant and Soil. 1996;182:339–343. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Van Cleve K, Barney R, Schlentner R. Evidence of temperature control of production and nutrient cycling in two interior Alaska black spruce ecosystems. Canadian Journal of Forest Research. 1981;11:258–273. [Google Scholar]
- Veneklaas EJ, Stevens T, Cawthray GR, Turner NC, Grigg AM, Lambers H. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant and Soil. 2003;248:187–197. [Google Scholar]
- Walkley A, Black IA. Estimation of soil organic carbon by the chromic acid titration method. Soil Science. 1934;37:29–38. [Google Scholar]
- Wertin TM, McGuire MA, Teskey RO. Higher growth temperatures decreased net carbon assimilation and biomass accumulation of northern red oak seedlings near the southern limit of the species range. Tree Physiology. 2011;31:1277–1288. doi: 10.1093/treephys/tpr091. [DOI] [PubMed] [Google Scholar]
- Wouterlood M, Lambers H, Veneklaas EJ. Plant phosphorus status has a limited influence on the concentration of phosphorus-mobilising carboxylates in the rhizosphere of chickpea. Functional Plant Biology. 2005;32:153–159. doi: 10.1071/FP04084. [DOI] [PubMed] [Google Scholar]