Abstract
Background and Aims
Delayed selfing is the predominant mode of autonomous self-pollination in flowering plants. However, few delayed selfing mechanisms have been documented. This research aims to explore a new delayed selfing mechanism induced by stigmatic fluid in Roscoea debilis, a small perennial ginger.
Methods
Floral biology and flower visitors were surveyed. The capacity of autonomous selfing was evaluated by pollinator exclusion. The timing of autonomous selfing was estimated by emasculation at different flowering stages. The number of seeds produced from insect-pollination was assessed by emasculation and exposure to pollinators in the natural population. The breeding system was also tested by pollination manipulations.
Key Results
Autonomous self-pollination occurred after flowers wilted. The stigmatic fluid formed a globule on the stigma on the third day of flowering. The enlarged globule seeped into the nearby pollen grains on the fourth flowering day, thus inducing pollen germination. Pollen tubes then elongated and penetrated the stigma. Hand-selfed flowers produced as many seeds as hand-crossed flowers. There was no significant difference in seed production between pollinator-excluded flowers and hand-selfed flowers. When emasculated flowers were exposed to pollinators, they produced significantly fewer seeds than intact flowers. Visits by effective pollinators were rare.
Conclusions
This study describes a new form of delayed autonomous self-pollination. As the predominant mechanism of sexual reproduction in R. debilis, delayed self-pollination ensures reproduction when pollinators are scarce.
Keywords: stigmatic fluid, autonomous selfing, reproductive assurance, Roecoea debilis, pollinator failure, ginger
INTRODUCTION
The transition from outcrossing to selfing has occurred repeatedly in angiosperms (Stebbins, 1974), and about 20 % of species have evolved selfing as a predominant mating strategy (Barrett, 2002). To understand why selfing evolves, both its costs and its benefits should be considered (Eckert and Herlihy, 2004). The major costs of selfing include inbreeding depression and gamete discounting (Darwin, 1876; Lande and Schemske, 1985; Lloyd, 1992; Harder and Wilson, 1998; Goodwillie et al., 2005). As a product of natural selection, selfing has adaptive values in different ecological environments, primarily providing two benefits: automatic selection advantage and reproductive assurance. The automatic selection advantage by selfing provides a 50 % gene transmission advantage (Fisher, 1941). Outcrossing plants are mother to their progeny on the same plant, while selfing plants are both mother and father to their own progeny. Reproductive assurance by selfing enables seed production when pollinators are absent or uncommon (Darwin, 1876; Stebbins, 1957; Lloyd, 1979, 1992). The reproductive assurance hypothesis can be determined experimentally by removing the anthers before dehiscence and permitting pollinator visits (Schoen and Lloyd, 1992; Fenster and Marten-Rodriguez, 2007). If selfing provides reproductive assurance, emasculated flowers should produce fewer seeds than intact flowers.
Autonomous selfing has been classified into three modes in angiosperm species: prior, competing and delayed selfing, according to its timing with respect to outcrossing (Lloyd, 1979; Lloyd and Schoen, 1992). Delayed selfing is the predominant mode of autonomous selfing (Fenster and Marten-Rodriguez, 2007). However, few mechanisms have been documented. Delayed selfing is achieved primarily through the change of relative positions between anther(s) and stigma(s) within a flower leading to anther–stigma contact (ASC). This ASC delayed selfing mechanism is classified into three categories (Fenster and Marten-Rodriguez, 2007): (1) style movement – autonomous selfing occurs through style(s) elongation or curl-down to contact the anther(s), for example in Collinsia verna (Kalisz et al., 1999), Hibiscus laevis (Klips and Snow, 1997) and Aquilegia canadensis (Eckert and Schaefer, 1998); (2) filament movement – the anther contacts the stigma through filament elongation or curve, for example in Gentianopsis paludosa (Duan et al., 2010) and Holcoglossum amesianum (Liu et al., 2006); and (3) corolla facilitation – ASC occurs through corolla facilitation, for example through corolla tube elongation in Hornstedtia scottiana (Ippolito and Armstrong, 1993), through corolla wilting in Pedicularis dunniana (Sun et al., 2005), and through corolla abscission in Incarvillea sinensis Lamarck var. sinensis (Qu et al., 2007) and Mimulus verbenaceus (Vickery, 2008). Non-ASC delayed selfing mechanisms are extremely rare. To our knowledge, there is only one report in Caulokaempferia coenobialis, in which delayed selfing occurs through the drop of pollen released from pollen sacs slowly flowing towards the stigma (Wang et al., 2004, 2005).
Here, we reported a new non-ASC delayed selfing mechanism in a small ginger, Roscoea debilis (Zingiberaceae), in which the stigma continuously secretes fluid, forming a large globule on the stigma cup on the third day of flowering. The large globule penetrates the nearby pollen grains on the fourth flowering day, inducing pollen grain germination and elongation of the pollen tubes into the stigma. Our preliminary observations revealed that R. debilis received few pollinator visits during peak blooming times, and we were consequently intrigued by how this species produced a high fruit-set. In this study, we focus primarily on the following questions: (1) Does R. debilis have the ability to fertilize itself? (2) How and when does self-pollination occur in this species? (3) Why is autonomous selfing favoured in R. debilis?
MATERIALS AND METHODS
Study sites and species
The natural population of Roscoea debilis was studied in Lincang, Yunnan province, south-western China (23°35′N, 100°04′E; altitude 1890 m) from August to September, 2010 and 2011. Individuals in this population grow on slopes in a pine forest with a large population size (more than 3000 flowering individuals).
R. debilis is a small perennial herb that usually inhabits mixed forests and moist stony pastures. Plants are 10–30 cm tall, with annual pseudostems from erect, reduced rhizomes. The inflorescence is on the pseudostem, with peduncle exserted from leaf sheaths. Its slender-tubed flowers are purple, pink or white. Flowers have one cucullate central lobe, two lateral lobes, two petaloid lateral staminodes and one large labellum. The anther is linear and its connective extends at the base to form a spur. The style is filiform. The stigma is funnelform, with margin ciliate (Cowley, 1982).
Floral biology
About 30 plants were randomly selected for investigation of flowering phenology and flower morphology. Time of anthesis, time of anther dehiscence, floral longevity, length of corolla tube, number of flowers per inflorescence and the number of flowers that opened on each plant each day were noted. In addition, the movement of floral organs was observed on each flowering day. Thirty flower buds were randomly selected and fixed in FAA solution (ethanol, acetic acid at 3 : 1, v/v) separately for pollen and ovule counts. A haemocytometer was used to estimate pollen production per flower following the method of Dafni (1992). The ovule number in each ovary was also counted under a dissecting microscope. The pollen-to-ovule (P/O) ratio was calculated as the number of pollen grains divided by the number of ovules for each flower.
To assess the nectar secretion patterns of flowers at each flowering stage, flower buds were tagged and covered with fine-mesh nylon bags. Flowers were divided into three stages based on the age of flowers: 1-, 2- and 3-d flowers. About 30 covered flowers from each flowering stage were randomly selected to measure volume and sucrose concentrations. Nectar volume and sugar concentrations were examined using micro-capillaries (5 and 10 µL) and a refractometer (Eclipse 45-81; Bellingham and Stanley Ltd, Tunbridge Wells, Kent, UK). Stigmatic fluid secretion patterns were also evaluated at each flowering stage. Because the amount of stigmatic fluid was small, it was not possible to evaluate the volume. Instead, the percentage of the flowers with stigmatic fluid surpassing the stigma (PSF) was measured.
To test the physiological activity of stigmas on the morning of first flowering, ten flowers were randomly chosen to conduct hand self-pollination experiments. Styles were removed 2 h after pollination and immediately fixed in FAA solution. Pollen tubes were measured after being stained with aniline blue, following the method of Dafni (1992). To determine stigma receptivity at each flowering stage, ten bagged flowers from each flowering stage were randomly selected. Dimethykthiazol-diphenyl-tetrazolium bromide (MTT) was used to test for the presence of dehydrogenase on the stigma (Rodriguez-Riano and Dafni, 2000). Stigmas with dehydrogenase stained dark purple–brown, suggesting the stigma was receptive.
Capacity for autonomous selfing and breeding system
To quantify the capacity for autonomous selfing and to determine the breeding system, single flowers from more than 150 individuals were randomly selected in 2010. Visitors were excluded using nylon mesh bags. Plants were evenly and randomly allocated to five pollination treatments: (1) flowers were hand pollinated using pollen grains from the same flower and bagged, (2) flowers were left intact and bagged, (3) flowers were emasculated before anthesis, hand pollinated using pollen grains from flowers of other individuals and bagged, (4) flowers were emasculated before anthesis and bagged, and (5) flowers were left intact. Fruits were collected 30 d after pollination, and seeds were counted. Treatments (1) and (2) were repeated in the flowering season of 2011.
To test for differences in seed number per fruit between hand self-pollination and autonomous selfing, a two-way ANOVA was used with year and treatment as fixed factors. Data for seed number were square-root transformed, to improve homoscedasticity. For fruit-set, the logistical model was computed to examine the effects of year, treatment and year × treatment interaction. To examine the difference in seed production between hand self-pollination and hand cross-pollination, a t-test was used to compare the difference in seed number, and a continuity-adjusted chi-square test was used to test fruit-set difference between hand self-pollinated flowers and hand cross-pollinated flowers. To test whether pollen limitation existed in the natural population, the differences of seed production among intact flowers, hand self-pollinated flowers and hand cross-pollinated flowers were compared. A one-way ANOVA was used to compute difference in seed numbers. For fruit-set, a chi-square test was used. All analyses in this study were performed in SAS 9.1 for Windows.
Timing of autonomous selfing
To determine the time of autonomous selfing, single flower buds from about 240 plants were selected and bagged to prevent visitors during flowering time. Plants were randomly assigned to one of eight groups: (1–7) anthers were removed 3, 15, 27, 39, 51, 63 and 75 h after anthesis, and (8) natural control – anthers were left intact. A one-way ANOVA was used to compare seed production at each flowering stage. A non-linear regression model was then used to test the relationship between timing of anther removal and seed production.
Pollinator observation and reproductive assurance
Observations of flower visitors were made in patches A, B and C in 2010, and in patches A, B and D in 2011. Patches A, B, C and D were four slopes in pine forest, in which there were more individuals of R. debilis than other patches. The distance between each two patches is about 1 km. More than 20 flowering individuals were observed from each patch. Observations were made on sunny days continuously from 0830 to 1700 h and 1830 to 2030 h for 3 d in each patch during the peak flowering period.
To evaluate the contribution of self-pollination to fruit-set and seed number, two groups were assigned: (1) emasculation and exposure to pollinators – flowers were emasculated before anther dehiscence. This treatment was designed to prevent autonomous self-pollination but allowed cross-pollination by pollinators. It is not possible to remove the anther for this treatment. Because the soft style is fixed to the anther, the stigma would fall off and deviate from its original position after removing the anther. Instead, it was manipulated according to the following method: the buds were first carefully opened by hand; the stigma was then removed from the anther, and all pollen sacs were scraped out; and lastly, the style was fixed to the original position of the anther with a string (i.e. Fig. 1C). (2) Natural pollination – flowers were left intact. More than 60 plants were evenly assigned to the two groups (one flower per plant per treatment). About 30 d later, fruits and seeds were collected and counted. If autogamy increased seed production, emasculated flowers would set fewer seeds than intact flowers. A t-test was used to test for differences in seed number. A chi-square test was used to test for differences in fruit-set.
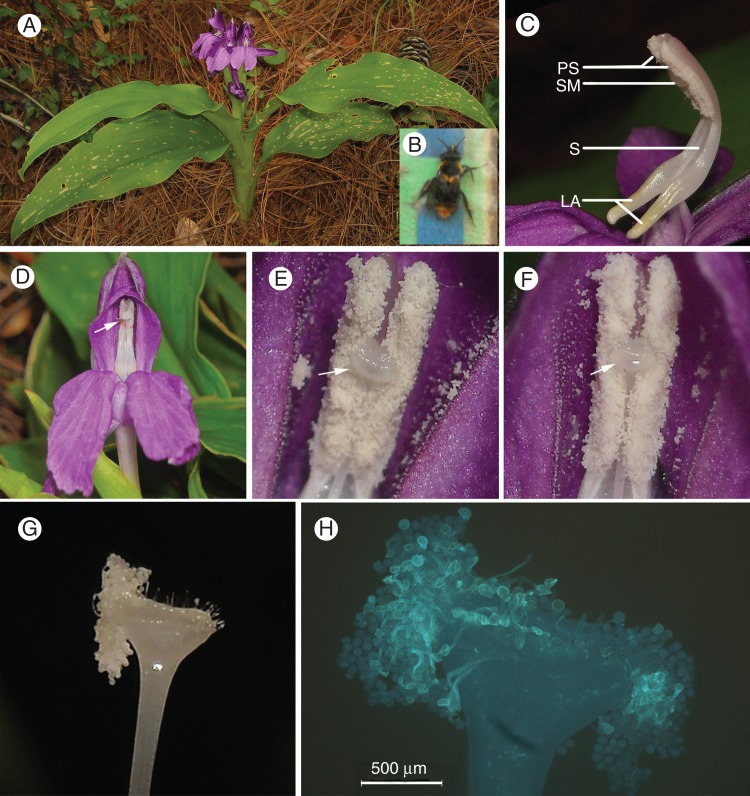
Fig. 1.
(A) Roscoea debilis in the field. (B) A yellow carpenter bee (Xylocopa confusa) caught while visiting flowers of R. debilis. (C) The inner structures of the flower: PS, pollen sacs; SM, stigma; S, style; LA, lever-like anther appendage. (D) Flower after emasculation and exposure to pollinators; the arrow indicates where the pollen sacs were scraped out before anthesis and the style was fixed to the anther with a string. (E–H) The process of autonomous selfing. (E) Stigma of flowers on the first flowering day; the arrow shows the stigma with limited stigmatic fluid. (F) Stigma of flowers on the third flowering day; the arrow shows the stigmatic fluid forming a large globule over the whole surface of the stigma. (G) Stigma of the wilted flowers on the fourth flowering day; the image shows the pollen grains consumed the stigmatic fluid and adhered to the corner of the stigma. (H) Fluorescence micrograph of the stigma of the wilted flowers on the fourth flowering day showing the adhered pollen grains germinate and tubes elongate into the style from corners of the stigma. The stigma was stained with aniline blue.
RESULTS
Floral biology
The flowering season of R. debilis persisted from mid-August to late September, with peak flowering occurring in early September in 2010 and 2011. This species typically has three to 12 purple flowers per inflorescence, with one to three flowers open at a time. Flowers began anthesis in the early morning and persisted for 3 d, wilting on the third night of flowering. The flowers wilted and remained on the inflorescence without abscission on the fourth flowering day. The corolla lobes of wilted flowers directly withered without closure, i.e. corolla wilting did not induce pollen–stigma contact. There were no floral organ movements detected during the entire flowering period. Anthers dehisced 2 h after flower opening. The average length of corolla tubes was 35·4 ± 3·4 mm (Table 1). The 2-d flowers produced more nectar than the 1-d flowers, while there was no significant difference between the 3-d flowers and the 2-d flowers (one-way ANOVA, F = 17·32, P < 0·0001). The average number of pollen grains per flower was 8031 ± 144 and the average ovule number was 34·1 ± 0·8; the P/O ratio was thus 239·2 ± 6·6 (Table 1).
Table 1.
Floral characters (mean ± s.e., with sample size in brackets) of Roscoea debilis
| Character | Value | 1-d flowers | 2-d flowers | 3-d flowers |
|---|---|---|---|---|
| Flowers per inflorescence | 4·67 ± 0·38 (39) | |||
| Flower longevity (d) | 3 (50) | |||
| Flowering intervals (d) | 0·77 (28 inflorescences) | |||
| Length of corolla tube (mm) | 35·4 ± 3·4 (30) | |||
| Pollen grains | 8031 ± 144 (30) | |||
| Ovules | 34·1 ± 0·8 (30) | |||
| P/O ratio | 239·2 ± 6·6 (30) | |||
| PTL (mm) | 2·96 ± 0·43 (10) | |||
| Nectar volume (μL) | 2·73 ± 0·27 (27) | 4·55 ± 0·29 (25) | 4·24 ± 0·18 (25) | |
| Sucrose concentration (%) | 18·75 ± 0·96 (27) | 17·04 ± 1·05 (25) | 20·14 ± 1·29 (25) | |
| PSF (%) | 0 (41) | 11·4 (35) | 83·7 (43) |
PTL, pollen tube length at 2 h after hand self-pollination in 1-d flowers. PSF, percentage of flowers with stigmatic fluid overtopping the stigma rim in each flowering stage.
The stigma produced stigmatic fluid and the anthers dehisced at the beginning of anthesis. The average pollen tube length of flowers (PTL) was 2·96 ± 0·43 mm at 2 h after hand self-pollination on the morning of first flowering. These results indicate that R. debilis is homogamous. All the stigmas of the flowers were stained dark purple–brown by MTT at each flowering stage, suggesting the stigma maintained viability during the entire flowering time.
Stigmatic fluid was continuously secreted during flowering, and this formed a large globule on the stigma on the third flowering morning (Fig. 1F). There were few flowers with stigmatic fluid surpassing the stigma in the 1-d flowers and the 2-d flowers, while the proportion of flowers with stigmatic fluid surpassing the stigma (PSF) was 83·7 % in the 3-d flowers (Table 1). The large globule on the stigma seeped into the nearby pollen grains on the fourth flowering day (flower wilted). Those pollen grains that were penetrated by stigmatic fluid germinated and the pollen tube entered into the style from the side of the stigma (Fig. 1G, H).
Capacity for autonomous selfing and breeding system
Bagged flowers set as many seeds and fruits as hand self-pollinated flowers, indicating a high capacity for autonomous selfing in R. debilis. Bagged flowers produced 18·7 ± 1·1 seeds per flower (n = 63) in 2010 and 21·8 ± 1·6 seeds per flower (n = 44) in 2011, which was not significantly different from hand self-pollinated flowers, which produced 19·8 ± 1·6 seeds per flower (n = 30) in 2010 and 19·7 ± 1·0 seeds per flower (n = 48) in 2011 (two-way ANOVA, Fig. 2, Table 2). Bagged flowers set 91·3 % (n = 69) and 88 % (n = 50) fruits in 2010 and 2011, respectively, showing no significant difference from that of hand self-pollinated flowers (for fruit-set: 90·9 %, n = 33, in 2010; for fruit-set: 94·1 %, n = 51, in 2011) (logistical model, Fig. 2, Table 2). Year and year × treatment interaction were not significant for either seed number or fruit-set (Fig. 2, Table 2).
Fig. 2.
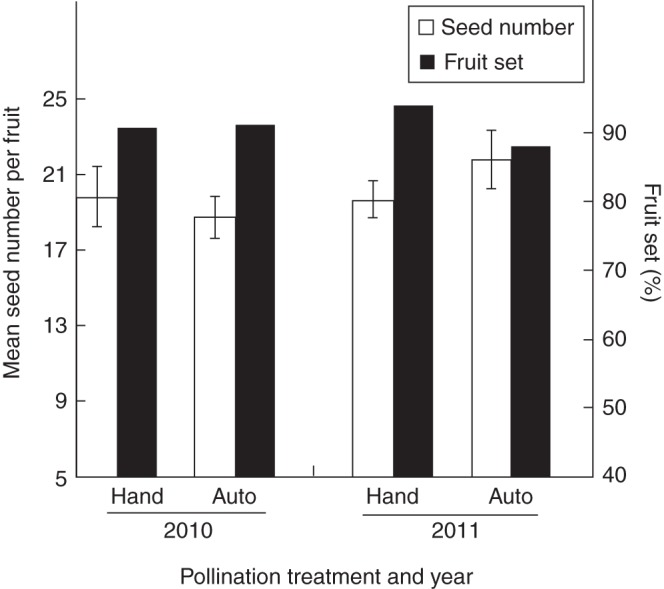
Capacity for autonomous selfing of Roscoea debilis. Autonomous self-pollinated flowers produced as many seeds and fruits as hand self-pollinated flowers in 2010 and 2011.
Table 2.
Results of an analysis of deviance (χ2) and a two-way ANOVA (F) examining the differences in fruit-set and seed number between hand self-pollination and autonomous selfing in Roscoea debilis
| Source | Fruit-set |
Seed number |
||||
|---|---|---|---|---|---|---|
| d.f. | χ2 | P | d.f. | F | P | |
| Year | 1 | 0·0029 | 0·9567 | 1 | 1·28 | 0·2592 |
| Treatment | 1 | 0·0132 | 0·9084 | 1 | <0·001 | 0·9475 |
| Year × treatment | 1 | 0·0199 | 0·8878 | 1 | 0·82 | 0·3663 |
The emasculated and bagged flowers did not set seed, indicating that no apogamy occurs in this species. Hand cross-pollinated flowers set 18·0 ± 1·6 seeds per flower (n = 30) and 88·2 % fruits, as many as hand self-pollinated flowers (19·8 ± 1·6 seeds per flower, n = 30, fruit set: 90·9 %) (for seed number: t-test, t = –0·89, P = 0·376; for fruit set: continuity adjusted chi-square test, χ2 < 0·0001, P = 1, Fig. 3). These results indicate that there was no self-incompatibility in R. debilis. Intact flowers produced as many seeds as hand-pollinated flowers (for seed number: one-way ANOVA, F = 0·89, P = 0·41; for fruit-set: chi-square test, χ2 = 0·2575, P = 0·88, Fig. 3), indicating that there was no pollen limitation in the natural population.
Fig. 3.
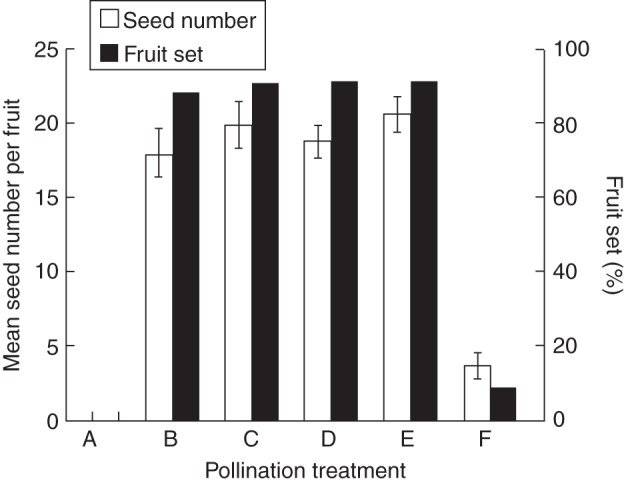
Tests for pollen limitation, self-compatibility and reproductive assurance. Treatments: A, apomixis; B, hand cross-pollination; C, hand self-pollination; D, autonomous self-pollination; E, natural pollination; F, emasculation and open pollination.
Timing of autonomous selfing
There was a significant exponential relationship between the timing of anther removal and mean seed number per flower (non-linear regression model, r = – 0·97, d.f. = 2, F = 170·4, P < 0·0001). A one-way ANOVA also showed that mean seed number per fruit differed significantly among the eight groups (F = 29·8, P < 0·001). Mean seed production per flower increased significantly when the anthers were removed at 75 h after anthesis (flowers wilted) (Fig. 4). This indicates that a majority of the seeds were produced after the flowers wilted.
Fig. 4.
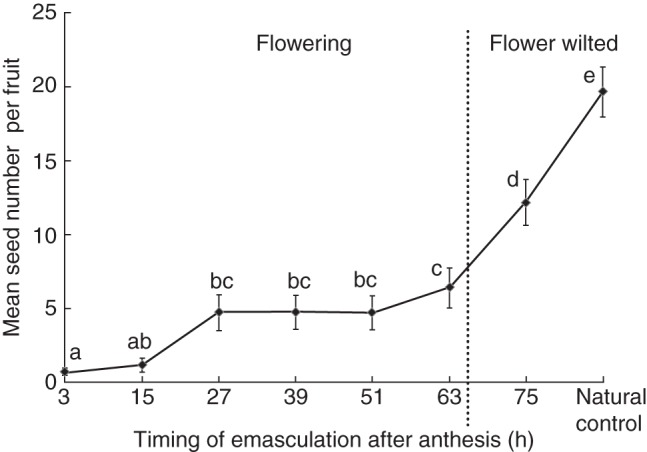
The relationship between the timing of anther removal and seed production by autonomous selfing in Roscoea debilis. Flowers were bagged during the entire flowering period. Values with the same letters indicate that differences are not significant (P > 0·05) using a Duncan post-hoc comparison.
Pollinator observation and reproductive assurance
Observations over two flowering seasons in 2010 and 2011 indicate that visitors to R. debilis are scarce in the natural population. No insects were observed visiting the flowers of R. debilis in patches A, B and C in either 2010 or 2011. In patch D (2011), 4 ± 0·58 visits were recorded by a yellow carpenter bee (Xylocopa confusa, Fig. 1B) per day (n = 3 d). When this carpenter bee landed on the large labellum and entered into the corolla tube to absorb nectar, it pushed against the elongated anther appendage centrally positioned in the entrance of the corolla tube, causing the anther to swing towards the insect's back, thus forming a lever mechanism for pollination. The pollen grains of flowers were left intact on the anthers in patches A, B and C during the entire flowering season.
Seed number (3·7 ± 0·9, n = 3) and fruit-set (9·4 %, n = 32) of emasculated flowers were both significantly lower than those of intact flowers (seed number: 20·5 ± 1·2, n = 53, fruit-set: 91·4 %, n = 58; for seed number: t-test, t = –11·35, P < 0·0001; for fruit set: chi-square test, χ2 = 59·0, P < 0·0001). This result suggests that most of the seed production was a result of autonomous selfing.
DISCUSSION
This study documents a previously unknown mechanism of delayed self-pollination. At the end of the window for pollination, stigmatic fluid forms a large globule and then seeps into the nearby pollen grains, inducing pollen germination and pollen tube elongation into the style. This process provides reproductive assurance when pollinator service is limited.
The autonomous selfing mechanism and reproductive assurance
Unlike most other species of Zingiberaceae in which the stigma is above the anther, the stigma of R. debilis lies in the middle of the anther between the two pollen sacs, and is enclosed by the pollen sacs (Fig. 1C). When the pollen sacs dehisce, the stigma is surrounded by pollen grains (Fig. 1E). Despite the close proximity of the stigma to the pollen grains, few pollen grains dropped into the stigma cup during the entire flowering period, because the flowers that were bagged and emasculated at 61 h after anthesis produced few seeds (Fig. 4). The stigma of R. debilis maintains receptivity during the entire flowering period, similar to two other species of Roscoea (Zhang et al., 2011). Stigmatic fluid is continuously secreted, which accumulates and forms a large globule on the stigma on the third morning of flowering (Fig. 1F). Large globules on the stigma have also been reported from Roscoea and 21 other genera in eight angiosperm families (Heslop-Harrison and Shivanna, 1977). In R. debilis, the large globule seeps into the nearby pollen grains on the fourth flowering day, inducing pollen germination and pollen tube elongation into the style from the corners of the stigma (Fig. 1G, H), and this has not previously been reported. The emasculation experiments also demonstrate that autonomous selfing occurs on the fourth flowering day (Fig. 4). The corolla lobes of wilted flowers directly withered without closure, and thus corolla wilting has little function in facilitating autonomous self-pollination.
The majority of the seeds (>70 %) were produced after the flowers wilted (Fig. 4), so the mode of autonomous selfing is considered as delayed selfing in R. debilis. Bagged flowers have the same seed production as hand self-pollinated flowers, indicating the well-developed capacity for autonomous selfing in the natural population. Intact flowers produce as many seeds as hand pollinated flowers (Fig. 3), suggesting that pollen limitation does not occur in the natural population. Emasculated flowers set significantly fewer fruits and seeds than intact flowers (Fig. 3), indicating that delayed self-pollination ensures seed production when pollinator services are absent or variable. Delayed selfing is generally considered as adaptive and will always be selected, because it provides an opportunity for outcrossing at first, then ensures seed production when outcrossing is unavailable, without pollen or seed discounting (Lloyd, 1992). Thus, a delayed selfing mechanism may be selected in R. debilis even in its original home with abundant pollinators.
The breeding system
This research reveals that R. debilis is a homogamous species, as anthers dehisced and stigmas were receptive at the beginning of anthesis. Hand self-pollinated flowers set as many seeds as hand cross-pollinated flowers, which is an indication that this species is completely self-compatible. The flowers that were emasculated and bagged before anthesis did not set seed, suggesting that apomixis does not occur in this species. All the stigmas of the flowers at each flowering stage were stained dark purple–brown by MTT, indicating the stigma maintains receptivity during the entire flowering period. The pollen-to-ovule ratio (P/O) indicated that R. debilis is facultatively autogamous, according to the standard suggested by Cruden (1977).
Pollination syndromes and pollinator failure
Similar to other Roscoea species, R. debilis retains the characteristics of insect-pollinated flowers, such as the purple, zygomorphic flowers with a landing platform, the lever-like anther appendage and nectar rewards. The character of long, slender corolla tubes (30–110 mm) of Roscoea species suggests a long-tongued insect floral syndrome. Fletcher and Son (1931) and Dierl (1968) observed long-proboscid flies (Corizoneura longirostris) visiting flowers of R. purpurea for nectar in the Himalayas of India and Nepal, which completely supports this pollination syndrome, suggesting that the original pollinators of Roscoea species are long-proboscid insects. Our field observations covering peak-flowering times over two years show that pollinators are scarce in this natural population. Given that all five Roscoea species investigated in the Chinese Himalayas undergo pollinator failure (Zhang and Li, 2008; Zhang et al., 2011), we speculate that pollinator scarcity may occur in other populations of R. debilis in China. Why Roscoea species experience pollinator failure in the Chinese Himalayas is a matter of debate. Zhang et al. (2011) proposed that the original long-proboscid pollinators were lost when Roscoea species spread from north-east India to the Chinese Himalayas, based on the phylogeny and distribution of Roscoea (Ngamriabsakul et al., 2000). According to this assumption, R. debilis is more likely to spread to the areas without pollinators, because the delayed selfing mechanism means that reproduction of R. debilis does not rely on pollinators.
The breakdown of a mutualism might occur when pollinators are lost from a specialized pollination system (Bond, 1994). When the original pollinators of a plant species disappear, unspecialized visitors may fill this vacant niche to reconstruct the pollination system (Schwartz-Tzachor et al., 2006). This situation happens in Himalayan species of Pedicularis (Scrophulariaceae) (Huang and Fenster, 2007) and Roscoea australis. Consequently, we infer that the mutualism between R. debilis and carpenter bees may be under the preliminary stage of reconstruction. However, this is based on limited observations and evidence. To fully understand the breakdown and the reconstruction of the pollination system of Roscoea species, long-term observations and large-scale investigations are needed both in the Indian Himalayas groups and in the Chinese Himalayas groups.
ACKNOWLEDGEMENTS
We thank Dr Jeffrey Karron and two anonymous reviewers for their valuable suggestions, Professor Yang Darong for insect identifications, Pelin Kayaalp and Professor Warren Thomas Kellie for corrections to the English text, and Zhu Xingfu and Dr Dharmalingam Mohandass for comments and helpful suggestions on an earlier draft of the manuscript. This work was supported by the NSFC (no. 31070340) and the CAS/SEFEA International Partnership Program for Creative Research Teams, and the Fund for Top One Hundred Young Scientists of the Chinese Academy of Sciences to Q.-J. Li.
LITERATURE CITED
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Bond W. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1994;344:83–90. [Google Scholar]
- Cowley E. A revision of Roscoea (Zingiberaceae) Kew Bulletin. 1982;36:747–777. [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Dafni A. Pollination ecology: a practical approach. Oxford: Oxford University Press; 1992. [Google Scholar]
- Darwin C. The effects of cross and self-fertilisation in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Dierl W. Zur Nahrungsaufnahme von Corizoneura longirostris (Hardwicke) (Diptera: Tabanidae) Khumbu Himal. 1968;3:76–81. [Google Scholar]
- Duan YW, Dafni A, Hou QZ, He YP, Liu JQ. Delayed selfing in an alpine biennial Gentianopsis paludosa (Gentianaceae) in the Qinghai–Tibetan Plateau. Journal of Integrative Plant Biology. 2010;52:593–599. doi: 10.1111/j.1744-7909.2010.00951.x. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Herlihy CR. Using a cost–benefit approach to understand the evolution of self-fertilization in plants: the perplexing case of Aquilegia canadensis (Ranunculaceae) Plant Species Biology. 2004;19:159–173. [Google Scholar]
- Eckert CG, Schaefer A. Does self-pollination provide reproductive assurance in Aquilegia canadensis (Ranunculaceae)? American Journal of Botany. 1998;85:919–924. [PubMed] [Google Scholar]
- Fenster CB, Marten-Rodriguez S. Reproductive assurance and the evolution of pollination specialization. International Journal of Plant Sciences. 2007;168:215–228. [Google Scholar]
- Fisher R. Average excess and average effect of a gene substitution. Annals of Eugenics. 1941;11:53–63. [Google Scholar]
- Fletcher T, Son S. A veterinary entomology for India, Part XIV. Indian Journal of Veterinary Science and Animal Husbandry. 1931;1:192–199. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics. 2005;36:47–79. [Google Scholar]
- Harder LD, Wilson WG. A clarification of pollen discounting and its joint effects with inbreeding depression on mating system evolution. The American Naturalist. 1998;152:684–695. doi: 10.1086/286199. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison Y, Shivanna K. The receptive surface of the angiosperm stigma. Annals of Botany. 1977;41:1233–1258. [Google Scholar]
- Huang SQ, Fenster CB. Absence of long-proboscid pollinators for long-corolla-tubed Himalayan Pedicularis species: implications for the evolution of corolla length. International Journal of Plant Sciences. 2007;168:325–331. [Google Scholar]
- Ippolito A, Armstrong JE. Floral biology of Hornstedtia scottiana (Zingiberaceae) in a lowland rain-forest of Australia. Biotropica. 1993;25:281–289. [Google Scholar]
- Kalisz S, Vogler D, Fails B, et al. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae) American Journal of Botany. 1999;86:1239–1247. [PubMed] [Google Scholar]
- Klips RA, Snow AA. Delayed autonomous self-pollination in Hibiscus laevis (Malvaceae) American Journal of Botany. 1997;84:48–53. [Google Scholar]
- Lande R, Schemske D. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Liu KW, Liu ZJ, Huang LQ, Li LQ, Chen LJ, Tang GD. Self-fertilization strategy in an orchid. Nature. 2006;441:945–946. doi: 10.1038/441945a. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist. 1979;113:67–79. [Google Scholar]
- Lloyd DG. Self-fertilization and cross-fertilization in plants.II. The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- Lloyd DG, Schoen DJ. Self-fertilization and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences. 1992;153:358–369. [Google Scholar]
- Ngamriabsakul C, Newman MF, Cronk QCB. Phylogeny and disjunction in Roscoea (Zingiberaceae) Edinburgh Journal of Botany. 2000;57:39–61. [Google Scholar]
- Qu R, Li X, Luo Y, Dong M, Xu H, Chen X, Dafni A. Wind-dragged corolla enhances self-pollination: a new mechanism of delayed self-pollination. Annals of Botany. 2007;100:1155–1164. doi: 10.1093/aob/mcm209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Riano T, Dafni A. A new procedure to asses pollen viability. Sexual Plant Reproduction. 2000;12:241–244. [Google Scholar]
- Schoen DJ, Lloyd DG. Self-fertilization and cross-fertilization in plants. III. Methods for studying modes and functional-aspects of self-fertilization. International Journal of Plant Sciences. 1992;153:381–393. [Google Scholar]
- Schwartz-Tzachor R, Dafni A, Potts SG, Eisikowitch D. An ancient pollinator of a contemporary plant (Cyclamen persicum): when pollination syndromes break down. Flora. 2006;201:370–373. [Google Scholar]
- Stebbins G. Self fertilization and population variability in the higher plants. American Naturalist. 1957;91:337–354. [Google Scholar]
- Stebbins G. Flowering plants: evolution above the species level. Cambridge, MA: Belknap Press; 1974. [Google Scholar]
- Sun SG, Guo YH, Gituru R, Huang SQ. Corolla wilting facilitates delayed autonomous self-pollination in Pedicularis dunniana (Orobanchaceae) Plant Systematics and Evolution. 2005;251:229–237. [Google Scholar]
- Vickery RK. How does Mimulus verbenaceus (Phrymaceae) set seed in the absence of pollinators? Evolutionary Biology. 2008;35:199–207. [Google Scholar]
- Wang YQ, Zhang DX, Renner SS, Chen ZY. A new self-pollination mechanism. Nature. 2004;431:39–40. doi: 10.1038/431039b. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Zhang DX, Renner SS, Chen ZY. Self-pollination by sliding pollen in Caulokaempferia coenobialis (Zingiberaceae) International Journal of Plant Sciences. 2005;166:753–759. [Google Scholar]
- Zhang ZQ, Li QJ. Autonomous selfing provides reproductive assurance in an alpine ginger Roscoea schneideriana (Zingiberaceae) Annals of Botany. 2008;102:531–538. doi: 10.1093/aob/mcn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZQ, Kress WJ, Xie WJ, Ren PY, Gao JY, Li QJ. Reproductive biology of two Himalayan alpine gingers (Roscoea spp., Zingiberaceae) in China: pollination syndrome and compensatory floral mechanisms. Plant Biology. 2011;13:582–589. doi: 10.1111/j.1438-8677.2010.00423.x. [DOI] [PubMed] [Google Scholar]