Abstract
Background and Aims
Patterns of ploidy variation among and within populations can provide valuable insights into the evolutionary mechanisms shaping the dynamics of plant systems showing ploidy diversity. Whereas data on majority ploidies are, by definition, often sufficiently extensive, much less is known about the incidence and evolutionary role of minority cytotypes.
Methods
Ploidy and proportions of endoreplicated genome were determined using DAPI (4',6-diamidino-2-phenylindole) flow cytometry in 6150 Gymnadenia plants (fragrant orchids) collected from 141 populations in 17 European countries. All widely recognized European species, and several taxa of less certain taxonomic status were sampled within Gymnadenia conopsea sensu lato.
Key Results
Most Gymnadenia populations were taxonomically and/or ploidy heterogeneous. Two majority (2x and 4x) and three minority (3x, 5x and 6x) cytotypes were identified. Evolution largely proceeded at the diploid level, whereas tetraploids were much more geographically and taxonomically restricted. Although minority ploidies constituted <2 % of the individuals sampled, they were found in 35 % of populations across the entire area investigated. The amount of nuclear DNA, together with the level of progressively partial endoreplication, separated all Gymnadenia species currently widely recognized in Europe.
Conclusions
Despite their low frequency, minority cytotypes substantially increase intraspecific and intrapopulation ploidy diversity estimates for fragrant orchids. The cytogenetic structure of Gymnadenia populations is remarkably dynamic and shaped by multiple evolutionary mechanisms, including both the ongoing production of unreduced gametes and heteroploid hybridization. Overall, it is likely that the level of ploidy heterogeneity experienced by most plant species/populations is currently underestimated; intensive sampling is necessary to obtain a holistic picture.
Keywords: Coexistence, contact zone, cytogeography, flow cytometry, fragrant orchid, Gymnadenia, Orchidaceae, hybridization, mixed-ploidy population, polyploidy, sympatry, unreduced gametes
INTRODUCTION
Polyploidy (the multiplication of complete chromosome sets in somatic cells above the diploid state) is a prominent and recurring process in the evolution of eukaryotic organisms (Otto and Whitton, 2000). Although polyploidy has been documented in all major lineages of eukaryotes, land plants show the highest incidence of polyploidy (Jiao et al., 2011). Karyological evidence suggests that at least 70 and 95 % of angiosperms and ferns, respectively, are polyploid (Masterson, 1994). Genomic data also support the near ubiquity of polyploidy, traces of ancient whole-genome duplication having been detected in virtually all angiosperms (Soltis et al., 2009). The success of polyploid plants can be related to different evolutionary transitions that may alter their genetic composition, phenotypic plasticity or ecological amplitude, and can ultimately lead to increased vigour and competitive superiority over diploid ancestors (Levin, 2002). Polyploid plants can combine genomes of two or more parental species (allopolyploids) or arise from the same parental species (autopolyploids). Whereas allopolyploids have long been assumed to prevail in situ, recent data suggest that the frequency of autopolyploids is much higher than previously considered and they play important evolutionary and ecological roles in natural populations (Soltis et al., 2007; Parisod et al., 2010). Autopolyploid derivates may originate through somatic chromosome doubling, but it is the formation of unreduced gametes that drives the dynamics of their genesis (Bretagnolle and Thompson, 1995; Ramsey and Schemske, 1998).
Although genome duplication is often associated with speciation (Wood et al., 2009), ploidy variation is also observed within traditionally delimited taxonomic species. This is especially true for autopolyploids, which more closely resemble their diploid/lower ploid progenitors than do allopolyploids and so are rarely recognized in formal classifications. For example, chromosomal data for the Californian flora indicate that approx. 13 % of the species listed are ploidy polymorphic and several of them possess more than two different cytotypes (Soltis et al., 2007). Based on a broad survey of species, Wood et al. (2009) reported that 12–13 % of angiosperm species and 17 % of fern species are variable for ploidy. In general, ploidy heterogeneity within species is likely to have been underestimated and is predicted to continue to increase with more intensive sampling. Indeed, ploidy screening across large spatial scales and in a representative number of individuals per population, made possible by the advent of flow cytometry (FCM), has resulted in a substantial increase in the number of ploidy-heterogeneous plant species recognized and in the number of different cytotypes recorded per species (Kron et al., 2007).
Fragrant orchids of the Gymnadenia conopsea aggregate constitute a highly ploidy-variable and taxonomically challenging species complex native to temperate Europe and Asia. Besides the karyological polymorphism (Marhold et al., 2005; Trávníček et al., 2011), members of the complex were also found to vary in morphology (Dworschak, 2002; Marhold et al., 2005; Vöth and Sontag, 2006; R. Bateman et al., unpubl. res.), floral scent biochemistry (Huber et al., 2005; Jersáková et al., 2010), flowering phenology (Soliva and Widmer, 1999; Gustafsson and Lönn, 2003) and preferred habitats (Dworschak, 2002). Investigations into phenotypic and genetic variation have often revealed strong genetic divergence among the recognized taxa but a lower level of morphological differentiation (e.g. Scacchi and de Angelis, 1989; Soliva and Widmer, 1999; Bateman et al., 2003; Gustafsson and Lönn, 2003; Stark et al., 2011; R. Bateman et al., unpubl. res.). Taxonomic delimitation is further complicated by weak pre-zygotic and post-zygotic barriers (Jersáková et al., 2010) that allow frequent formation of spontaneous hybrids at both intrageneric and intergeneric levels (e.g. Hedrén et al., 2000; Lönn et al., 2006).
Setting aside the former genus Nigritella, recent classifications of Gymnadenia in Europe mostly recognize five major taxa at different taxonomic levels, depending on the author's preferred concept. Most recent British authors have followed Bateman et al. (2003) in recognizing all of these taxa as full species, whereas the most influential Continental monographers (e.g. Kreutz, 2004; Delforge, 2006) have treated most of these taxa as varieties only. In addition to the widespread G. conopsea (L.) R.Br. sensu stricto (s.s.), G. densiflora (Wahlenb.) A.Dietr. and G. odoratissima (L.) Rich., G. frivaldii Hampe ex Griseb. is a Balkan endemic only recently confirmed as assignable to Gymnadenia (Bateman et al., 2006). Originally described from a type locality in Cumbria, G. borealis (Druce) R.M.Bateman, Pridgeon & M.W.Chase is regarded by some authors as being confined to Britain and Ireland, though morphologically identical plants also occur along the Scandinavian mountain chain (Strann and Bjerke, 2010). Several local morphotypes with a more questionable taxonomic status have also been described, including the compact, late-flowering G. conopsea var. friesica Schlechter from sand dunes on the Friesian Islands (Schlechter, 1919; Kreutz and Dekker, 2000) and the slender alpine ecotype referred to as G. conopsea var. alpina Rchb.f. ex Beck (1893). Robust plants from the Pyrenees that resemble the short-spurred G. odoratissima but have a spur about one-third longer than the ovary have been recognized as var. pyrenaica (Philippe) P.Delforge (2005). A substantially longer spur is also supposedly diagnostic of G. odoratissima subsp. longicalcarata C.E.Hermosilla & J.Sabando (1996) from northern Spain. Several additional taxa from the Bavarian Alps were recently described on the basis of morphological observations (Dworschak, 2002): G. graminea Dworschak, G. conopsea subsp. serotina (Schönh.) Dworschak, G. splendida Dworschak and G. vernalis Dworschak.
Our previous study (Trávníček et al., 2011) provided new insights into ploidy variation but only at population and regional scales, being confined to the Czech Republic plus Slovakia. We found a surprisingly high proportion of mixed-ploidy populations, consisting of different combinations of two majority and three minority cytotypes. In addition, unique FCM profiles (i.e. different levels of progressively partial endoreplication; see Discussion for detailed explanation) were observed for G. conopsea s.s. and G. densiflora. The present study builds on our previous research, aiming to assess ploidy variation across much larger spatial scales and encompassing all major European Gymnadenia species. Patterns of ploidy variation, both among and within populations, can provide useful insights into the evolutionary mechanisms that shape the dynamics of these polyploid systems.
Specifically, we address the following questions. (1) Which patterns of progressively partial endoreplication can be found among the investigated plants? Is this variation geographically or taxonomically structured? (2) Where is the geographical centre of ploidy variation located? (3) How frequent are mixed-ploidy populations? Do different Gymnadenia taxa differ in this respect? (4) How common and how widespread are minority cytotypes? Do they preferentially occur in populations with a particular composition of majority ploidies?
MATERIALS AND METHODS
Field sampling
Plant samples were collected in 17 European countries between 2004 and 2011, spanning the geographical range 40 °57′N–59 °17′N and 06 °01′W–30 °30′E (for locality details, see Supplementary Data Table S1) and totalling 6150 individuals from 141 populations. The number of localities and individuals sampled for specific countries were as follows: Austria, 9/318; Belgium, 1/26; Bulgaria, 3/36; Estonia, 3/91; France, 20/958; Germany, 20/877; Italy, 10/594; Macedonia, 1/6; The Netherlands, 1/48; Poland, 2/58; Romania, 9/209; Russia, 7/130; Scotland, 19/600; Slovakia, 5/266; Spain, 1/13; Sweden, 15/1348; and Switzerland, 15/572. Although taxonomic revision of the Gymnadenia conopsea aggregate was beyond the scope of this study, we aimed to encompass most of the taxonomic and phenotypic diversity recognized in Europe. In addition to traditionally accepted species, we also sampled known localities for recently described taxa of questionable taxonomic status (e.g. Dworschak, 2002; Supplementary Data Table S1). Due to taxonomic uncertainties, some plants from France with distinct FCM profiles and morphology were not assigned to any particular taxon and instead are provisionally named ‘French diploid’ and ‘French tetraploid’. The taxonomic composition of our data set is summarized in Table 1.
Table 1.
Flow cytometric results for five major European Gymnadenia species and two undetermined taxa from France
| Species | Ploidy level | Relative fluorescence intensity against internal reference standard, Pisum sativum (mean ± s.d.)* | Proportion of replicated genome (mean ± s.d., %)* | No. of FCM analyses | No. of individuals |
|---|---|---|---|---|---|
| G. borealis | 2x | 0·956 ± 0·017c | 53·7 ± 1·7e | 139 | 599 |
| G. conopsea (incl. subsp. serotina p.p., var. alpina, G. graminea, G. splendida p.p., G. vernalis) | 2x | 0·853 ± 0·021f | 58·1 ± 1·9c | 496 | 2114 |
| 4x | 1·588 ± 0·029b | 60·7 ± 2·3b | 161 | 528 | |
| G. densiflora (incl. G. conopsea subsp. serotina p.p., G. conopsea var. friesica, G. splendida p.p.) | 2x | 0·748 ± 0·014g | 74·4 ± 2·4a | 362 | 1538 |
| G. frivaldii | 2x | 0·857 ± 0·031f | 50·8 ± 1·9e | 10 | 32 |
| G. odoratissima | 2x | 0·906 ± 0·019e | 56·8 ± 1·8cd | 106 | 464 |
| French diploid | 2x | 0·923 ± 0·018d | 56·2 ± 1·7d | 163 | 565 |
| French tetraploid | 4x | 1·673 ± 0·026a | 60·6 ± 2·0b | 90 | 192 |
*Different letters indicate groups of taxa that are significantly different at α = 0·05.
Whenever possible, leaf tissue from at least 50 individuals was collected at each locality (the actual number of samples per locality varied from one to 191; Supplementary Data Table S1). The number of samples chosen per locality reflected (1) population size; (2) taxonomic composition (more intensive sampling in mixed-species populations); and (3) morphological/phenological variation (more intensive sampling in populations showing high phenotypic variation or supporting multiple variants with contrasting flowering periods). Leaf tissue was wrapped in moist paper towels, placed in plastic bags and transported rapidly to the FCM laboratory. Because one or more Gymnadenia species rank among threatened plants in several European countries, we preferred images to herbarium specimens as vouchers. Plants were imaged at each locality (Supplementary Data Fig. S2), and herbarium specimens (kept in PRC or CBFS) were taken only from selected representative sites (Supplementary Data Table S1). Because the majority of diagnostic characters are located on floral parts, two flowers per plant were collected at each locality and stored in 70 % ethanol.
Flow cytometry
Relative fluorescence intensities of plant samples were determined by DAPI (4',6-diamidino-2-phenylindole) FCM following the methodology detailed by Trávníček et al. (2011). Up to five individuals were processed together. Each plant was re-analysed separately in cases of mixed-ploidy samples or if the coefficient of variation of either the unknown sample or the internal standard peaks exceeded 5 %. Pisum sativum ‘Ctirad’ (2C = 9·09 pg) was selected as a primary reference standard, as it has a genome size close to, but not overlapping, that of most Gymnadenia samples. Vicia faba ‘Inovec’ served as a reference standard for measurements of G. borealis; the relative nuclear DNA amount of Vicia was calibrated against Pisum (3·14× greater; Suda et al., 2007). Karyologically counted (2n = 40 and 2n = 80) plants of G. conopsea from the Czech Republic were used as reference points when interpreting the FCM results. Some data, such as the incidence of individuals with putatively 50 somatic chromosomes among FCM-screened progeny of our experimental crosses (J. Jersáková et al., unpubl. res.; see also Trávníček et al., 2011) may indicate that x = 10 is the basic chromosome number in the G. conopsea aggregate. Nonetheless, in line with the generally accepted view (e.g. Marhold et al., 2005; Stark et al., 2011), we interpreted here plants with 2n = 40 and 2n = 80 as diploids and tetraploids, respectively, pending any stronger cytological evidence for x = 10.
Statistical analyses
Flow cytometry data were analysed using the SAS 8·1 statistical package (SAS Institute, Cary, NC, USA). Interspecific differences in relative fluorescence intensities and proportions of endoreplicated genome were tested by GLM (general linear model) because of unbalanced data design, and Tukey's procedure was applied to compare mean values.
Binomial multiple regression (LOGISTIC procedure in SAS) was used to test whether polyploids (i.e. 3x–6x) or tetraploids specifically are linked to geographical parameters of sampled populations (latitude, longitude, altitude and their combinations; Manzaneda et al., 2011). The presence/absence of polyploids or tetraploids in populations fitted a binomial distribution, which was therefore used with the logit link function as parameters of the model.
RESULTS
Genome characteristics
Flow cytometric analysis of 6150 plants (Fig. 1) resulted in five distinct groups of fluorescence intensities, corresponding to diploids (5312 individuals; 86·4 %), triploids (94 individuals; 1·5 %), tetraploids (720 individuals; 11·7 %), pentaploids (17 individuals; 0·3 %) and hexaploids (seven individuals; 0·1 %). Table 1 shows FCM characteristics of the majority (2x and 4x) ploidies for five species and two undetermined Gymnadenia taxa. Two groups of tetraploids with significantly different relative nuclear DNA contents were found; one corresponded to G. conopsea s.s. (Trávníček et al., 2011), whereas the other was not assigned to any species; it is provisionally referred to simply as ‘French tetraploid’. Disregarding minority ploidies, all other species were diploid. Their mean relative fluorescence intensities (setting the value for the reference standard P. sativum to unity) varied 1·278-fold, ranging from 0·748 in G. densiflora to 0·956 in G. borealis. With the exception of G. conopsea vs. G. frivaldii, the remaining diploids possessed significantly different relative amounts of nuclear DNA (Table 1). The proportions of endoreplicated genome also differed significantly among several Gymnadenia taxa (Table 1). Gymnadenia frivaldii was the species with the lowest level of progressively partial endoreplication (50·8 % on average), whereas G. densiflora showed the highest level (74·4 % on average). Flow cytometric profiles (a combination of relative fluorescence values together with the proportion of endoreplicated genome) therefore offer a reliable method of distinguishing between all major Gymnadenia species recognized in the more accurate of the recent European classifications.
Fig. 1.
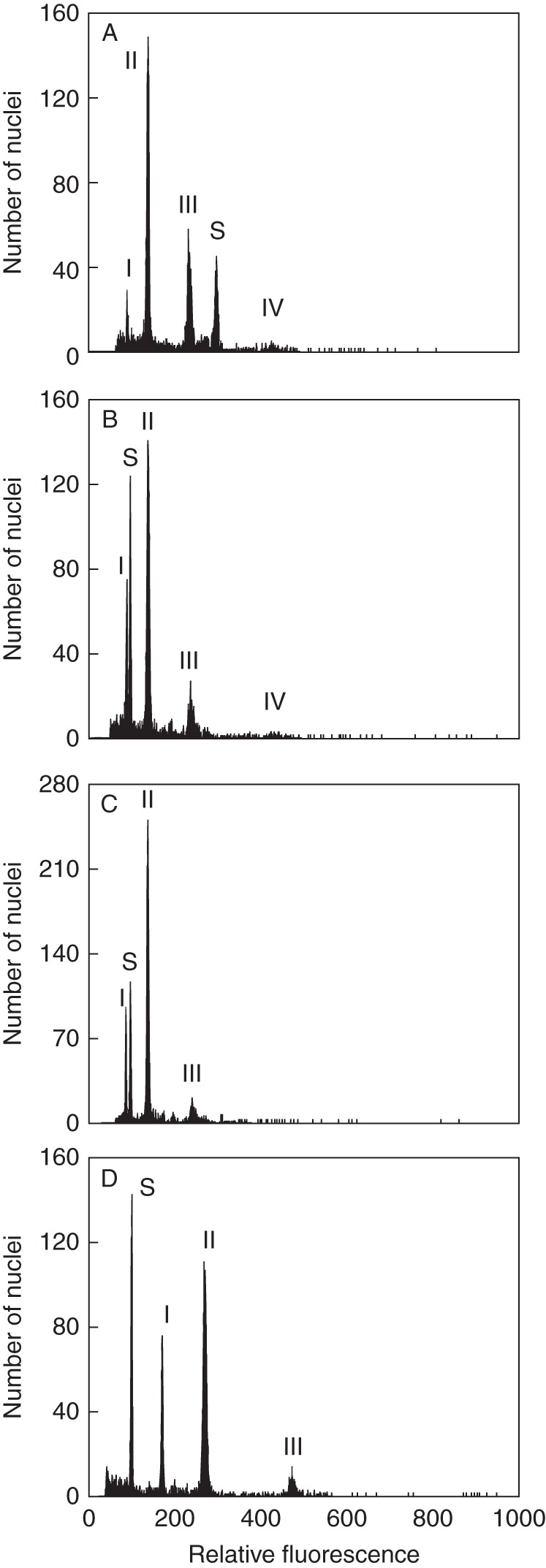
Representative flow cytometric histograms of the studied Gymnadenia taxa (analysed together with the internal reference standard). Nuclei of both the sample and standard were isolated, stained with DAPI and simultaneously run on the flow cytometer. (A) Diploid G. borealis (loc. GB05) – ratios between individual Gymnadenia peaks 1 : 1·54 : 2·63 : 4·81; (B) diploid G. odoratissima (loc. IT05) – peak ratios 1 : 1·56 : 2·71 : 4·91; (C) French diploid (loc. FR04) – peak ratios 1 : 1·58 : 2·78; (D) French tetraploid (loc. FR 04) – peak ratios 1 : 1·58 : 2·78. I, II, III and IV, peaks of Gymnadenia nuclei undergoing different numbers of partial endoreplication cycles. S, internal standard: Vicia faba in (A), Pisum sativum in (B–D).
Cytogeography and population structure
Half of the Gymnadenia populations sampled (71 of 141) were deemed complex in terms of species composition, karyological variation or both (Table 2). Up to three different taxa and five different cytotypes coexisted at a single site. In total, we found 22 different species–majority ploidy combinations (Table 2), and the frequent occurrence of one or more minority cytotypes further increased the intrapopulation heterogeneity. Diploids and tetraploids were recorded in 133 and 25 populations, respectively; however, only 83 and four populations, respectively, were homogeneous for ploidy. The most common type of ploidy mixture involved sympatry of diploids and triploids, suggesting regular formation of unreduced gametes. Some form of ploidy variation was observed in 54 (38·3 %) populations; two, three and four different cytotypes coexisted in 40, ten and three populations, respectively. All five cytotypes grew together in population FR04 near Sainte-Maure-de-Touraine in France (Supplementary Data Table S1), which also maintained two coexisting taxa. In total, more than two taxa were observed in nearly one-third (41) of the populations analysed, the most common combination being 2x G. conopsea, 2x G. densiflora and 2x G. odoratissima (ten populations), followed by sympatry of the two former species (nine populations; Table 2).
Table 2.
Taxonomic and ploidy composition of the 141 Gymnadenia populations investigated
| No. of populations for a given taxonomic composition harbouring minority cytotypes |
||||||||
|---|---|---|---|---|---|---|---|---|
| Taxonomic composition (majority ploidies) | Total no. of populations with the given taxonomic composition | 3x | 5x | 6x | 3x + 5x | 5x + 6x | 3x + 6x | 3x + 5x + 6x |
| 2C | 34 | 9 | ||||||
| 2D | 21 | 6 | ||||||
| 2O | 2 | |||||||
| 2Sp | 8 | 3 | ||||||
| 2B | 19 | 1 | ||||||
| 2F | 2 | 1 | ||||||
| 2C + 2D | 9 | 3 | ||||||
| 2C + 2O | 6 | 2 | ||||||
| 2C + 2Sp | 2 | 2 | ||||||
| 2D + 2Sp | 1 | |||||||
| 2O + 2Sp | 1 | |||||||
| 2C + 2D + 2O | 10 | 6 | ||||||
| 2C + 2D + 2Sp | 1 | |||||||
| 4C | 7 | 1 | 1 | 1 | ||||
| 4Sp | 1 | 1 | ||||||
| 4C + 2C | 6 | 3 | 1 | 1 | ||||
| 4C + 2D | 4 | 1 | 1 | |||||
| 4C + 2C + 2D | 2 | 1 | 1 | |||||
| 4C + 2C + 2O | 1 | 1 | ||||||
| 4C + 2D + 2O | 1 | 1 | ||||||
| 4Sp + 2Sp | 2 | 1 | ||||||
| 4Sp + 2Sp + 2D | 1 | 1 | ||||||
2B, 2x G. borealis; 2C, 2x G. conopsea; 2D, 2x G. densiflora; 2F, 2x G. frivaldii; 2O, 2x G. odoratissima; 2Sp, undetermined diploid from France; 4C, 4x G. conopsea; 4Sp, undetermined tetraploid from France.
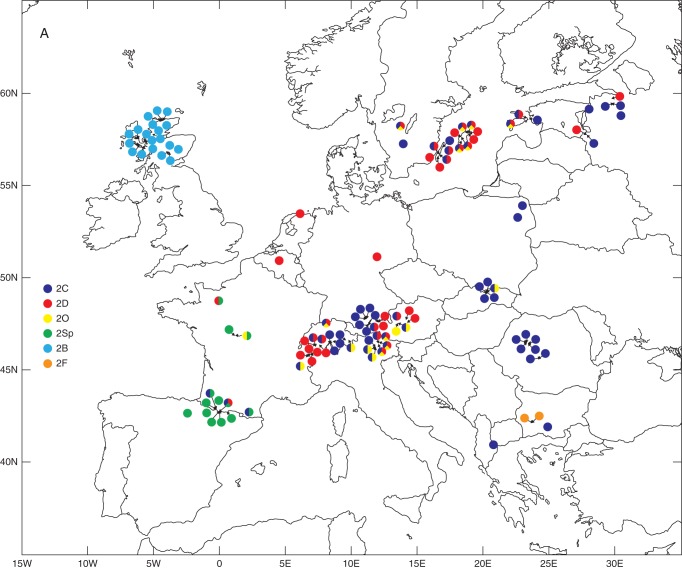
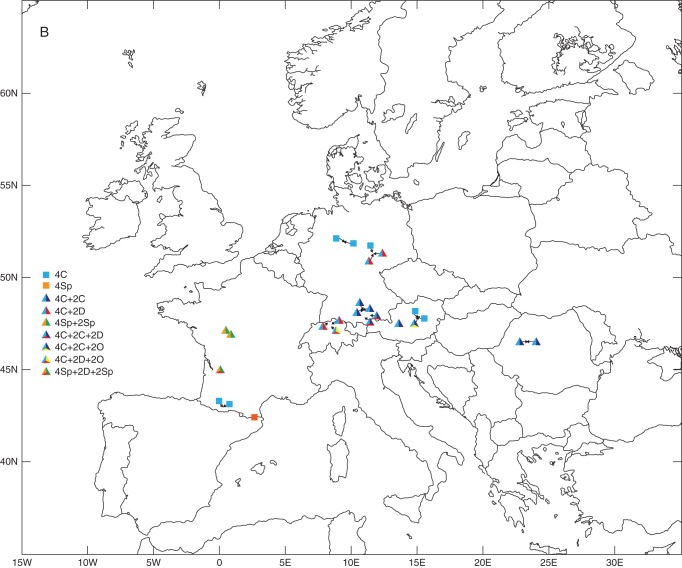
Diploids were recorded in all 17 countries (Fig. 2A), whereas tetraploids were restricted to just five of these countries: Austria, France, Germany, Romania and Switzerland (Fig. 2B). The multiple regression analysis showed a significant negative relationship between the incidence of polyploids and latitude (β ± s.d. = –0·294 ± 0·095; d.f. 1,133; P = 0·0021). Tetraploids were strongly negatively associated with latitude and also less strongly with altitude (β ± s.d. = –0·607 ± 0·188; d.f. 1,133; P = 0·0013 and β ± s.d. = –0·036 ± 0·018; d.f. 1,133; P = 0·0433, respectively).
Fig. 2.
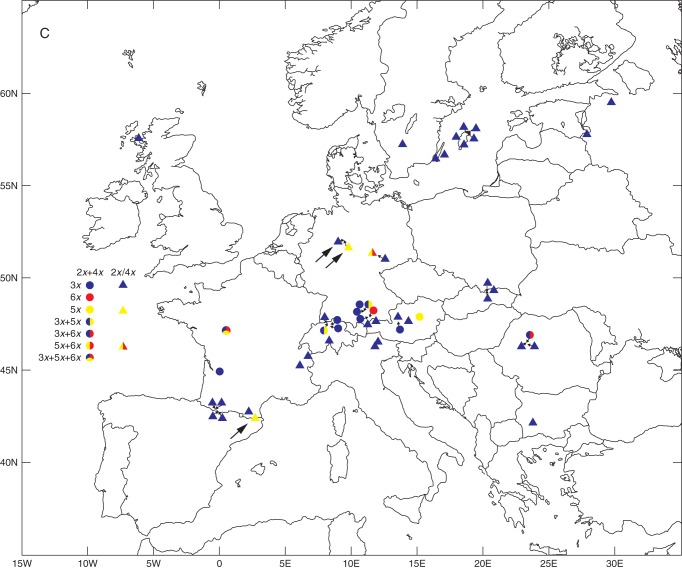
Ploidy variation and taxonomic composition of 141 studied populations of the Gymnadenia conopsea complex in Europe. (A) Diploid populations (either ploidy-uniform or with the presence of minority cytotypes). Intrapopulation taxonomic heterogeneity is indicated by mixed colours. (B) Tetraploid (squares) and mixed 2x–4x (triangles) populations. Intrapopulation taxonomic heterogeneity is indicated by mixed colours. (C) Populations harbouring minority cytotypes (3x, blue; 5x, yellow; 6x, red). The presence of both majority ploidies (2x and 4x) is illustrated by a circle, whereas triangles illustrate exclusive di- or tetraploid populations. Co-occurrence of different minority cytotypes is indicated by mixed colours. Arrows indicate populations in which an additional cytotype (most probably diploid) is predicted (sympatry of 3x + 4x or 4x + 5x). Taxa abbreviations in (A) and (B): 2B, 2x G. borealis; 2C, 2x G. conopsea; 2D, 2x G. densiflora; 2F, 2x G. frivaldii; 2O, 2x G. odoratissima; 2Sp, undetermined diploid from France; 4C, 4x G. conopsea; 4Sp, undetermined tetraploid from France.
Minority cytotypes
Minority ploidies constituted <2 % of all samples, but they were present in more than one-third (50 of 141) of our study populations, distributed across the area investigated (Table 2, Fig. 2C). Triploids, pentaploids and hexaploids occurred in 45, seven and four populations, respectively. Although it is difficult to determine the taxonomic identity of minority cytotypes in multispecies populations, our data indicate that they were formed in all widely recognized taxa (Table 2). Most triploids were recorded in otherwise exclusively diploid populations (33 populations), although in 11 populations they co-occurred with diploids and tetraploids. Significantly higher proportions of triploid individuals occurred in mixed 2x–4x populations than in otherwise uniform 2x populations (Mann–Whitney U-test: 6·9 % vs 3·2 %, n = 44, P = 0·0037 and 7·1 % vs 2·2 %, n = 39, P <0·001, as assessed for, respectively, all populations and only populations yielding >30 analysed individuals). These observations suggest that, in addition to the formation of unreduced gametes, interploidy hybridization was also involved in the genesis of triploids. This inference can also be reached from the proportion of populations of different ploidy composition that harboured triploids; although triploids were present in 64·7 % of 2x–4x populations, this proportion fell to 28·4 % if only 2x populations were considered. Higher polyploids (5x and 6x) were always associated with tetraploids, and in six out of nine of these populations, diploids were also present.
DISCUSSION
This study represents by far the most comprehensive investigation of ploidy variation in the G. conopsea complex in terms of taxonomic coverage, geographical scale and the number of cytotyped plants.
Genome characteristics
Somatic tissues of at least some orchids are known to undergo ‘progressively partial endoreplication’, a phenomenon that was first described in Vanilla planifolia by Bory et al. (2008). Unlike conventional whole-genome endoreplication, which has been documented in plant species from a range of families (Barow, 2006), only part of the genome is duplicated during progressively partial endoreplication. Consequently, the ratio between the first and second peaks in FCM histograms is substantially less than 2:1. Previously (Trávníček et al., 2011), we observed differences in the proportion of endoreplicated genome between the two Gymnadenia species native to the Czech Republic and Slovakia, G. conopsea (mean value 58·5 %) and G. densiflora (mean value 74·7 %). The present study confirmed the validity of interspecific differences between G. conopsea and G. densiflora across Europe (Table 1) and revealed new species-specific profiles for G. borealis (53·7 % of endoreplicated genome) and G. frivaldii (50·8 % of endoreplicated genome). With the exception of G. frivaldii, there is a negative relationship between the proportion of endoreplicated genome and the total amount of nuclear DNA (Table 1). It is therefore possible that the level of endoreplication has an adaptive role and contributes to shaping, either directly or indirectly, optimal genome size and/or cell size (Gregory, 2005).
Genome characteristics of the less well known taxa (e.g. Dworschak, 2002) were indistinguishable from those of the major Gymnadenia species. Because their morphological delineation also remains ambiguous, we have provisionally synonymized G. conopsea var. alpina, G. graminea and G. vernalis with the nominate variety of G. conopsea and G. conopsea var. friesica with G. densiflora. On the basis of FCM results, individuals corresponding to G. conopsea subsp. serotina and G. splendida sensu Dworschak (2002) were classified as either G. conopsea or G. densiflora (Table 1).
Cytogeography and population structure
The results provided new insights into cytotype variation at different spatial scales, from transcontinental to intrapopulational. Five different ploidies (2x, 3x, 4x, 5x, and 6x) were found among the present samples, reflecting our previous smaller scale study confined to the Czech Republic and Slovakia (Trávníček et al., 2011). (Note that previously we referred to these cytotypes as tetraploid, hexaploid, octoploid, etc.; Trávníček et al., 2011.) The evolution of the G. conopsea complex proceeded mostly at the diploid level, which was detected in all five recognized species plus one undetermined taxon (Table 1, Fig. 2A, Supplementary Data Fig. S1A). Tetraploids were more restricted, both taxonomically and spatially. Although polyploidy is generally more frequent at higher latitudes (Brochmann et al., 2004), the binomial multiple regression provided evidence that tetraploids (and polyploids in general) in Gymnadenia tended to occur in southern parts of the investigated area. The most common category of tetraploids corresponded to G. conopsea; it extends latitudinally from its centre of distribution in Central Europe at least as far as France and Romania (Fig. 2B, Supplementary Data Fig. S1B). France is also the home of tetraploids that possess slightly larger amounts of nuclear DNA and were not assigned by us to a particular pre-existing species. Potentially, they may correspond to G. conopsea var. pyrenaica (a full species according to Bournérias and Prat, 2005), but for the present we refrain from any taxonomic conclusion. Most published records of tetraploid fragrant orchids have been made in Austria (Groll, 1965; Mrkvička, 1993; Marhold et al., 2005; Stark et al., 2011), Germany (Wegener, 1966; Stark et al., 2011) and the Czech Republic and Slovakia (Marhold et al., 2005; Trávníček et al., 2011). More recently, Stark et al. (2011) observed tetraploids at one locality in France, Heusser (1938) having earlier reported this cytotype from Switzerland. Our new discoveries from two sites in Romania (Fig. 2B, Supplementary Data Fig. S1B), and published counts from the Caucasus (Sokolovskaya and Strelkova, 1940) and Armenia (Torosyan, 1990), demonstrate that tetraploids extend from Central to Eastern Europe and further into Asia Minor. In contrast, they appear to be absent from northern Europe, as we did not find any tetraploid plants among samples from Sweden, Estonia or Russia.
Several species can co-occur at the sample locality, in particular when different microhabitats are present; we observed two and three different Gymnadenia taxa at 28 and 13 sites, respectively (Table 2). Mixed-species populations clearly prevailed in G. odoratissima (90·5 %) and G. densiflora (58·0 %), and were also common in G. conopsea (43·4 %). The coexistence of multiple species opens up obvious possibilities for interspecific hybridization. We occasionally observed morphotypes intermediate between 2x G. conopsea and 2x G. odoratissima (e.g. localities IT04, IT06; Supplementary Data Table S1). In addition, a few plants from mixed populations of G. conopsea and G. densiflora yielded unusual FCM profiles that might indicate hybridization (e.g. population AT07 from the Dachstein Mts.; Supplementary Data Table S1). Such individuals were excluded from the present study and will be subjected to further investigation using detailed molecular techniques. Although only species-uniform populations of G. borealis and G. frivaldii were recorded in our study, we regard this outcome as an artefact of sampling; only two populations were available for G. frivaldii and all of our numerous collections of G. borealis originated from Scotland. Mixed sites of G. borealis and G. conopsea have been reported from more southern parts of the UK (Campbell et al., 2007). However, the only mixed-species populations in Britain and Ireland detected by one of us during 35 years of fieldwork involved G. borealis plus G. densiflora in central Scotland and G. borealis plus G. conopsea s.s. in western Ireland (R. Bateman et al., unpubl. res.).
Minority cytotypes
Large-scale population screenings, made possible by FCM, have changed our perception of intraspecific and intrapopulation ploidy heterogeneity (Kron et al., 2007; Suda et al., 2007). Previously overlooked minority cytotypes (often occurring at frequencies <1 %), such as odd ploidy levels or high polyploids, have recently been discovered in several plant species; these include Parasenecio auriculata (0·4 % triploids; Nakagawa, 2006), Vicia cracca (0·1 % triploids; Trávníček et al., 2010), Actinidia chinensis (0·6 % pentaploids; Li et al., 2010), Pilosella officinarum (0·3 % heptaploids; Mráz et al., 2008) and Senecio carniolicus (0·1, 0·7, 0·1 and 0·1 % tri-, penta-, hepta- and nonaploids, respectively; Sonnleitner et al., 2010).
Three minority cytotypes (3x, 5x and 6x) with a cumulative frequency of approx. 2·7 % have also been found in the G. conopsea complex in the Czech Republic and Slovakia (Trávníček et al., 2011). The substantial extension of the investigated area and much more intensive sampling in the present study did not lead to the discovery of further minority cytotypes. However, although the minority ploidies accounted for only 1·9 % of all samples (118 out of 6150 individuals), they markedly increased estimates of both intraspecific and intrapopulation variation. Without minority cytotypes, only one species (G. conopsea) and 17 out of 141 populations (approx. 12 %) would be categorized as mixed ploidy. In reality, however, ploidy variation (mostly caused by the incidence of minority cytotypes) occurred in all recognized taxa and in 54 (approx. 38 %) study populations (Table 2). The number of populations with sympatric 2x + 3x cytotypes was almost double the number of populations where the two majority ploidies (2x + 4x) co-occurred (33 vs. 17). In addition, rare triploids also occupied much wider ranges in Europe than their more common tetraploid counterparts (cf. Fig. 2B, C; and Supplementary Data Fig. S1B, C).
A recent survey of ploidy diversity in natural plant populations (Husband et al., 2012) revealed that although mixed-ploidy sites occur commonly in some species (e.g. Burton and Husband, 1999; Sonnleitner et al., 2010), this pattern largely reflects the coexistence of two or more majority ploidies. Gymnadenia is thus far unique in that it is the incidence of rare minority cytotypes that largely drives intrapopulation ploidy variation. One of the few plant systems known to possess a similar population structure is the daisy Aster amellus (Mandáková and Münzbergová, 2006), which, however, maintains a much lower proportion of populations that show sympatry of a majority and a minority cytotype.
Conclusions
Although several chromosomal counts have been published for the G. conopsea aggregate (e.g. Marhold et al., 2005, and references therein), only large data sets such as that presented here, requiring a sampling scheme that is both extensive (many sites throughout the distribution range) and intensive (many plants per site), can generate a genuinely holistic picture of ploidy variation of complex systems and thereby provide deeper insights into the population dynamics of the studied systems. We have shown that most Gymnadenia populations exhibit considerable cytogenetic (and, to a lesser degree, taxonomic) heterogeneity, which should be considered in any future research to avoid biases introduced by pooling data from coexisting but nonetheless cytogenetically distinct populations. We suggest that ongoing production of unreduced gametes in the majority (2x and 4x) cytotypes, together with their hybridization in contact zones, led to the establishment of the minority ploidies (3x, 5x and 6x). All of the minority cytotypes occur only at low frequencies. We assume that they most probably always originate de novo and that their reproductive potential is limited. Nonetheless, minority cytotypes substantially increase intraspecific and intrapopulation ploidy diversity estimates for fragrant orchids. Our ongoing research aims to explore, using morphometric, molecular and experimental approaches, the evolutionary history of populations with ploidy heterogeneity and mechanisms maintaining co-occurring mixtures of cytotypes.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank L. Berger, J. Cambecèdes, W. Dworschak, O. Gerbaud, S. Gustafsson, J.-M. Lewin, W. Mohrmann, D. Prat, M.-A. Selosse, M. Štech, T. Urfus and E. Vicherová for their help in the field. We are grateful to the Nature Conservation Agencies of Aargau, Grison, Ticino and Valais cantons in Switzerland, National Park Schiermonnikoog, Conservatoire Botanique National des Pyrénées et de Midi-Pyrénées and Landesamt für Umweltschutz Sachsen-Anhalt for issuing collection permits. This study was supported by the Czech Science Foundation (project 206/09/0843). Additional support was provided by the Academy of Science of the Czech Republic (long-term research development project no. RVO 67985939) and institutional resources of the Ministry of Education, Youth and Sports of the Czech Republic for the support of science and research, the Grant Agency of University of South Bohemia (projects GAJU 145/2010/P and GAJU 064/2010/Z), the Estonian Science Foundation (grant 8584) and Herbarium TAA.
LITERATURE CITED
- Barow M. Endopolyploidy in seed plants. BioEssays. 2006;28:271–281. doi: 10.1002/bies.20371. [DOI] [PubMed] [Google Scholar]
- Bateman RM, Hollingsworth PM, Preston J, Luo Y-B, Pridgeon AM, Chase MW. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Botanical Journal of the Linnean Society. 2003;142:1–40. [Google Scholar]
- Bateman RM, Rudall PJ, James KE. Phylogenetic context, generic affinities and evolutionary origin of the enigmatic Balkan orchid Gymnadenia frivaldii Hampe ex Griseb. Taxon. 2006;55:107–118. [Google Scholar]
- Beck G. Flora von Niederösterreich. 1893 II/2. Wien. [Google Scholar]
- Bory S, Catrice O, Brown S, et al. Natural polyploidy in Vanilla planifolia (Orchidaceae) Genome. 2008;51:816–826. doi: 10.1139/G08-068. [DOI] [PubMed] [Google Scholar]
- Bournérias M, Prat D, editors. Les Orchidées de France, Belgique et Luxembourg. 2nd edn. Mèze: Biotope Press; 2005. [Google Scholar]
- Bretagnolle F, Thompson JD. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytologist. 1995;129:1–22. doi: 10.1111/j.1469-8137.1995.tb03005.x. [DOI] [PubMed] [Google Scholar]
- Brochmann C, Brysting AK, Alsos IG, et al. Polyploidy in arctic plants. Biological Journal of the Linnean Society. 2004;82:521–536. [Google Scholar]
- Burton TL, Husband BC. Population cytotype structure in the polyploid Galax urceolata (Diapensiaceae) Heredity. 1999;82:381–390. doi: 10.1038/sj.hdy.6884910. [DOI] [PubMed] [Google Scholar]
- Campbell VV, Rowe G, Beebee TJC, Hutchings MJ. Genetic differentiation amongst fragrant orchids (Gymnadenia conopsea s.l.) in the British Isles. Botanical Journal of the Linnean Society. 2007;155:349–360. [Google Scholar]
- Delforge P. Guide des Orchidées d'Europe, d'Afrique du Nord et du Proche-Orient. 3rd edn. Lausanne: Delachaux & Niestlé; 2005. [Google Scholar]
- Delforge P. Orchids of Europe, North Africa and the Middle East. London: A & C Black; 2006. [Google Scholar]
- Dworschak W. Gliederung der verschiedenen Erscheinungsformen der Mücken-Händelwurz in Südbayern. Jahresberichte des Naturwissenschaftlichen Vereins Wuppertal. 2002;55:27–45. [Google Scholar]
- Gregory TR. The evolution of the genome. Burlington: Elsevier Academic Press; 2005. [Google Scholar]
- Groll M. Fruchtansatz, Bestäubung und Merkmalsanalyse bei diploiden und polyploiden Sippen von Dactylorchis (Orchis) maculata und Gymnadenia conopsea. Österreichische Botanische Zeitschrift. 1965;112:657–700. [Google Scholar]
- Gustafsson S, Lönn M. Genetic differentiation and habitat preference of flowering-time variants within Gymnadenia conopsea. Heredity. 2003;91:284–292. doi: 10.1038/sj.hdy.6800334. [DOI] [PubMed] [Google Scholar]
- Hedrén M, Klein E, Teppner H. Evolution of polyploids in the European orchid genus Nigritella: evidence from allozyme data. Phyton-Annales Rei Botanicae A. 2000;40:239–275. [Google Scholar]
- Hermosilla CE, Sabando J. Notas sobre orquídeas II. Estudios del Museo de Ciencias Naturales de Álava. 1996:123–128. 10–11. [Google Scholar]
- Heusser K. Chromosomenverhältnisse bei schweizerischen basitonen Orchideen. Berichte der Schweizerischen Botanischen Gesellschaft. 1938;48:562–605. [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae) Oecologia. 2005;142:564–575. doi: 10.1007/s00442-004-1750-9. [DOI] [PubMed] [Google Scholar]
- Husband BC, Baldwin SJ, Suda J. The incidence of polyploidy in natural plant populations: major patterns and evolutionary processes. In: Leitch IJ, Greilhuber J, Doležel J, Wendel JF, editors. Plant genome diversity. Vol. 2. New York: Springer Verlag (in press); 2012. [Google Scholar]
- Jersáková J, Castro S, Sonk N, et al. Absence of pollinator-mediated premating barriers in mixed-ploidy populations of Gymnadenia conopsea s.l. (Orchidaceae) Evolutionary Ecology. 2010;24:1199–1218. [Google Scholar]
- Jiao YN, Wickett NJ, Ayyampalayam S, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- Kreutz CAJ. Kompendium der Europäischen Orchideen. Landgraaf: Kreutz Publishers; 2004. [Google Scholar]
- Kreutz CAJ, Dekker H. De orchideeën van Nederland – ecologie, verspreiding, bedreiging, beheer. Raalte and Landgraaf: BJ Seckel and CAJ Kreutz; 2000. [Google Scholar]
- Kron P, Suda J, Husband BC. Applications of flow cytometry to evolutionary and population biology. Annual Review of Ecology, Evolution, and Systematics. 2007;38:847–876. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. New York: Oxford University Press; 2002. [Google Scholar]
- Li D, Liu Y, Zhong C, Huang H. Morphological and cytotype variation of wild kiwifruit (Actinidia chinensis complex) along an altitudinal and longitudinal gradient in central-west China. Botanical Journal of the Linnean Society. 2010;164:72–83. [Google Scholar]
- Lönn M, Alexandersson R, Gustafsson S. Hybrids and fruit set in a mixed flowering-time population of Gymnadenia conopsea (Orchidaceae) Hereditas. 2006;143:222–228. doi: 10.1111/j.2006.0018-0661.01958.x. [DOI] [PubMed] [Google Scholar]
- Mandáková T, Münzbergová Z. Distribution and ecology of cytotypes of the Aster amellus aggregate in the Czech Republic. Annals of Botany. 2006;98:845–856. doi: 10.1093/aob/mcl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzaneda AJ, Rey PJ, Bastida JM, Weiss-Lehman CW, Raskin E, Mitchell-Olds T. Environmental aridity is associated with cytotype segregation and polyploidy occurrence in Brachypodium distachyon (Poaceae) New Phytologist. 2011;193:797–805. doi: 10.1111/j.1469-8137.2011.03988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhold K, Jongepierová I, Krahulcová A, Kučera J. Morphological and karyological differentiation of Gymnadenia densiflora and G. conopsea in the Czech Republic and Slovakia. Preslia. 2005;77:159–176. [Google Scholar]
- Masterson J. Stomatal size in fossil plants – evidence for polyploidy in majority of angiosperms. Science. 1994;264:421–424. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- Mráz P, Šingliarová B, Urfus T, Krahulec F. Cytogeography of Pilosella officinarum (Compositae): altitudinal and longitudinal differences in ploidy level distribution in the Czech Republic and Slovakia and the general pattern in Europe. Annals of Botany. 2008;101:59–71. doi: 10.1093/aob/mcm282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkvička AC. Statistische Untersuchungen an Gymnadenia conopsea (L.) R. Br. s.l. Mitteilungsblatt Arbeitskreis Heimische Orchideen Baden-Württemberg. 1993;25:361–367. [Google Scholar]
- Nakagawa M. Ploidy, geographical distribution and morphological differentiation of Parasenecio auriculata (Senecioneae; Asteraceae) in Japan. Journal of Plant Research. 2006;119:51–61. doi: 10.1007/s10265-005-0239-x. [DOI] [PubMed] [Google Scholar]
- Otto SP, Whitton J. Polyploid incidence and evolution. Annual Review of Genetics. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- Parisod C, Holderegger R, Brochmann C. Evolutionary consequences of autopolyploidy. New Phytologist. 2010;186:5–17. doi: 10.1111/j.1469-8137.2009.03142.x. [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Schemske W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Scacchi R, De Angelis G. Isoenzyme polymorphism in Gymnadenia conopsea and its inferences for systematics within this species. Biochemical Systematics and Ecology. 1989;17:25–33. [Google Scholar]
- Schlechter FRR. Mitteilungen über europäische und mediterrane Orchideen II. Feddes Repertorium. 1919;16:279. [Google Scholar]
- Sokolovskaya AP, Strelkova OS. Kariologicheskoe issledovanie vysokogornoi flory Glavnogo Kavkazskogo khrebta i problema geograficheskogo rasprostraneniya poliploidov. Doklady Akademii Nauk SSSR. 1940;29:413–416. [Google Scholar]
- Soliva M, Widmer A. Genetic and floral divergence among sympatric populations of Gymnadenia conopsea s.l. (Orchidaceae) with different flowering phenology. International Journal of Plant Sciences. 1999;160:897–905. doi: 10.1086/314192. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, et al. Polyploidy and angiosperm diversification. American Journal of Botany. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Schemske DW, et al. Autopolyploidy in angiosperms: have we grossly underestimated the number of species? Taxon. 2007;56:13–30. [Google Scholar]
- Sonnleitner M, Flatscher R, García PE, et al. Distribution and habitat segregation on different spatial scales among diploid, tetraploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps. Annals of Botany. 2010;106:967–977. doi: 10.1093/aob/mcq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark C, Michalski SG, Babik W, Winterfeld G, Durka W. Strong genetic differentiation between Gymnadenia conopsea and G. densiflora despite morphological similarity. Plant Systematics and Evolution. 2011;293:213–226. [Google Scholar]
- Strann KB, Bjerke JW. Orkideer i Nord-Norge. Østfold: Arctic Research and Consulting DA; 2010. [Google Scholar]
- Suda J, Kron P, Husband BC, Trávníček P. Flow cytometry and ploidy: applications in plant systematics, ecology and evolutionary biology. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Analysis of genes, chromosomes and genomes. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2007. pp. 103–130. [Google Scholar]
- Torosyan GK. Chromosome numbers of some Armenian Orchidaceae. Biologicheskii Zhurnal Armenii. 1990;43:256–259. [Google Scholar]
- Trávníček P, Eliášová E, Suda J. The distribution of cytotypes of Vicia cracca in Central Europe: the changes that have occurred over the last four decades. Preslia. 2010;82:149–163. [Google Scholar]
- Trávníček P, Kubátová B, Čurn V, et al. Remarkable coexistence of multiple cytotypes of the Gymnadenia conopsea aggregate (the fragrant orchid): evidence from flow cytometry. Annals of Botany. 2011;107:77–87. doi: 10.1093/aob/mcq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöth W, Sontag S. Die intraspezifischen Varietäten der Gymnadenia conopsea (L.) R. Br. Journal Europäischer Orchideen. 2006;38:581–624. [Google Scholar]
- Wegener KA. Ein Beitrag zur Zytologie von Orchideen aus dem Gebiet der DDR. Wissenschaftliche Zeitschrift der Ernst-Moritz-Arndt-Universität Greifswald, Mathematisch-Naturwissenschaftliche Reihe. 1966;15:1–7. [Google Scholar]
- Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. The frequency of polyploid speciation in vascular plants. Proceedings of the National Academy of Sciences, USA. 2009;106:13875–13879. doi: 10.1073/pnas.0811575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.