Abstract
Background and Aims
Photosynthetic thermotolerance (PT) is important for plant survival in tropical and sub-tropical savannas. However, little is known about thermotolerance of tropical and sub-tropical wild plants and its association with leaf phenology and persistence. Longer-lived leaves of savanna plants may experience a higher risk of heat stress. Foliar Ca is related to cell integrity of leaves under stresses. In this study it is hypothesized that (1) species with leaf flushing in the hot-dry season have greater PT than those with leaf flushing in the rainy season; and (2) PT correlates positively with leaf life span, leaf mass per unit area (LMA) and foliar Ca concentration ([Ca]) across woody savanna species.
Methods
The temperature-dependent increase in minimum fluorescence was measured to assess PT, together with leaf dynamics, LMA and [Ca] for a total of 24 woody species differing in leaf flushing time in a valley-type savanna in south-west China.
Key Results
The PT of the woody savanna species with leaf flushing in the hot-dry season was greater than that of those with leaf flushing in the rainy season. Thermotolerance was positively associated with leaf life span and [Ca] for all species irrespective of the time of flushing. The associations of PT with leaf life span and [Ca] were evolutionarily correlated. Thermotolerance was, however, independent of LMA.
Conclusions
Chinese savanna woody species are adapted to hot-dry habitats. However, the current maximum leaf temperature during extreme heat stress (44·3 °C) is close to the critical temperature of photosystem II (45·2 °C); future global warming may increase the risk of heat damage to the photosynthetic apparatus of Chinese savanna species.
Keywords: Photosynthetic thermotolerance, foliar calcium concentration, chlorophyll fluorescence, critical temperature, leaf life span, leaf mass per area, thermostability, woody savanna species, global warming
INTRODUCTION
Savannas consist of a discontinuous upper layer of trees above a generally continuous layer of grasses. Globally, savanna is an important biome, occurring in four continents (Asia, Africa, South America and Australia), and covering near one-third of the world's land surface (Huntley and Walker, 1982). It is characterized by a hot climate throughout the year with a distinct dry season for about half of the year. High temperatures may lead to deactivation of photosynthetic enzymes and bleaching of chlorophyll, reducing carbon gain, growth and reproduction, and thus influencing vegetation productivity and species distribution (Sainz et al., 2010; Hüve et al., 2011). Global warming may, by the end of this century, result in a temperature increase of 2–4 °C in the tropics and thus an increased risk of heat stress to tropical plants (Corlett, 2011). However, photosynthetic thermotolerance [PT; the change in the excitation capacity of photosystem II (PSII)] has been assessed for only a tiny and non-random selection of tropical species (Corlett, 2011). In this context, it is critically important to understand the thermotolerance of tropical plants.
One of the intriguing phenological features of savannas is that the leaves of a portion of the woody species flush in the late dry season in Chinese savannas (Zhang et al., 2007) and other tropical savannas (Williams et al., 1997; Franco et al., 2005; Chapotin et al., 2006). By flushing before the onset of the rainy season, plants will photosynthesize for a longer period, they may avoid rain-induced nutrient leaching from young immature leaves and escape herbivory (Lieberman and Lieberman, 1984). However, early flushing requires plants to possess greater PT to protect new leaves from thermal damage. Drought induces stomatal closure so that reduces leaf transpiration and increases leaf temperature (e.g. Zhang et al., 2007).
Heat stress can destroy the reaction centres of PSII, which can be detected by chlorophyll fluorescence emission. The minimum chlorophyll fluorescence (Fo)–temperature curve technique was proposed by Schreiber and Berry (1977) as a rapid assay of PT, and has been frequently used for this (e.g. Valladares and Pearcy, 1997; Knight and Ackerly, 2002; Weng and Lai, 2005). Fluorescence is emitted from leaves during the rapid de-excitation of excited electrons. A disruption of electron transport caused by high temperatures results in a greater proportion of excited electrons being de-excited and thus increasing fluorescence emission (e.g. Yamane et al., 2000). Several physiological stress responses lead to increased fluorescence at high temperatures, such as increased membrane fluidity, the dissociation of primary electron acceptors QA and QB, and the separation of the light-harvesting complex from the reaction centres of PSII (see also Knight and Ackerly, 2002).
Photosynthetic thermotolerance may be associated with other leaf traits, such as leaf life span, leaf mass per unit area (LMA) or foliar Ca concentration ([Ca]). Longer lived leaves may have a higher probability of experiencing heat stress during their life span, and thus require a greater PT to be able to function during and after a period of elevated temperature. Leaf life span also plays a pivotal role in the leaf economics spectrum, which varies from species with short-lived productive leaves with quick returns on carbon and nutrient investment to species with long-lived leaves with slow returns on resource investment but a longer revenue period. A long leaf life span is an important plant adaptation to unproductive environments (e.g. Reich et al., 1998; Wright et al., 2004; Escudero et al., 2008). Another important component of the leaf economics spectrum is LMA. Knight and Ackerly (2003) found in a comparative study of six chaparral shrub species that species with a high LMA had a high expression of heat shock proteins and thus greater PT. The leaf chemical composition may also be correlated with stress resistance. For example, [Ca] is related to the membrane stability and cell integrity (Gong et al., 1998), playing an important role in defence against freezing, disease and dehydration (McLaughlin and Wimmer, 1999), but it is unclear whether [Ca] is related to heat stress and PT.
The river valleys in south-west China particularly in Yunnan and Sichuan Provinces are characterized by a hot-dry climate because of the rain-shadow effect of mountains (see also Supplementary Data Fig. S1) and host a valley-type savanna (Wu, 1995; Jin and Ou, 2000). Few studies have been carried out on the ecological adaptation of plants from savannas and particularly these Chinese savannas (e.g. Zhang et al., 2007; Zhu et al., 2009). Here, we evaluate the PT and associated leaf traits (leaf life span, LMA and foliar [Ca]) of 24 woody savanna species from south-west China. We tested the hypotheses that (1) species with leaf flushing in the hot-dry season show greater PT than those with leaf flushing in the rainy season; and (2) PT correlates positively with leaf life span, LMA and [Ca]. The traditional correlation analysis on traits across species neglects the potential influence of ancestors on traits and autocorrelation of plant phylogeny. For rigorous analysis of correlation and to characterize the correlated evolution between PT and leaf traits, the comparative approach (phylogenetically independent contrast analysis; Felsenstein, 1985) was used in the present study.
MATERIALS AND METHODS
Site and species
This study was carried out in a valley savanna (23°41′N, 101°59'E, altitude 770 m) of the Yuanjiang River (the upper Red River), 10 km north-west of Yuanjiang City, Yunnan Province, south-west China. Mean annual temperature is 23·8 °C and the mean annual precipitation is 802 mm (according to the records from the Yuanjiang Meteorological Station located at 396 m altitude). There are three distinct seasons: a cool-dry (November–February), hot-dry (March–April) and hot-rainy (May–October) season (Supplementary Data Fig. S1). The soil is a ferralic Cambisol, with pH between 5·2 and 6·3. The upper soil horizons (0–20 cm) contain about 1·1 % organic matter, 0·1 % nitrogen, 0·04 % phosphorus and 2·6 % potassium.
The vegetation is dominated by deciduous woody shrub species and has been protected since the 1980s, but has occasionally been disturbed by grazing. Twenty-four woody species were studied, representing about 80 % of the savanna woody flora in this county (Table 1). These species differ in leaf flushing time, with five species flushing in the hot-dry season (Fig. 1A) and the 19 other species flushing at the beginning of the rainy season (Fig. 1B). Leaf fall of most species is concentrated from the middle of the cool dry season to the late dry season.
Table 1.
Photosynthetic thermotolerance and leaf traits for 24 woody species
| Species | Family | LFT | TS20 (°C) | Tc (°C) | T50 (°C) | Tmax (°C) | Leaf life span (d) | LMA (g m−2) | [Ca] (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Antidesma bunius | Euphorbiaceae | R | 44·4 ± 0·3 | 44·7 ± 0·1 | 46·2 ± 0·1 | 48·8 ± 0·1 | 91 ± 7 | 94 ± 6 | 7·6 ± 2·4 |
| Bridelia stipularis | Euphorbiaceae | R | 44·0 ± 0·4 | 44·9 ± 0·2 | 47·3 ± 0·5 | 50·5 ± 0·7 | 172 ± 11 | 95 ± 6 | NA |
| Buchanania latifolia | Anacardiaceae | D | 45·7 ± 0·7 | 46·1 ± 0·6 | 47·5 ± 0·6 | 49·8 ± 0·6 | 251 ± 9 | 126 ± 4 | 13·9 ± 2·6 |
| Callicarpa nudiflora | Verbenaceae | R | 45·0 ± 0·9 | 45·4 ± 0·7 | 47·1 ± 0·7 | 49·6 ± 0·6 | NA | 65 ± 7 | 7·9 ± 0·4 |
| Cyclobalanopsis helferiana | Fagaceae | R | 44·9 ± 0·8 | 45·0 ± 0·9 | 46·3 ± 0·4 | 49·8 ± 0·5 | NA | 150 ± 6 | 5·9 ± 0·5 |
| Dendrolobium triangulare | Papilionaceae | D | 45·5 ± 0·6 | 46·7 ± 0·6 | 49·3 ± 0·4 | 52·7 ± 0·6 | 257 ± 12 | 78 ± 7 | 16·0 ± 0·2 |
| Ehretia corylifolia | Boraginaceae | R | 42·9 ± 0·6 | 43·4 ± 0·4 | 46·6 ± 0·5 | 49·9 ± 0·6 | NA | 125 ± 4 | 13·9 ± 1·6 |
| Eriobotrya henryi | Rosaceae | R | 43·8 ± 0·6 | 44·6 ± 0·5 | 47·2 ± 0·6 | 50·1 ± 0·6 | NA | 132 ± 5 | NA |
| Ficus semicordata | Moraceae | R | 45·2 ± 0·6 | 46·5 ± 0·6 | 48·6 ± 0·7 | 52·2 ± 0·8 | 189 ± 32 | 87 ± 2 | 21·0 ± 2·9 |
| Fraxinus malacophylla | Oleaceae | R | 45·5 ± 0·5 | 46·0 ± 0·2 | 48·0 ± 0·3 | 50·5 ± 0·5 | 216 ± 16 | 113 ± 6 | 7·1 ± 0·8 |
| Gochnotia decora | Compositae | R | 44·9 ± 0·5 | 45·0 ± 0·5 | 47·0 ± 0·5 | 49·1 ± 0·6 | 145 ± 9 | 109 ± 3 | 8·4 ± 0·2 |
| Grewia celtidifolia | Tiliaceae | D | 45·8 ± 0·7 | 45·8 ± 0·5 | 48·0 ± 0·7 | 51·0 ± 0·7 | 150 ± 9 | 105 ± 4 | 16·3 ± 2·9 |
| Haldina cordifolia | Rubiaceae | R | 44·3 ± 0·3 | 45·3 ± 0·4 | 47·4 ± 0·5 | 51·5 ± 0·4 | 123 ± 13 | 81 ± 5 | 12·0 ± 0·7 |
| Lannea coromandelica | Anacardiaceae | D | 46·3 ± 0·3 | 46·3 ± 0·6 | 48·4 ± 0·5 | 50·8 ± 0·5 | 204 ± 8 | 95 ± 5 | 10·0 ± 1·3 |
| Pittosporum kerrii | Pittosporaceae | R | 43·7 ± 0. 9 | 44·1 ± 0·8 | 45·9 ± 0·7 | 48·2 ± 0·5 | NA | 97 ± 5 | NA |
| Polyalthia cerasoides | Annonaceae | R | 45·7 ± 0·9 | 46·3 ± 0·7 | 48·2 ± 0·6 | 50·8 ± 0·8 | 149 ± 8 | 76 ± 3 | 8·4 ± 0·7 |
| Psidium guajava | Myrtaceae | R | 43·5 ± 0·4 | 43·9 ± 0·2 | 45·8 ± 0·2 | 49·0 ± 0·3 | NA | 114 ± 4 | NA |
| Quercus aliena | Fagaceae | R | 45·1 ± 0·7 | 45·4 ± 0·6 | 48·1 ± 0·9 | 51·5 ± 1·1 | 220 ± 4 | 104 ± 3 | 9·6 ± 0·8 |
| Quercus cocciferoides | Fagaceae | R | 42·3 ± 1·9 | 43·8 ± 1·1 | 45·5 ± 1·3 | 49·8 ± 0·5 | NA | 108 ± 6 | NA |
| Symplocos racemosa | Symplocaceae | D | 46·5 ± 1·2 | 46·8 ± 0·9 | 48·8 ± 0·6 | 51·2 ± 0·4 | 222 ± 10 | 144 ± 5 | 19·3 ± 4·9 |
| Terminalia franchetii | Combretaceae | R | 44·7 ± 0·5 | 45·0 ± 0·2 | 46·4 ± 0·2 | 48·3 ± 0·3 | 198 ± 11 | 99 ± 4 | NA |
| Terminthia paniculata | Anacardiaceae | R | 43·7 ± 0·8 | 44·6 ± 0·6 | 47·2 ± 0·3 | 50·8 ± 0·1 | 123 ± 9 | 110 ± 2 | 10·4 ± 1·7 |
| Wendlandia tinctoria | Rubiaceae | R | 44·3 ± 0·3 | 44·4 ± 0·3 | 46·4 ± 0·2 | 47·9 ± 0·3 | 114 ± 26 | 109 ± 4 | 4·9 ± 0·6 |
| Woodfordia fruticosa | Lythraceae | R | 44·0 ± 0·4 | 44·3 ± 0·3 | 46·2 ± 0·2 | 49·2 ± 0·4 | 89 ± 12 | 122 ± 5 | 10·1 ± 0·4 |
See Fig. 2 for definitions of the four thermotolerance parameters.
LFT, leaf flushing time (D = dry season; R = rainy season); LMA, leaf mass per area; NA, data not available. The nomenclature follows the database of the Missouri Botanical Garden (W3TROPICOS; www.mobot.org).
Data are means ± s.e. for 5–9 replicates per species.
Fig. 1.
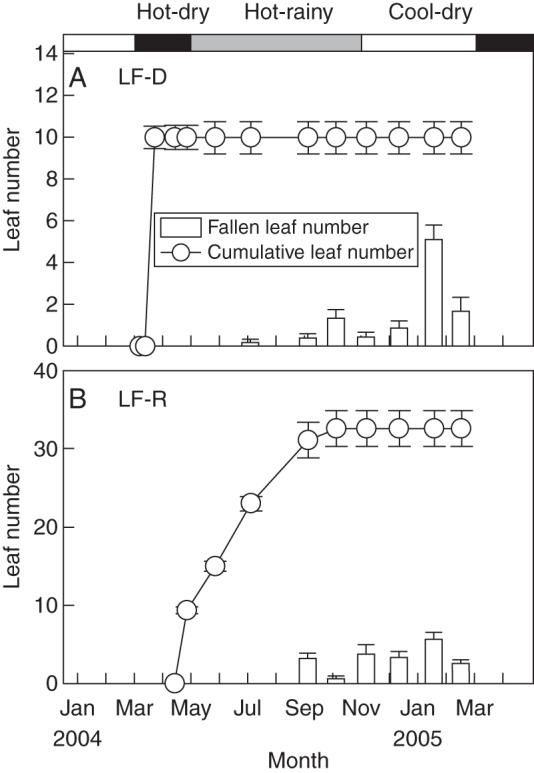
Leaf dynamics of two representative species, (A) Buchanania latifolia that flushes in the dry season (LF-D) and (B) Woodfordia fruticosa that flushes from the beginning of the rainy season onwards (LF-R). Cumulative leaf number and the number of fallen leaves are shown, as indicated in the key; values are means ± s.e. At the top of (A), the climatic conditions during the study period are indicated: cool-dry season (white), hot-dry season (grey) and hot-rainy season (black).
Previous studies have shown that thermostability can be influenced by ambient temperature, leaf age and drought stress (e.g. Havaux, 1993). To avoid the artefacts arising due to these factors, we selected mature and healthy leaves and measured PT, LMA and [Ca] within 1 week (15–22 September 2004) in the rainy season when plants were growing under optimal conditions.
Photosynthetic thermotolerance
The PT of PSII was analysed using the temperature-dependent increase in minimum fluorescence (T – Fo, Fig. 2; Bilger et al., 1984; Knight and Ackerly, 2002). Early in the morning, one shoot of five individuals per species was collected from the exposed, upper part of the crown, immediately inserted in water and brought to a nearby field laboratory. After dark adaptation for at least 1 h, one leaf or leaf segment from each of these shoots was placed above wet filter papers in a small metal chamber that was submerged in a water bath. The leaves were continuously heated from 25 to 60 °C, with a temperature increase of about 1 °C min−1. Leaf temperature was measured with a thermocouple attached to the underside of the leaves, and recorded continuously with a datalogger (LI-1400, Li-Cor, Lincoln, NE, USA). Fo was monitored continuously every 30 s using a portable fluorometer (FMS2, Hansatech, Norwich, UK). The slow rise in Fo is related to the reversible inactivation of reaction centres of PSII, whereas the rapid rise is related to irreversible destruction of the reaction centres (Bilger et al., 1984). Therefore, using the method of Knight and Ackerly (2002), four parameters (Fig. 2) were derived from the T – Fo curves to describe PT: TS20, the temperature at which the slope of the T – Fo curve reaches 20 % of its maximum; Tc, the critical temperature, defined as the intersection of the lines extrapolated from the slow and fast rise portion of the T – Fo response curve; T50, the temperature at which Fo reaches 50 % of its maximum; and Tmax, the temperature at which Fo reaches its maximum. We checked the reliability of the T – Fo method for the assessment of PT, by measuring the temperature-dependent leaf ion leakage for four common species of this savanna as well (cf. Chen et al., 1982). The lethal temperature for the leaf assessed by this method was highly correlated with Tmax as determined by the T – Fo method (r = 0·98, P < 0·05, n = 4, Supplementary Data Fig. S2), indicating that the T – Fo method is reliable for assessing the thermotolerance.
Fig. 2.
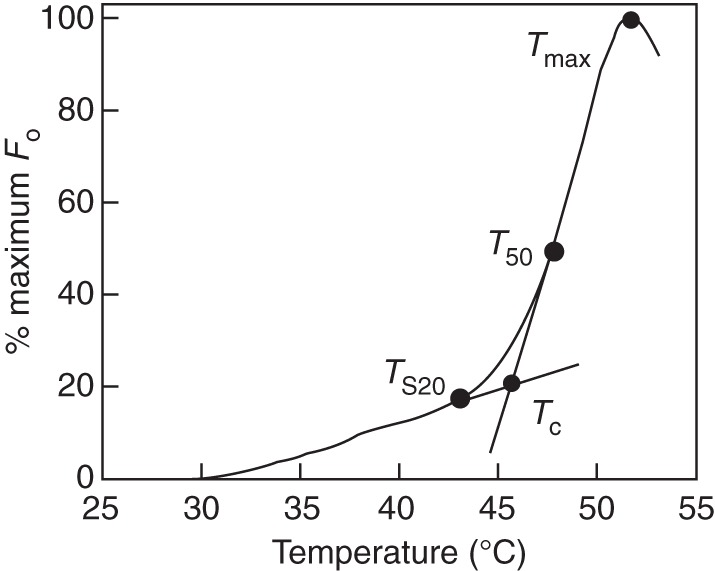
Temperature-dependent increase in minimum fluorescence (T – Fo) for Terminthia paniculata, a common species, as an example. Four parameters were calculated from the T – Fo curve: TS20, the temperature at which the slopes of the response curve reach 20 % of its maximum; Tc, critical temperature, the intersection of the lines extrapolated from the slow and fast rise portion of the T – Fo curve; T50, the temperature at which Fo reaches 50 % of its maximum; and Tmax, the temperature at which Fo reaches its maximum.
Leaf traits
The area of leaves used for thermotolerance measurement were measured using a portable leaf area meter (LI-3000A, Li-Cor), and then oven-dried at 80 °C for 48 h. The petiole and midrib were removed before leaf area measurements. LMA was calculated as leaf dry mass divided by leaf area. For an additional set of leaf samples collected from the same five plants per species, leaves were oven-dried and pulverized for measurement of foliar [Ca]. Foliar [Ca] was analysed with an inductively coupled plasma atomic-emission spectrometer (IRIS Advantage-ER, Thermo Jarrell Ash Corporation, Franklin, MA, USA) after leaf samples were digested by concentrated HNO3–HClO4.
Leaf dynamics were monitored for 9–27 shoots of 5–9 individuals per species from the late dry season (March 2004) to the start of the rainy season (May 2005). At the first census, a sunlit stem was selected and marked with a coloured tag. The last mature leaf of that stem was marked with ink. During the following censuses, which took place every 1 or 2 months, the last mature leaf was marked, the number of leaves produced since the previous census was noted and the total number of living leaves remaining on the stem above the first marked leaf was recorded. A leaf was considered to be dead when it had turned completely brown or had fallen. Leaf life span (LL) was then calculated as (Navas et al., 2003):
where TP is the duration of leaf flushing in days, TL is the duration of leaf fall and T is the time lag in days between the end of leaf flushing and the beginning of leaf fall. T is negative when the period between leaf flushing and fall is overlapping, otherwise it is positive.
Statistical analysis
Differences in PT between species flushing in the dry season and species flushing in the wet season were analysed using a t-test. The associations of PT with leaf life span, LMA and [Ca] were analysed with Pearson's correlation. To test whether PT is associated with leaf life span after considering the effect of leaf flushing season, analysis of covariance (ANCOVA) was carried out, with leaf life span as a dependent variable, flushing season as a factor and PT as a covariate. Phylogenetically independent contrast (PIC) analysis was used to eliminate the phylogenetic effect and test whether these phenotypic relationships were evolutionarily correlated (Felsenstein, 1985). An APG3-derived megatree was selected as the base tree (APG III, 2009) for our study taxa. The BLADJ algorithm in the program Phylocom (Webb et al., 2008) was applied to adjust the branch lengths of the phylogeny using known ages of the plant fossils (Wikström et al., 2001). The ‘ape’ library was then used to calculate the coefficients of PIC correlations (Paradis, 2004; R Development Core Team, 2009).
RESULTS
When averaged across all species, TS20, Tc, T50 and Tmax were 44·7, 45·2, 47·2 and 50·1 °C, respectively (Table 1). All parameters describing thermotolerance were highly correlated across species (Table 2). Species with leaf flushing in the late dry season had significantly greater TS20, Tc, T50 and Tmax than those with leaf flushing in the rainy season (P < 0·05 in each case, Fig. 3). Leaf life span varied from 89 to 257 d across the species studied (Table 1). Thermotolerance indices (TS20, Tc and T50) were positively correlated with leaf life span (Fig. 4A; Table 2) and T50 and Tmax were positively correlated with [Ca] (Fig. 4B; Table 2). Based on ANCOVA results, the associations of PT (TS20, Tc and T50) and leaf life span are still significant (P < 0·05) after considering the effect of flushing season. None of the thermotolerance indices was significantly correlated with LMA (Table 2).
Table 2.
Phenotypic correlations (above diagonal) and phylogenetic-independent contrast correlations (below diagonal) between photosynthetic thermotolerance parameters and leaf traits across the woody savanna species
| TS20 | Tc | T50 | Tmax | LL | LMA | Ca | |
|---|---|---|---|---|---|---|---|
| TS20 | – | 0·92*** | 0·777*** | 0·42* | 0·67** | –0·16 | 0·28 |
| Tc | 0·92*** | – | 0·90*** | 0·66*** | 0·75*** | –0·30 | 0·47 |
| T50 | 0·86*** | 0·89*** | – | 0·83*** | 0·71** | –0·28 | 0·62** |
| Tmax | 0·52* | 0·70*** | 0·82*** | – | 0·48 | –0·26 | 0·67** |
| LL | 0·66** | 0·66** | 0·64** | 0·21 | – | 0·04 | 0·46 |
| LMA | –0·11 | –0·30 | –0·26 | –0·38 | 0·09 | – | 0·40 |
| Ca | 0·18 | 0·49* | 0·58* | 0·71** | 0·13 | 0·02 | – |
See Fig. 2 for definitions of the four thermotolerance parameters.
LL, leaf life span; LMA, leaf mass per area.
Significance level: *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 3.
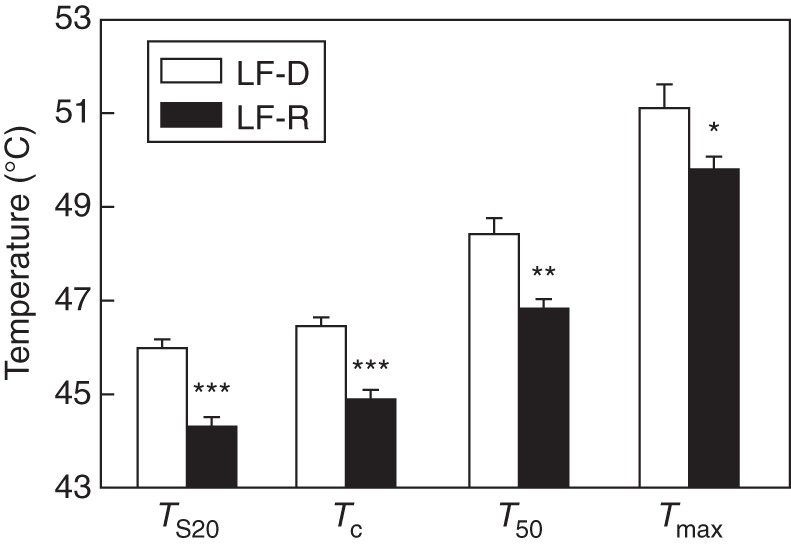
Differences in TS20, Tc, T50 and Tmax between species with leaf flushing in the late dry season (LF-D, n = 5) and species with leaf flushing in the rainy season (LF-R, n = 19). See Fig. 2 for definitions of the four photosynthetic thermotolerance parameters. Data are means ± s.e. *P < 0·05; **P < 0·05; ***P < 0·001.
Fig. 4.
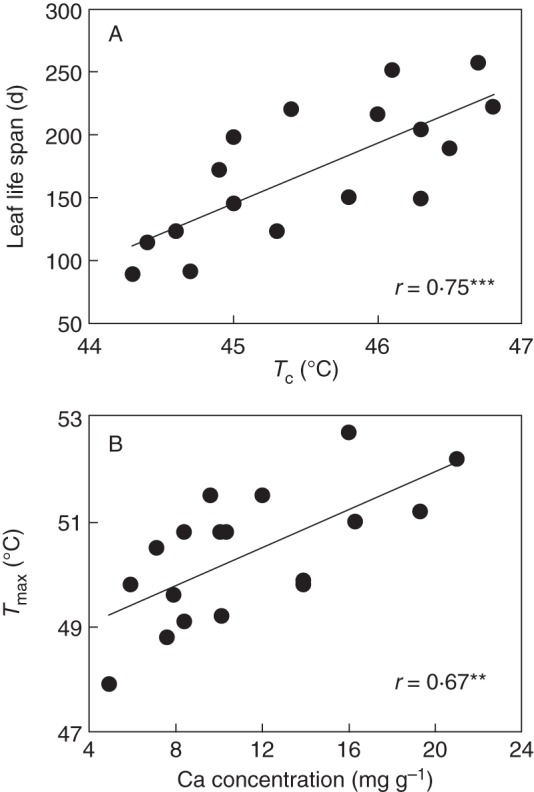
Relationships between photosynthetic thermotolerance, leaf life span and Ca concentration across species. See Fig. 2 for definitions of Tc and Tmax. **P <0·01; ***P <0·001.
Nearly all cross-species correlations (18 from 19) between thermotolerance and leaf traits were also underlain by evolutionary correlations (Table 2). Thermotolerance parameters, for instance, showed a positive pattern of evolutionary correlations with leaf life span and [Ca]. Thermotolerance, however, was evolutionarily independent of LMA.
DISCUSSION
Comparison of thermotolerance data between this (Table 1) and previous studies (Valladares and Pearcy, 1997; Knight and Ackerly, 2002; Weng and Lai, 2005) suggests that the PT of the woody savanna species in south-western China is moderate. Average Tc values of this study (44·7 °C), for example, were lower than those of Californian chaparral plants (49·9 °C; Knight and Ackerly, 2002) and a Mediterranean shrub species (53·5 °C; Valladares and Pearcy, 1997), but higher than those of 22 tropical or sub-tropical origin herbaceous and woody plants grown in a common garden (42·4 °C; Weng and Lai, 2005). Tc represents the change from inactivation to irreversible destruction of photosynthetic reaction centres as a consequence of increased leaf temperature (Bilger et al., 1984), and varied from 44 to 47 °C across the study species (Table 1). This range of Tc is close to the maximum leaf temperatures (approx. 44·3 °C) detected from five common woody species at the study site during an extreme hot and dry period in June 2006 (Zhu et al., 2009). Together, our results suggest that the leaves of the species can be well adapted to the usual high temperatures of the site, without irreversible destruction of photosynthetic reaction centres (Zhang et al., 2007).
Species that flush their leaves in the hot-dry season have greater PT compared with species flushing leaves in the rainy season (Fig. 3), consistent with the first hypothesis. A greater PT could protect the new leaves from irreversible thermal damage in the hot-dry season, thus maintaining the stability of the photosynthetic apparatus. In fact, there was no irreversible photoinhibitory damage in the new leaves that flushed in the hot-dry season even during two extremely dry hot periods (Zhu et al., 2009).
Thermotolerance is evolutionarily correlated with several key leaf traits. The positive correlation between thermotolerance and leaf life span (Fig. 4A; Table 2) suggests that greater PT is associated with longer persistence, supporting our second hypothesis that longer lived leaves have a higher probability to encounter heat stress during their lifetime, and that they need to be well protected to avoid irreversible damage. PT was, both in the regular cross-species correlations and in the evolutionary correlations, uncoupled from LMA (Table 2). This contrasts with the significant positive correlation between PT and LMA found by Knight and Ackerly (2003) across six chaparral plants and with the general concept that, within and across species, plants respond to stressful conditions by having a high LMA (Wright et al., 2004; Poorter et al., 2009). One reason is that the data set included a smaller range of LMA (see also Table 1) and leaf habits than those studies, and, hence, it is therefore more difficult to find a relationship of photosynthetic leaf traits with LMA. Another reason may be that a high LMA in arid environments is usually due to a higher leaf density, which limits transpiration and confers higher water use efficiency during drought (Gratani and Bombelli, 2001). Thus, the independence of PT from LMA may indicate that heat resistance and drought resistance may be associated with independent axes of ecological adaptation in this unique savanna habitat.
The PT was positively related with foliar [Ca] (Fig. 4B), in line with the second hypothesis. The positive correlation of thermotolerance with foliar [Ca] indicates that Ca-rich leaves are more tolerant to heat stress. Perhaps one reason for this is that Ca plays an important role in the maintenance of cell integrity (McLaughlin and Wimmer, 1999; Hirschi, 2004; Hepler, 2005). Another reason is that Ca-rich leaves probably have a high capacity to increase cytosolic [Ca] through mobilization of Ca from both intracellular and extracellular sources (Tan et al., 2011). This process seems to act as a signal to trigger some of the biochemical and physiological processes, such as improved thermostability of the oxygen-evolving complex and the reaction centre of PSII and enhanced antioxidative enzyme activities, for plants to promote thermal tolerance (Gong et al., 1998; Tan et al., 2011).
In summary, the results presented here extend earlier studies (Zhang et al., 2007; Zhu et al., 2009) in which the physiological adaptation of Chinese savanna woody species to dry and hot environmental conditions was assessed. This is the first time that PT has been shown to be associated with earlier leaf emergence, persistence (longer leaf life span) and increased Ca concentration across a large number of woody savanna species. Our results suggest that woody species from Chinese savannas are adapted to hot-dry habitats. The fact that the current maximum leaf temperature that they experience during extreme heat stress (44·3 °C) is close to their critical PT temperature (45·2 °C) indicates that future global warming of 2–4 °C (Corlett, 2011) may enhance the risk of irreversible heat damage. This may lead to a shift in species composition, from species that flush in the rainy season, towards more heat-tolerant species, which have longer lived leaves and flush in the dry season.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The Biogeochemistry Laboratory of the Xishuangbanna Tropical Botanical Garden carried out the analyses of soil and foliar nutrient concentration of the present study. We thank two anonymous reviewers for helpful comments. This study was financially supported by the National Natural Science Foundation of China (30900174, 90302013) and the West Light Foundation of Chinese Academy of Sciences to J.L.Z.
LITERATURE CITED
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Bilger H-W, Schreiber U, Lange OL. Determination of leaf heat resistance: comparative investigation of chlorophyll fluorescence changes and tissue necrosis methods. Oecologia. 1984;63:256–262. doi: 10.1007/BF00379886. [DOI] [PubMed] [Google Scholar]
- Chapotin SM, Razanameharizaka JH, Holbrook NM. Water relations of baobab trees (Adansonia spp. L.) during the rainy season: does stem water buffer daily water deficits? Plant, Cell and Environment. 2006;29:1021–1032. doi: 10.1111/j.1365-3040.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- Chen H-H, Shen Z-Y, Li PH. Adaptability of crop plants to high temperature stress. Crop Science. 1982;22:719–725. [Google Scholar]
- Corlett RT. Impacts of warming on tropical lowland rainforests. Trends in Ecology and Evolution. 2011;26:606–613. doi: 10.1016/j.tree.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Escudero A, Mediavilla S, Heilmeier H. Leaf longevity and drought: avoidance of the costs and risks of early leaf abscission as inferred from the leaf carbon isotopic composition. Functional Plant Biology. 2008;35:705–713. doi: 10.1071/FP08037. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Franco AC, Bustamante M, Caldas LS, et al. Leaf functional traits of Neotropical savanna trees in relation to seasonal water deficit. Trees: Structure and Function. 2005;19:326–335. [Google Scholar]
- Gong M, van der Luit AH, Knight MR, Trewavas AJ. Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiology. 1998;116:429–437. [Google Scholar]
- Gratani L, Bombelli A. Differences in leaf traits among Mediterranean broad-leaved evergreen shrubs. Annales Botanici Fennici. 2001;38:15–24. [Google Scholar]
- Havaux M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant, Cell and Environment. 1993;16:461–467. [Google Scholar]
- Hepler PK. Calcium: a central regulator of plant growth and development. The Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiology. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley BJ, Walker BH. Ecology of tropical savannas. Berlin: Springer; 1982. [Google Scholar]
- Hüve K, Bichele I, Rasulov B, Niinemets Ü. When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant, Cell and Environment. 2011;34:113–126. doi: 10.1111/j.1365-3040.2010.02229.x. [DOI] [PubMed] [Google Scholar]
- Jin ZZ, Ou XK. Vegetations in the hot and dry valleys along the Yuanjiang, Nujiang, Jinshajiang, and Lanchangjiang Rivers. Kunming: Yunnan University Press; 2000. [Google Scholar]
- Knight CA, Ackerly DD. An ecological and evolutionary analysis of photosynthetic thermotolerance using the temperature-dependent increase in fluorescence. Oecologia. 2002;130:505–514. doi: 10.1007/s00442-001-0841-0. [DOI] [PubMed] [Google Scholar]
- Knight CA, Ackerly DD. Evolution and plasticity of photosynthetic thermal tolerance, specific leaf area and leaf size: congeneric species from desert and coastal environments. New Phytologist. 2003;160:337–347. doi: 10.1046/j.1469-8137.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- Lieberman D, Lieberman M. The causes and consequences of synchronous flushing in a dry tropical forest. Biotropica. 1984;16:193–201. [Google Scholar]
- McLaughlin SB, Wimmer R. Calcium physiology and terrestrial ecosystem processes. New Phytologist. 1999;142:373–417. [Google Scholar]
- Navas M-L, Ducout B, Roumet C, Richarte J, Garnier J, Garnier E. Leaf lifespan, dynamics and construction cost of species from Mediterranean old-fields differing in successional status. New Phytologist. 2003;159:213–228. doi: 10.1046/j.1469-8137.2003.00790.x. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar H. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2009 www.r-project.org . [Google Scholar]
- Reich PB, Walters M, Ellsworth DS, et al. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia. 1998;114:471–482. doi: 10.1007/s004420050471. [DOI] [PubMed] [Google Scholar]
- Sainz M, Díaz P, Monza J, Borsani O. Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought–heat stressed Lotus japonicus. Physiologia Plantarum. 2010;140:46–56. doi: 10.1111/j.1399-3054.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Berry JA. Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus. Planta. 1977;136:529–538. doi: 10.1007/BF00385990. [DOI] [PubMed] [Google Scholar]
- Tan W, Meng QW, Brestic M, Olsovska K, Yang XH. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. Journal of Plant Physiology. 2011;168:2063–2071. doi: 10.1016/j.jplph.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Valladares F, Pearcy R. Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll. Heteromeles arbutifolia. Plant, Cell and Environment. 1997;20:25–36. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- Weng J-H, Lai M-F. Estimating heat tolerance among plant species by two chlorophyll fluorescence parameters. Photosynthetica. 2005;43:439–444. [Google Scholar]
- Wikström N, Savolainen V, Chase MW. Evolution of angiosperms: calibrating the family tree. Proceedings of the Royal Society B: Biological Sciences. 2001;268:2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D. Leaf phenology of woody species in a north Australian tropical savanna. Ecology. 1997;78:2542–2558. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wu ZY. The vegetation of China. Beijing: Science Press; 1995. [Google Scholar]
- Yamane Y, Shikanai T, Kashino Y, Koike H, Satoh K. Reduction of QA in the dark: another cause of fluorescence Fo increases by high temperatures in higher plants. Photosynthesis Research. 2000;63:23–34. doi: 10.1023/A:1006350706802. [DOI] [PubMed] [Google Scholar]
- Zhang J-L, Zhu J-J, Cao K-F. Seasonal variation in photosynthesis in six woody species with different leaf phenology in a valley savanna in southwestern China. Trees: Structure and Function. 2007;21:631–643. [Google Scholar]
- Zhu J-J, Zhang J-L, Liu H-C, Cao K-F. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiologia Plantarum. 2009;135:62–72. doi: 10.1111/j.1399-3054.2008.01171.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


