Abstract
Background and Aims
A pollen grain contains a number of esterases, many of which are released upon contact with the stigma surface. However, the identity and function of most of these esterases remain unknown. In this work, esterases from olive pollen during its germination were identifided and functionally characterized.
Methods
The esterolytic capacity of olive (Olea europaea) pollen was examined using in vitro and in-gel enzymatic assays with different enzyme substrates. The functional analysis of pollen esterases was achieved by inhibition assays by using specific inhibitors. The cellular localization of esterase activities was performed using histochemical methods.
Key Results
Olive pollen showed high levels of non-specific esterase activity, which remained steady after hydration and germination. Up to 20 esterolytic bands were identified on polyacrylamide gels. All the inhibitors decreased pollen germinability, but only diisopropyl fluorophosphate (DIFP) hampered pollen tube growth. Non-specific esterase activity is localized on the surface of oil bodies (OBs) and small vesicles, in the pollen intine and in the callose layer of the pollen tube wall. Acetylcholinesterase (AChE) activity was mostly observed in the apertures, exine and pollen coat, and attached to the pollen tube wall surface and to small cytoplasmic vesicles.
Conclusions
In this work, for the first time a systematic functional characterization of esterase enzymes in pollen from a plant species with wet stigma has been carried out. Olive pollen esterases belong to four different functional groups: carboxylesterases, acetylesterases, AChEs and lipases. The cellular localization of esterase activity indicates that the intine is a putative storage site for esterolytic enzymes in olive pollen. Based on inhibition assays and cellular localization of enzymatic activities, it can be concluded that these enzymes are likely to be involved in pollen germination, and pollen tube growth and penetration of the stigma.
Keywords: Acetylcholinesterases, acetylesterases, carboxylesterases, DIFP, ebelactones, germination, lipases, neostigmine, olive, Olea europaea, pollen, pollen tube, sulfydryl reagents
INTRODUCTION
Successful pollination in flowering plants depends on complex interactions between the pollen grain and stigmatic surface. The stigma can be dry or wet, depending on the absence or presence of copious secretions during the receptive period (Heslop-Harrison and Shivanna, 1977). In wet stigmas, pollen adhesion is mediated by the stickiness and surface tension of the stigmatic secretion (Swanson et al., 2004). The surface of receptive-wet stigmas is characterized by a high rate of activity of some enzymes, notably peroxidases and esterases (Dafni and Maués, 1998). The dry-type stigma, which is present in some Angiosperm families such as Brassicaceae, is enclosed by a continuous layer of cuticle of a lipidic nature. Characteristically, the stigma cuticle is overlaid with a thin proteinaceous pellicle, which displays strong esterase activity (Mattsson et al., 1974). In dry stigmas, pollen adhesion depends on the pollen coat, and the proteinaceous pellicle of the stigma (Edlund et al., 2004).
A mature pollen grain contains numerous enzymes, many of which are released upon contact with the stigmatic surface (Brewbaker, 1971; Heslop-Harrison, 1987). Among them, carboxylic-ester hydrolases [Enzyme Commission (EC) number: EC 3·1.1; Webb, 1992] are esterases (EC 3·1) that catalyse the hydrolysis of carboxylic acid esters that result in the formation of an alcohol and a carboxylic acid anion (Mäkinen and Brewbaker, 1967; Mäkinen and McDonald, 1968; Lavithis and Bhalla, 1995). Three active cutinases (EC 3·1.1·74) have been characterized in the pollen of Tropaeolum majus (Shaykh et al., 1977; Maiti et al., 1979), Brassica napus (Hiscock et al., 1994) and Arabidopsis thaliana (Takakashi et al., 2010). These specialized esterases break down the waxy polymers of cutin present in the stigma cuticle, allowing the pollen tube to breach this barrier (Hiscock et al., 1994; Takakashi et al., 2010). Several carboxylesterases (EC 3·1.1·1), which possess the catalytic triad ‘Ser 153, Tyr167 and Lys171 (S-D-H)’, at their active site are required for pollen tube penetration of the stigma in Brassica napus (Hiscock et al., 2002a). The pollen coat of arabidopsis and sunflower also possesses several active lipases (EC 3·1.1·3) that might be involved in the degradation of the stigma's lipidic structures such as the cuticle (Mayfield et al., 2001; Shakya and Bhatla, 2010).
Once the cuticle has been breached, the pollen tube penetrates the stigma tissues and grows through the intercellular spaces of the stylar transmitting tissue. In some species, the hollow style is filled with a mucilaginous matrix, and the pollen tube grows adhered to one layer of the secretory tissue that covers the inner surface of the cylinder (Lord, 2003). The pollen tube wall consists of an inner layer of callose and cellulose and an outer coating of pectins (Geitmann and Steer, 2006). The pollen tube tip lacks the callose layer (Ferguson et al., 1998) and is enriched with esterified pectins (Li et al., 1994; Jauh and Lord, 1996; Parre and Geitmann, 2005), which provide the apex with sufficient strength and elasticity to support polarized tip growth. Pollen pectin methylesterases (EC 3·1.1·11) have a relevant function in regulating pollen tube wall dynamics in pistil tissues (Bosch et al., 2005; Jiang et al., 2005). Esterified pectins from the pollen tube tip are gradually de-esterified by pectin methylesterases, which cleave the methoxyester groups of homogalacturonans (Catoire et al., 1998). Then, the exposed carboxyl residues can be cross-linked by calcium ions, forming a pectate gel (Goldberg et al., 1996).
In addition to cutinases, lipases and pectinesterases, pollen from most species contains many other esterases (Mäkinen and Brewbaker, 1967; Knox and Heslop-Harrison, 1970; Lavithis and Bhalla, 1995; Hiscock et al., 2002b). These enzymes are ubiquitous in nature and exist in multiple forms and have broad substrate specificity. When the substrate is a simple ester such as naphthyl acetate, the enzyme is termed a non-specific esterase. Currently, the functional classification of non-specific esterases mostly relies on their behaviour in the presence of different classes of inhibitors. Based on their sensitivity to carbamates, organophosphorus (OP) compounds and sulfydryl reagents, non-specific esterases are classified into three groups (Pearse, 1972). Carboxylesterases (EC 3·1.1·1) are inhibited by OP compounds [e.g. diisopropyl fluorophosphate (DIFP)]. Arylesterases (EC 3·1.1·2) are not affected by either carbamates or OP compounds but are inhibited by sulfydryl reagents [e.g. p-chloromercuribenzoate (pCMB)] and EDTA. Acetylesterases (EC 3·1.1·6) are not sensitive to any of the inhibitors mentioned above.
There is a considerable overlap between non-specific esterases and other more specific esterases, such as acetylcholinesterases (AChEs; EC 3·1.1·7) and lipases, which are also capable of hydrolysing simple esters. However, AChEs are differentiated from non-specific esterases by the action of carbamates (e.g. eserine and neostigmine bromide). Thus, the hydrolysing capacity of AChEs is destroyed by these inhibitors while the activity of non-specific esterases is not affected. On the other hand, lipases are capable of hydrolysing long-chain esters while non-specific esterases lack this ability.
The isozyme pattern of non-specific esterases in plants is independent of external factors and allows interspecific and intraspecific species identification (Bílkowá et al., 1999). The isozyme composition is also known to be tissue specific and was shown to change during ontogenesis. Esterases are also used as markers of somatic embryogenesis induction, different embryonic developmental stages and the degree of stigma receptivity (Stejskal and Griga, 1995; Dodeman and Ducreux, 1996; Heslop-Harrison et al., 1975a, b). The identity and function of many of the pollen esterases are still unknown. Indeed, no data regarding the systematic functional classification of esterases from the pollen grain are available as yet. In the present work, we have identified and functionally characterized the esterases of olive (Olea europaea L.) pollen.
MATERIALS AND METHODS
Plant material
Olive (Olea europaea L.) pollen grains were collected from dehiscent anthers at the end of the flowering period by vigorously shaking the flowering shoots inside paper bags. Sampling was carried out from five selected trees (cv. ‘Lechín de Granada’) belonging to the olive germplasm bank of the Centro de Investigación y Formación Agraria (CIFA) ‘Venta del Llano’ (Mengíbar, Jaén, Spain). Samples were sieved through a set of meshes to remove floral debris. Pollen viability was routinely assessed by staining pollen with fluorescein diacetate before each experiment (Heslop-Harrison and Heslop-Harrison, 1970). Pollen viability rates varied between 34·9 and 37·2 % depending on the pollen batch (i.e. tree).
In vitro pollen germination
Freshly collected pollen samples were rehydrated by incubation in a humid chamber at room temperature for 30 min and then transferred to Petri dishes (0·1 g of pollen per dish) containing 10 mL of germination liquid medium [10 % (w/v) sucrose, 0·03 % (w/v) Ca(NO3)2, 0·01 % (w/v) KNO3, 0·02 % (w/v) MgSO4 and 0·01 % (w/v) H3BO3]. Petri dishes were maintained at room temperature in the dark for 6 h under gentle agitation. Pollen sampling was carried out at 1 and 6 h after the onset of the culture.
Preparation of protein extracts
Freshly collected desiccated pollen (0·1 g) was suspended in 1·5 mL of extraction buffer (0·05 m phosphate buffer, pH 7·0). Pollen proteins were eluted under continuous and vigorous stirring at 4 °C for 1 h. The suspension was spun at 13 500 rpm for 30 min at 4 °C and the resulting supernatants were filtered through a PD10 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and concentrated by centrifugation through Amicon Ultra-15 centrifugal filter devices (Millipore, Billerica, USA). After culture, pollen was filtrated through a set of meshes, in order to separate germinated from non-germinated pollen grains. Proteins were extracted from 1 and 6 h germinated pollen as described above. Total protein content was estimated for each sample using the Detergent Compatible (DC™) reagent (Bio-Rad, Hercules, USA) and bovine serum albumin (BSA) as standard following the manufacturer's instructions. All the samples were aliquoted and immediately processed, or stored at –80 °C until use.
In vitro assay of non-specific esterase activity
Non-specific esterase activity was spectrophotometrically assayed by measuring the formation of p-nitrophenol from p-nitrophenyl butyrate (PNB) ester according to Purdy and Kolattukudy (1973) with minor modifications. The effect of pH, temperature and time of incubation was determined. The optimal reaction mixture consisted of 880 µL of 50 mm Tris–HCl (pH 8·0), 100 µL of 0·4 % (v/v) Triton X-100, 10 µL of 1·76 % PNB (v/v in acetonitrile) and 10 µL of pollen protein extract (approx. 30 µg). This mixture was incubated at 30 °C for 30 min, and the PNBase activity was measured at 405 nm using a Shimadzu 1800 spectrophotometer (Shimadzu, Kyoto, Japan).
Changes in pollen PNBase activity during in vitro germination were measured as described above. For each sample, five independent experiments with three replicates each (n = 15) were performed, and the mean and standard deviation were computed. Esterase activity rates were expressed as relative percentages referred to the maximum mean value of A405. Control reactions were performed as described above by either omitting the pollen protein extract from the reaction mixture or heating the protein extract at 100 °C for 10 min.
SDS–PAGE
Pollen proteins (approx. 25 µg per sample) were mixed with an equal volume of 2× SDS sample buffer (Laemmli, 1970), boiled for 3 min prior to gel loading, and separated by SDS–PAGE on 12 % (w/v) polyacrylamide gel slabs using a Mini-PROTEAN 3 Electrophoretic Cell (Bio-Rad). After electrophoresis, the resulting gels were stained with Coomassie brilliant blue (CBB) according to standard procedures. Gel documentation was carried out in an ImageScanner III (GE Healthcare Bio-Sciences) using the LabScan 6·0 software (GE Healthcare Bio-Sciences).
In-gel detection of non-specific esterase activity
The non-specific esterase profile was studied in mature pollen. SDS–PAGE was performed as above, but the sample boiling step was omitted. After electrophoresis, SDS was removed from the polyacrylamide gels by washing them three times for 30 min each in a solution containing 0·05 m phosphate buffer (pH 7·0) and 2·5 % (v/v) Triton X-100. Esterase activity was revealed by incubating gels for 3 h at 37 °C in a developing solution containing 30 mg of α-naphthyl acetate prepared in 0·5 mL of acetone and 100 mg of Fast blue RR salt in 50 mL of 0·1 m phosphate buffer (pH 7·0). The reproducibility of esterase profiles was confirmed first by carrying out three independent experiments and, secondly, by running each protein sample in triplicate (n = 9 gels). The apparent molecular masses of the resulting esterase bands were estimated using the Precision Plus protein standards (Bio-Rad). Control reactions were performed by omitting either the substrate or salt from the developing solution. All enzyme substrates were purchased from Sigma-Aldrich (St Louis, USA).
In-gel detection of lipase activity
SDS–PAGE was performed as above. After SDS removal, lipase activity was revealed by incubating the gels for 30 min at 37 °C in a developing solution containing 40 mg of α-naphthyl palmitate, prepared in 16 mL of N, N-dimethylformamide, and 80 mg of Fast blue BB salt in 144 mL of 0·1 m phosphate buffer (pH 7·0). Control reactions were performed by omitting either the substrate or the salt from the developing solution. The reproducibility of lipase activity profiles was confirmed as above.
Inhibition studies
In vitro inhibition assays for non-specific esterase activity were carried out using protein extracts of mature pollen grains. Briefly, protein samples were prepared as above, and the reaction mixture lacking the substrate (PNB) but containing the inhibitor at different concentrations was pre-incubated for 30 min at 30 °C. After this period, 1·76 µL of PNB was added to the mixture and the incubation of samples was extended for 30 min at 30 °C. The esterase activity was measured as above. Five independent experiments with three replicates each (n = 15) were performed, and the mean and standard deviation were computed.
To perform in-gel inhibition assays, mature pollen protein extracts were prepared as above but the inhibitor at a final concentration of 2 mm was added to the extraction buffer. Protein samples were then subjected to SDS–PAGE, and esterase enzymes were detected by incubating gels for 3 h at 37 °C in a developing solution as above. The reproducibility of the results was confirmed first by carrying out three independent experiments and, secondly, by running each protein sample in triplicate (n = 9 gels). A control gel was incubated as above but without inhibitors.
Inhibition assays were also performed during pollen germination. For this purpose, pollen samples were germinated in vitro, and the corresponding inhibitor was added at the onset of the culture. Pollen was sampled after 6 h of germination and fixed in a mixture of acetic acid and ethanol (3:1). The germination rate (%) was calculated from approx. 2250 pollen grains randomly counted (200 grains per count × 5 independent experiments × 3 replicates for each experiment) under an Axioplan microscope (Nikon, Tokyo, Japan) using a palm-held counting device. Pollen grains were counted as germinated when the length of the pollen tube was at least 2-fold the pollen diameter (Pinney and Polito, 1990). Pollen tube length was measured from 750 germinated (i.e. pollen tube length ≥2× the pollen diameter) pollen grains (50 grains per count × 5 independent experiments × 3 replicates for each experiment) using Image J v.1·43 software. The mean and standard deviation for each parameter were calculated and plotted using the SigmaPlot software (Systat Software GmbH, Erkrath, Germany).
The following inhibitors (purchased from Sigma-Aldrich) were used in all the experiments described above: neostigmine (dissolved in water), DIFP (dissolved in isopropanol) and pCMB (dissolved in ethanol and water, 1:1). In addition, the inhibitor ebelactone B (dissolved in ethanol and water, 1:1) was used for carrying out inhibition assays of lipase activity.
Transmission electron microscopy
Mature and germinated pollen samples (a 1:1 mixture) were pre-fixed in 100 mm sodium cacodylate buffer (pH 7·2) containing 2·5 % (w/v) glutaraldehyde for 1 h at 4 °C. Samples were then washed in 100 mm sodium cacodylate buffer (pH 7·2) three times for 1 h each. After washing, non-specific esterase activity was detected by incubating samples at 37 °C for 30 min in a 1·5 mL solution of 100 mm phosphate buffer (pH 7·0) containing 30 µL of 60 mg mL−1 α-naphthyl acetate in acetone and 60 µL of 2 mg mL−1 Fast blue RR salt.
In order to detect AChE activity, pollen samples were processed as above and incubated at 40 °C for 25 min in a 1·5 mL solution containing 0·1 m acetate buffer (pH 6·0), 0·1 m sodium citrate, 30 mm copper sulfate, 5 mm potassium ferricyanide and 0·002 g of acetylthiocholine iodide. After enzyme reactions, the samples were rinsed in 100 mm cacodylate buffer (pH 7·2) three times for 30 min each and post-fixed in 1 % (w/v) OsO4 for 1 h at 4 °C. After dehydration in a graded ethanol series, pollen grains were embedded in epoxy resin (Sigma-Aldrich).
Ultrathin sections (thickness, 70 nm) were cut with a Reichert-Jung Ultracut microtome (Leica Microsystems AG, Wetzlar, Germany) and mounted on 200-mesh formvar-coated nickel grids. Grids with both mature and germinated pollen sections were stained with 5 % (w/v) uranyl acetate and lead citrate (1·33 g of lead nitrate, 1·67 g of sodium citrate and 8 mL of 1 M sodium hydroxide in 50 mL of dH2O). Observations were carried out in a JEM-1011 transmission electron microscope (JEOL, Tokyo, Japan). Images were obtained with a MegaView III camera (Olympus, Tokyo, Japan) using iTEM software (JEOL). The reproducibility of the results was confirmed by performing three independent experiments. Control reactions were carried out as above but the enzyme substrate was not added.
RESULTS
Detection of esterolytic activity in olive pollen during germination
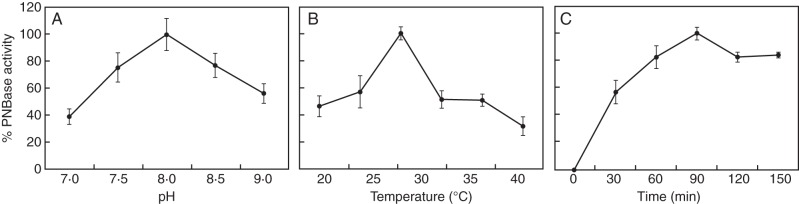
The effect of pH, temperature and time of incubation on the non-specific esterase activity rate in mature olive pollen was measured in vitro (Fig. 1). The PNBase activity was found to be optimal at pH 8·0, at 30 °C and after 90 min of incubation. These assay conditions were further applied to measure the PNBase activity in pollen grains during in vitro germination (Fig. 2). The esterolytic activity remained steady after hydration and during pollen in vitro germination, showing similar values to that of mature pollen (Fig. 2). We also detected the presence of non-specific esterase activity in the culture medium, but the values barely represented 20 % of the activity detected in mature pollen grains after 1 h of germination. Then, the PNBase activity significantly decreased after 6 h of pollen culture (Fig. 2).
Fig. 1.
Effect of (A) pH, (B) temperature and (C) incubation time on non-specific esterase activity in the mature olive pollen. The optimal conditions giving maximum PNBase activity are: pH 8·0, 30 °C and 90 min of incubation.
Fig. 2.
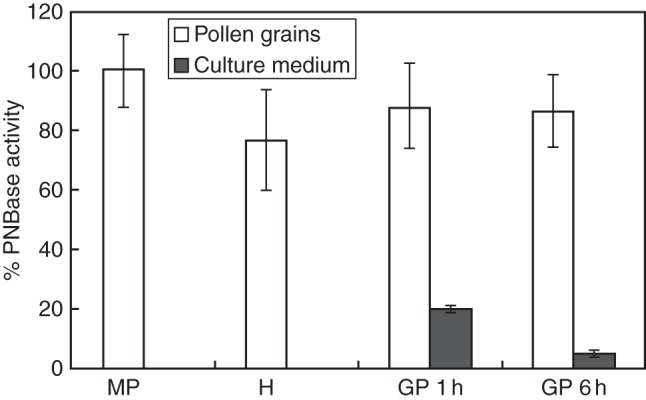
Non-specific esterase activity profile in olive pollen grains and the culture medium during in vitro germination. MP, mature pollen; H, hydrated pollen; GP 1 h and GP 6 h, germinated pollen after 1 and 6 h of culture, respectively.
Profiling of esterase activity in olive pollen during germination
Figure 3A shows the esterase activity profile in mature olive pollen grains after total protein separation on an SDS–polyacrylamide gel and after incubation with α-naphthyl acetate as substrate. These olive pollen esterase enzymes were generically named OeEst and numbered consecutively beginning from the anodic end of the gel (Fig. 3B). The resulting esterase activity profile was essentially the same after using either naphthyl acetate (2 C atoms) or naphthyl butyrate (4 C atoms) esters (data not shown) as substrates in the reaction mixture. Up to 14 different esterolytic bands were observed, having molecular weights ranging from 26 to 245 kDa (Table 1). No differences were found after using either the α or β isomers of naphthyl acetate ester in the reaction (data not shown).
Fig. 3.
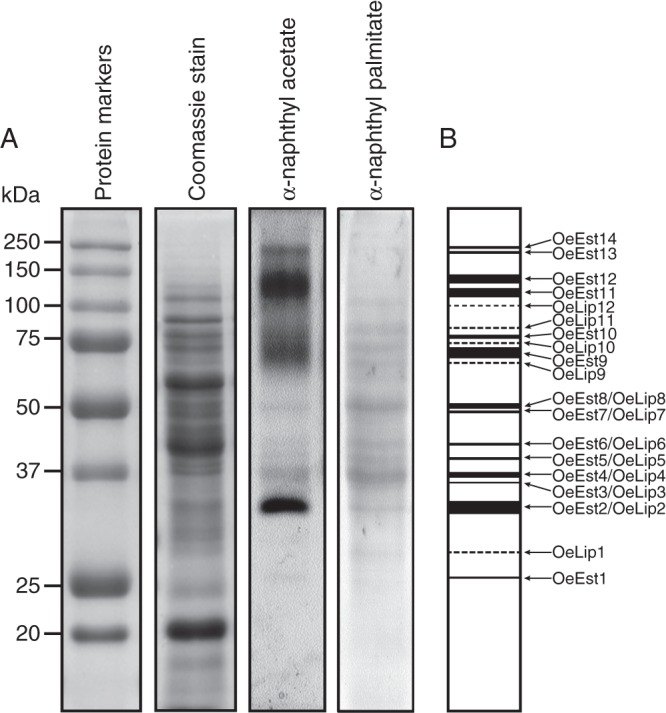
(A) In-gel detection of esterase activities in mature olive pollen. Esterase activity was developed in a solution containing α-naphthyl acetate or β-naphthyl palmitate as enzyme substrate. (B) Zymogram of all the esterolytic bands detected in (A). Dashed lines in the zymogram indicate bands only detected with β-naphthyl palmitate.
Table 1.
Molecular weight of unspecific esterase (Est) and lipase (Lip) enzymes identified in mature olive pollen, calculated from gels shown in Fig. 3A
| Enzyme | Mol. wt (kDa) |
|---|---|
| OeEst1 | 25·7 |
| OeLip1 | 28·0 |
| OeLip2 | 32·4 |
| OeEst2 | 32·8 |
| OeEst3/OeLip3 | 35·7 |
| OeEst4/OeLip4 | 38·8 |
| OeEst5/OeLip5 | 39·7 |
| OeEst6/OeLip6 | 42·4 |
| OeEst7/OeLip7 | 49·3 |
| OeEst8/OeLip8 | 51·6 |
| OeLip9 | 66·5 |
| OeEst9 | 70·6 |
| OeLip10 | 75·0 |
| OeEst10 | 78·0 |
| OeLip11 | 84·9 |
| OeLip12 | 102·1 |
| OeEst11 | 117·4 |
| OeEst12 | 136·6 |
| OeEst13 | 218·4 |
| OeEst14 | 242·6 |
After incubation with a long-chain fatty acid ester such as β-naphthyl palmitate (16 C atoms), the profile differed significantly in quantitative and qualitative terms (Fig. 3A). The resulting bands were generically named OeLip and numbered consecutively beginning from the anodic end of the gel (Fig. 3B). Up to 12 esterolytic bands were visible, five of which (OeLip1 and OeLip9–OeLip12) were specific for the β-naphthyl palmitate substrate (Fig. 3B). The remaining bands, except OeLip2, showed a higher intensity compared with the same α-naphthyl acetate-derived bands. This fact suggests that OeLip2 and OeEst2, although they have similar molecular weights, are not the same protein.
Functional classification of esterases present in olive pollen
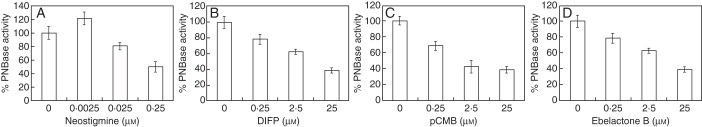
The functional classification of esterolytic bands was carried out using a set of esterase inhibitors, i.e. neostigmine, DIFP, pCMB and ebelactone B. When assayed in vitro, neostigmine slightly stimulated esterolytic activity when applied at very low concentrations and inhibited PNBase activity at higher doses (Fig. 4A). A similar pattern was observed when the inhibitor eserine was used (data not shown). The other three inhibitors produced a significant decrease in the PNBase activity of mature pollen in a dose-dependent manner (Fig. 4B, C).
Fig. 4.
In vitro inhibition assays of esterolytic activity in olive pollen. Effect of (A) neostigmine, (B) DIFP, (C) pCMB and (D) ebelactone B inhibitors on PNBase activity in the total protein extracts prepared from mature olive pollen. All the inhibitors induced a reduction in the PNBase activity in vitro.
Figure 5A shows pollen esterase activity profiles on polyacrylamide gels after treatment with the inhibitors mentioned above. Using α-naphthyl acetate as an enzyme substrate, we found that the esterolytic bands OeEst1 and OeEst2 were both inhibited by DIFP and pCMB, but were unaffected after exposure to carbamates (Fig. 5A). This inhibition pattern suggests that OeEst1 and OeESt2 are carboxylesterases. The inhibitory effect of pCMP may reflect the close proximity of a cysteine residue to the active site. The bands from OeEst9 to OeEst14 were identified as AChEs since they were completely inhibited by the action of neostigmine and DIFP. None of the chemical inhibitors affected the activity of esterolytic bands OeEst3–OeEst8, so these enzymes are likely to be acetylesterases. Finally, treatments with the inhibitor ebelactone B resulted in the inhibition of all esterolytic activities detected on polyacrylamide gels when β-naphthyl palmitate was used as an enzyme substrate (Fig. 5B).
Fig. 5.
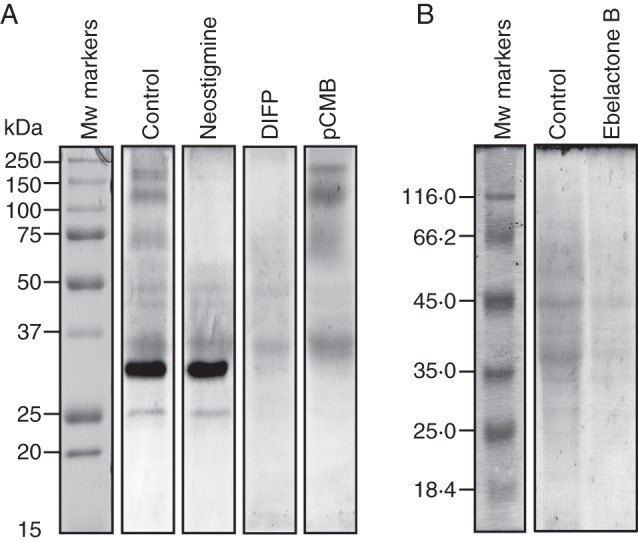
In-gel inhibition assays of esterolytic activity in olive pollen. (A) Effect of neostigmine (2 mm), DIFP (2 mm) and pCMB (2 mm) on esterolytic activity in polyacrylamide gels when using α-naphthyl acetate as the enzyme substrate. (B) Effect of ebelactone B (2 mm) on esterolytic activity when using β-naphthyl palmitate as the enzyme substrate. Protein markers are displayed on the left.
Effect of esterase inhibitors on pollen performance
Olive pollen was allowed to germinate in vitro in the presence of different esterase inhibitors, and both germinability and pollen tube growth rates were recorded (Fig. 6). All the inhibitors produced a strong reduction in the capacity of olive pollen to germinate when compared with controls after 6 h of culture (Fig. 6A). Neostigmine caused the strongest inhibitory effect on pollen germination, decreasing values up to 5-fold compared with non-treated controls (Fig. 6A; Supplementary Data Fig. S1). On the other hand, only the organophosphate DIFP seriously hampered the pollen tube growth rate, whereas neither neostigmine nor pCMB seemed to affect the pollen tube length significantly after 6 h of culture (Fig. 6B; Supplementary Data Fig. S1).
Fig. 6.
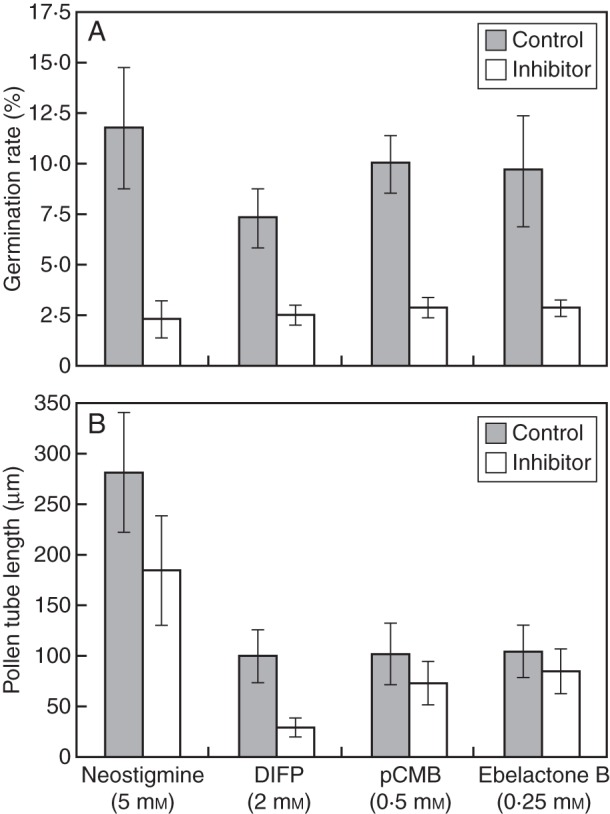
(A) Effect of several esterase inhibitors on olive pollen capacity to germinate in vitro. After 6 h of culture, all the inhibitors significantly decreased the germination rate compared with non-treated controls. (B) Effect of several esterase inhibitors on pollen tube growth in vitro. After 6 h of culture, only DIFP induced a significant decrease in the pollen tube length (μm) compared with a non-treated control.
Sub-cellular localization of esterolytic activity in olive pollen
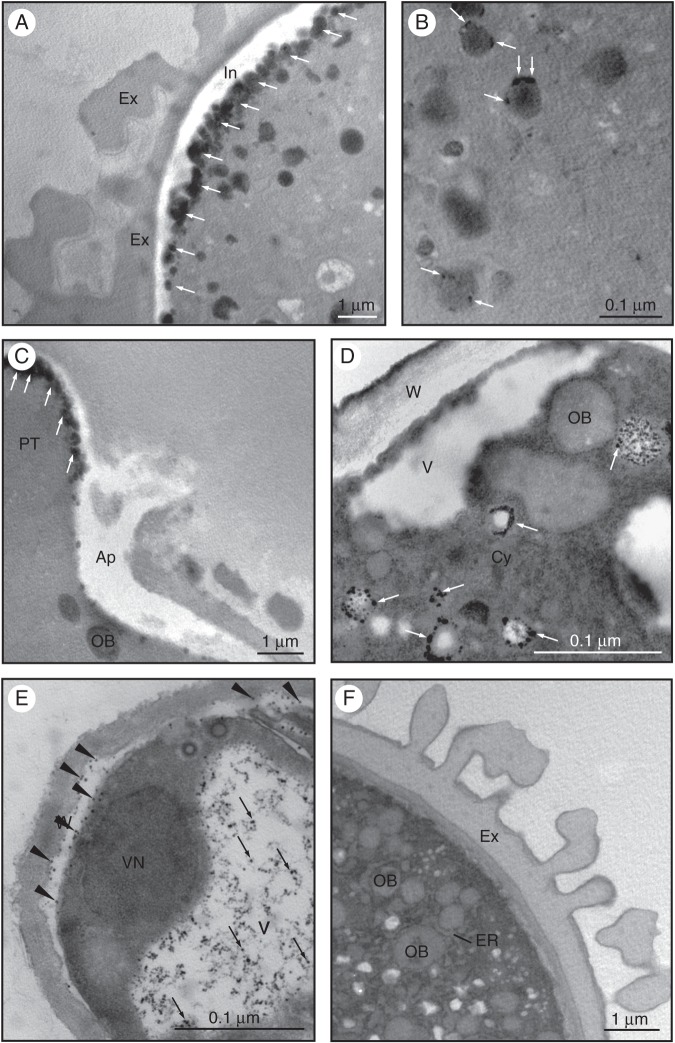
Non-specific esterase activity was first localized in mature pollen grains at the ultrastructural level using α-naphthyl acetate as a substrate for the enzyme reaction (Fig. 7). We observed the accumulation of electron-dense precipitates in the intine, particularly at the aperture (Fig. 7A, arrows). In the cytoplasm of the vegetative cell, precipitates were also localized to a lesser extent at the periphery of round-shaped electron-dense structures that probably correspond to oil bodies (OBs; Fig. 7B, arrows). In the germinating pollen, precipitates were accumulated at the tip of the pollen tube (Fig. 7C, arrows) as well as at the boundaries of small vesicles scattered throughout the cytoplasm (Fig. 7D, arrows). In the distal region of the pollen tube, far from the apex, precipitates were heavily accumulated inside vacuoles and in the inner callose layer of the pollen tube wall (Fig. 7E, arrows). The control reaction did not show any labelling (Fig. 7F).
Fig. 7.
Ultrastructural localization of non-specific esterase activity in olive pollen. (A) In mature pollen, we observed the accumulation of electron-dense precipitates in the intine, particularly at the apertural region (arrows). (B) In the cytoplasm of the vegetative cell, electron-dense precipitates localized in round-shaped structures that correspond to oil bodies (arrows). (C) During pollen germination, precipitates were visible in the pectin wall at the tip of the pollen tube (arrows). (D) An intense esterase activity was also found around small vesicles spread throughout the cytoplasm (D, arrows). (E) At the distal zone of the pollen tube, precipitates were observed in the inner callose layer of the pollen tube wall (arrowheads) and inside the vacuole (arrows). (F) Control reaction as in (A–E) but without the enzyme substrate. Abbreviations: Ap, aperture; Cy, cytoplasm; ER, endoplasmic reticulum; Ex, exine; In, intine; OB, oil body; PT, pollen tube; W, pollen wall; V, vacuole; VN, vegetative nucleus.
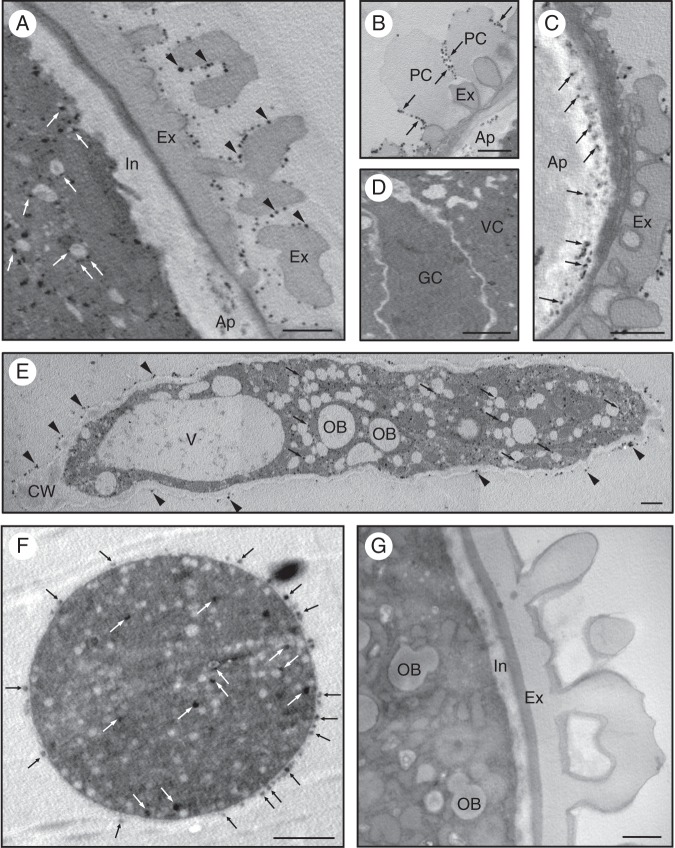
We also carried out cytochemical studies in order to localize AChE activity at the ultrastructural level in the mature and germinating pollen of the olive. In the mature pollen grain, electron-dense precipitates were mainly observed in the exine (Fig. 8A, arrowheads) as well as in the pollen coat (Fig. 8B, arrows). To a lesser extent, electron-dense precipitates were also located in the vegetative cell, attached to small vesicles scattered in the cytoplasm (Fig. 8A, arrows). After pollen hydration, an intense labelling was found specifically in the swollen apertures (Fig. 8C, arrows). The generative cell did not show any significant labelling (Fig. 8D). During pollen germination, numerous electron-dense precipitates were observed in the pollen tube cytoplasm, that were often attached to short endoplasmic reticulum (ER) cisternae and small vesicles that were fused with the plasma membrane (Fig. 8E, arrows). An intense labelling was also observed attached to the pollen tube wall surface (Fig. 8E, arrowheads). In the sub-apical region of the pollen tube, a similar sub-cellular localization was observed (Fig. 8F). We did not find electron-dense precipitates in the controls (Fig. 8G).
Fig. 8.
Ultrastructural localization of acetycholinesterase (AChE) activity in olive pollen. (A) In the hydrated pollen, AChE activity was observed on the exine surface (arrowheads) and attached to small vesicles spread in the cytoplasm (arrows). (B) Electron-dense precipitates were also localized in the pollen coat (arrows). (C) An intense AChE activity was found in the intine layer at the three apertural regions (arrows). (D) No enzyme activity was observed in the generative cell. (E) Longitudinal section of an olive pollen tube showing intense AChE activity in the cytoplasm (arrows), located mainly attached to ER cisternae and vesicles as well as in the pectin layer of the pollen tube wall (arrowheads). (F) Transversal section of the sub-apical zone of an olive pollen tube. Precipitates were localized in the outermost layer of the pollen tube wall (black arrows), while a few precipitates were also present attached to small cytoplasmic vesicles (white arrows). (G) Control reaction as in Fig. 8A but without enzyme substrate. Abbreviations: Ap, aperture; Cy, cytoplasm; Ex, exine; GC, generative cell; In, intine; OB, oil body; PC, pollen coat; V, vacuole; VC, vegetative cell. Scale bars = 0·2 µm.
DISCUSSION
A small number of pollen enzymes with esterolytic capacity have been identified and characterized to date, mainly from plants possessing a dry stigma (Hiscock et al., 1994, 2002a; Lavithis and Bhalla, 1995; Shakya and Bhatla, 2010) and also from species with a wet-type stigma (Shivanna and Sastri, 1981; Mu et al., 1994). However, to our knowledge, this is the first report involving a systematic functional classification of esterases obtained from pollen grains of a plant with a wet-type stigma.
Mature olive pollen grains contain 14 different esterase enzymes with a capacity to hydrolyse non-specific ester substrates such as α-naphthyl acetate. These enzymes were functionally classified as carboxylesterases, acetylesterases and AChEs. Olive pollen did not show arylesterase activity. First, we identified two putative carboxylesterases of 25·7 and 32·8 kDa. In Brassica, up to six carboxylesterases were identified in pollen grains (Hiscock et al., 1994, 2002a), while five isoenzymes were detected in pollen grains of sunflower (Shakya and Bhatla, 2010). The sub-cellular localization pattern of non-specific esterase activity in the olive pollen grain and pollen tube was similar to that previously reported in Vicia faba (Bednarska, 1992). The presence of esterase enzymes in the intine is in good agreement with the idea that this structure serves as a reservoir for esterolytic enzymes, which are released upon contact with the stigma (Hiscock et al., 1994). A 22 kDa esterase was shown to be an active cutinase involved in stigma cuticle penetration (Hiscock et al., 1994). It is unlikely that any of the olive pollen carboxylesterases are cutinase homologues since the wet stigma of the olive is not cuticularized (Serrano et al., 2008). Interestingly, we found that the capacity of olive pollen to germinate in vitro was seriously hampered and pollen tube growth was slowed down when these enzymes were chemically inhibited. Inhibition assays demonstrated that these esterases are required in vivo for pollen tube penetration of the stigmatic papillae (Hiscock et al., 2002a). Our data suggest that in addition to its function in pollen tube penetration of the stigma reported in Brassica napus, these carboxylesterases might also be required for pollen tube growth itself. Esterase-specific inhibitors such as ebelactone B also produced a decrease in pollen germination on B. napus stigmas (Hiscock et al., 2002). This fact strongly suggests that the inhibitory effects we observed in vitro are likely to occur on olive stigmas as well, although this hypothesis needs to be experimentally verified.
A second group of olive pollen non-specific esterases comprised six acetylesterases. This class of enzymes was previously identified in leaf and fruit tissues (Nielsen et al., 2002; Orasmo et al., 2007). Acetylesterases have also been detected in reproductive tissues. Nielsen et al. (2002) found a strong acetylesterase activity within the lime (Citrus aurantifolia) anther, which was located in the tapetum and pollen grains. During pollination, acetylesterase activity was detected in the cytoplasm of both dispersed mature pollen grains and stigmatic papillae. Interestingly, some galacturonyl residues in the backbone of wall pectic compounds are often O-acetylated at the C2 or C3 hydroxyl group (Perrone et al., 2002; Ralet et al., 2008). Recently, an acetylesterase (PtPAE1) from black cotton wood (Populus trichocarpa), which mediates pectin deacetylation, was functionally characterized in tobacco (Gou et al., 2012). Overexpression of this enzyme impaired pollen development within the anther, and hindered pollen germination and pollen tube elongation, resulting in severe male sterility. Tentatively, we can speculate that olive pollen acetylesterases might be involved in a similar function, but experimental data are needed to support this hypothesis.
A third group of olive pollen esterases included six putative AChEs. As expected, these enzymes were completely inhibited by OP compounds and carbamates (Sagane et al., 2005). The existence of cholinesterase activity in mature pollen was first evidenced in V. faba by means of histochemical techniques (Bednarska, 1992). Similarly, olive pollen AChE activity was mainly located extracellularly, closely attached to the exine surface, or associated with the pollen coat material. Our data suggest that olive pollen AChEs might have a double sporophytic and gametophytic origin. This idea is supported by the presence of AChE activity in the anther tapetum remnants, which are deposited onto the pollen wall surface and fill the exine cavities (unpublished data). During germination, the ultrastructural localization of AChE activity in the pollen tube suggests that AChE is released by exocytosis into the extracellular space through the ER/Golgi pathway. Further, we have identified for the first time pollen AChE enzymes on polyacrylamide gels, allowing us to determine the number of isoforms. The molecular masses of these bands were also coincident with the molecular masses of AChEs described in other plant species (Luppa and Andrä, 1983). These results are in good agreement with previous studies reporting in vitro biochemical detection of cholinesterase activity in aqueous homogenates from anther and pollen of several plant species (Semenova and Roshchina, 1993; Roshchina and Semenova, 1994). We found that neostigmine negatively affects the germination capacity of olive pollen. A recent study has pointed out a role for acetylcholine (ACh) in plant reproduction. The study showed that elongation of pollen tubes in the pistils of lily after self-incompatible pollination is promoted by ACh (Tezuka et al., 2007). These authors proposed that endogenous levels of ACh in pistils might control elongation of pollen tubes after pollination; therefore, self-incompatibility results from a decrease in ACh concentration. Moreover, the activity of AChE in self-compatible pistils and pollen grains is higher than that after cross-pollination (Roshchina, 1991; Kovaleva and Roshchina, 1997; Tezuka et al., 2007). Whether a similar mechanism exists in the olive still lacks experimental evidence. However, the extracellular localization of this enzyme supports its function as a signal transducer.
Using a long-chain fatty acid ester as an enzyme substrate, we also identified a group of 12 putative lipases in olive pollen, six of which also had the ability to hydrolyse non-specific ester substrates. Lipases have been localized in the extracellular pollen coat using both biochemical and microscopy methods (Mayfield et al., 2001; Shakya and Bhatla 2010). However, there is no information about the cellular localization of these enzymes inside pollen grains. Interestingly, we detected a strong non-specific esterase activity in the boundaries of OBs. These organelles accumulate during olive pollen ontogeny and supply the pollen with energy for early pollen tube growth (Zienkiewicz et al., 2010, 2011). It is generally accepted that triacylglycerides (TAGs) stored in seed OBs are hydrolysed to free fatty acids and glycerol by the sequential action of one or more lipases (Quettier and Eastmond, 2009). Similarly, we could expect that lipases that reside on the surface of pollen OBs might be involved in TAG hydrolysis.
To conclude, we have carried out for a first time a systematic functional classification of esterase enzymes in pollen grains from a plant species with wet stigma. Olive pollen esterases can be classified into four different functional groups, namely carboxylesterases, acetylesterases, AChEs and lipases. The cellular localization of esterase activity indicates that the intine is a putative storage site for esterolytic enzymes in the olive pollen. Based on inhibition assays, we can conclude that these enzymes are likely to be involved in pollen germination, and pollen tube growth and penetration of the stigma.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Ms C. Martínez-Sierra for her expert technical assistance, and the CIFA ‘Venta del Llano’ (Mengíbar, Jaén, Spain) for kindly providing us with the pollen material. This work was supported by the Spanish Ministry of Science and Innovation (MICINN) (ERDF-cofinanced project AGL2008-00517) and the Junta de Andalucía (ERDF-cofinanced project P2010-CVI5767). A.Z. thanks the CSIC for providing JAEdoc grant funding.
LITERATURE CITED
- Bednarska E. The localization of nonspecific esterase and cholinesterase activity in germinating pollen and in pollen tube of Vicia faba L. The effect of actinomycin D and cycloheximide. Biologia Plantarum. 1992;34:229–240. [Google Scholar]
- Bílková J, Albrechtová J, Opatrná J. Histochemical detection and image analysis of non-specific esterase activity and the amount polyphenols during annual bud development in Norway spruce. Journal of Experimental Botany. 1999;35:1129–1138. [Google Scholar]
- Bosch M, Cheung AY, Hepler PK. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiology. 2005;138:1334–1346. doi: 10.1104/pp.105.059865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker JL. Pollen enzymes and isoenzymes. In: Heslop- Harrison J, editor. Pollen: development and physiology. London: Butterworths; 1971. pp. 156–170. [Google Scholar]
- Catoire L, Pierron M, Morvan C, du Penhoat CH, Goldberg R. Investigation of the action patterns of pectin methylesterase isoforms through kinetic analyses and NMR spectroscopy. Journal of Biological Chemistry. 1998;50:33150–33156. doi: 10.1074/jbc.273.50.33150. [DOI] [PubMed] [Google Scholar]
- Dafni A, Maués MM. Rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction. 1998;11:177–180. [Google Scholar]
- Dodeman VL, Ducreux G. Isozyme patterns in zygotic and somatic embryogenesis of carrot. Plant Cell Reports. 1996;16:101–105. doi: 10.1007/BF01275460. [DOI] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D. Pollen and stigma structure and function: the role of diversity in pollination. The Plant Cell. 2004;16:S84–S97. doi: 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson C, Teeri TT, Siika-aho M, Read SM, Bacic A. Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta. 1998;206:452–460. [Google Scholar]
- Geitmann A, Steer M. The architecture and properties of the pollen tube cell wall. In: Malhó R, editor. The pollen tube. A cellular and molecular perspective. Berlin: Springer-Verlag; 2006. pp. 177–200. [Google Scholar]
- Goldberg R, Morvan C, Jauneau A, Jarvis MC. Methyl-esterification, de-esterification and gelation of pectins in the primary cell wall. Progress in Biotechnology. 1996;14:151–172. [Google Scholar]
- Gou JY, Miller LM, Hou G, Yu XH, Chen XY, Liu CJ. Acetylesterase-mediated deacetylation of pectin impairs cell elongation, pollen germination, and plant reproduction. The Plant Cell. 2012;24:50–65. doi: 10.1105/tpc.111.092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J. Pollen germination and pollen tube growth. International Review of Cytology. 1987;107:1–78. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technology. 1970;45:115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y, Barber J. The stigma surface in incompatibility responses. Proceedings of the Royal Society B: Biological Sciences. 1975;188:287–297. [Google Scholar]
- Heslop-Harrison J, Knox RB, Heslop-Harrison Y, Mattson O. Pollen wall proteins: emission and role in incompatibility response. Biological Journal of the Linnaean Society. 1975;7:189–202. [Google Scholar]
- Heslop-Harrison Y, Shivanna KR. The receptive surface of the Angiosperm stigma. Annals of Botany. 1977;41:1233–1258. [Google Scholar]
- Hiscock SJ, Dewey FM, Coleman JOD, Dickinson HG. Identification and localization of an active cutinase in the pollen of Brassica napus L. Planta. 1994;193:377–384. [Google Scholar]
- Hiscock SJ, Bown D, Sarah J, Dickinson HG. Serine esterases are required for pollen tube penetration of the stigma in Brassica. Sexual Plant Reproduction. 2002;15:65–74. [Google Scholar]
- Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG. The stigma surface and pollen–stigma interactions in Senecio squalidus L. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. International Journal of Plant Science. 2002;163:1–16. [Google Scholar]
- Jauh GY, Lord EM. Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta. 1996;199:251–261. [Google Scholar]
- Jiang L, Yang SL, Xie LF, et al. VANGUARD1 encodes a pectin methylesterases that enhances pollen tube growth in the Arabidopsis style and transmitting tract. The Plant Cell. 2005;17:584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RB, Heslop-Harrison J. Pollen wall proteins: localization and enzymic activity. Journal of Cell Science. 1970;6:1–27. doi: 10.1242/jcs.6.1.1a. [DOI] [PubMed] [Google Scholar]
- Kovaleva LV, Roshchina VV. Does cholinesterase participate in the intercellular interactions in pollen–pistil system? Biologia Plantarum. 1997;39:207–213. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavithis M, Bhalla PL. Esterases in pollen and stigma of Brassica. Sexual Plant Reproduction. 1995;8:289–298. [Google Scholar]
- Li YQ, Chen F, Linskens HF, Cresti M. Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sexual Plant Reproduction. 1994;7:145–152. [Google Scholar]
- Lord EM. Adhesion and guidance in compatible pollination. Journal of Experimental Botany. 2003;54:47–54. doi: 10.1093/jxb/erg015. [DOI] [PubMed] [Google Scholar]
- Luppa H, Andrä J. The histochemistry of carboxylester hydrolases: problems and possibilities. Histochemical Journal. 1983;15:111–137. doi: 10.1007/BF01042281. [DOI] [PubMed] [Google Scholar]
- Maiti IB, Kolattukudy PE, Shaykh M. Purification and characterization of a novel cutinase from nasturtium (Tropaeolum majus) pollen. Archives of Biochemistry and Biophysics. 1979;196:412–423. doi: 10.1016/0003-9861(79)90292-3. [DOI] [PubMed] [Google Scholar]
- Mäkinen Y, Brewbaker JL. Isozyme polymorphism in flowering plants I. Diffusion of enzymes out of intact pollen grains. Physiologia Plantarum. 1967;20:477–482. [Google Scholar]
- Mäkinen Y, MacDonald T. Isoenzyme polymorphism in flowering plants II. Pollen enzymes and isoenzymes. Physiologia Plantarum. 1968;21:477–486. [Google Scholar]
- Mattsson O, Knox RB, Heslop-Harrison J, Heslop-Harrison Y. Protein pellicle of stigmatic papillae as a probable recognition site in incompatibility reactions. Nature. 1974;247:298–300. [Google Scholar]
- Mayfield JA, Fiebig A, Johnstone S, Preuss D. Gene families from Arabidopsis thaliana pollen coat proteome. Science. 2001;292:2482–2485. doi: 10.1126/science.1060972. [DOI] [PubMed] [Google Scholar]
- Mu JH, Stains JP, Kao TH. Characterization of a pollen-expressed gene encoding a putative pectin esterase of Petunia inflata. Plant Molecular Biology. 1994;25:539–544. doi: 10.1007/BF00043881. [DOI] [PubMed] [Google Scholar]
- Nielsen JE, Christensen TMIE. Distribution of pectin methyl esterase and acetylesterase in the genus Citrus visualized by tissue prints and chromatography. Plant Science. 2002;162:799–807. [Google Scholar]
- Orasmo GR, Oliveira-Collet SA, Lapenta AS, Machado MFPS. Biochemical and genetic polymorphisms for carboxylesterase and acetylesterase in grape clones of Vitis vinifera (Vitaceae) cultivars. Biochemical Genetics. 2007;45:663–670. doi: 10.1007/s10528-007-9103-0. [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005;220:582–592. doi: 10.1007/s00425-004-1368-5. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry: theoretical and applied. 3rd edn. Vol. 2. Edinburgh: Churchill-Livingstone; 1972. [Google Scholar]
- Perrone P, Hewage CM, Thomson AR, Bailey K, Sadler IH, Fry SC. Patterns of methyl and O-acetyl esterification in spinach pectins: new complexity. Phytochemistry. 2002;60:67–77. doi: 10.1016/s0031-9422(02)00039-0. [DOI] [PubMed] [Google Scholar]
- Pinney K, Polito VS. Olive pollen storage and in vitro germination. Acta Horticulture. 1990;286:207–209. [Google Scholar]
- Purdy RE, Kolattukudy PE. Depolymerization of a hydroxyl fatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi: isolation and some properties of the enzyme. Archives of Biochemistry and Biophysics. 1973;159:61–69. doi: 10.1016/0003-9861(73)90429-3. [DOI] [PubMed] [Google Scholar]
- Quettier AL, Eastmond PJ. Storage oil hydrolysis during early seedling growth. Plant Physiology and Biochemistry. 2009;47:485–490. doi: 10.1016/j.plaphy.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Ralet MC, Crépeau MJ, Bonnin E. Evidence for a blockwise distribution of acetyl groups onto homogalacturonans from a commercial sugar beet (Beta vulgaris) pectin. Phytochemistry. 2008;69:1903–1909. doi: 10.1016/j.phytochem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Roshchina VV. Biomediators in plants: acetylcholine and biogenic amines. Pushchino: Biological Center of USSR Academy of Sciences; 1991. [Google Scholar]
- Roshchina VV, Semenova MN. Plant cholinesterases: activity and substrate-inhibitory specificity. Journal of Evolutionary Biochemistry and Physiology. 1994;26:644–651. [Google Scholar]
- Sagane Y, Nakagawa T, Yamamoto K, Michikawa S, Oguri S, Momonoki Y. Molecular characterization of maize acetylcholinesterase. A novel enzyme family in the plant kingdom. Plant Physiology. 2005;138:1359–1371. doi: 10.1104/pp.105.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova MN, Roshchina VV. The occurrence of cholinesterase in anthers of higher plants. Russian Journal of Plant Physiology. 1993;40:255–259. [Google Scholar]
- Serrano I, Suárez C, Olmedilla A, Rapoport HF, Rodríguez-García MI. Structural organization and cytochemical features of the pistil in Olive (Olea europaea L.) cv. ‘Picual’ at anthesis. Sexual Plant Reproduction. 2008;21:99–111. [Google Scholar]
- Shakya R, Bhatla SCh. A comparative analysis of the distribution and composition of lipidic constituents and associated enzymes in pollen and stigma of sunflower. Sexual Plant Reproduction. 2010;23:163–172. doi: 10.1007/s00497-009-0125-0. [DOI] [PubMed] [Google Scholar]
- Shaykh M, Kolattukudy PE, Davis R. Production of a novel extracellular cutinase by the pollen and the chemical composition and ultrastructure of the stigma cuticle of nasturtium (Tropaeolum majus) Plant Physiology. 1977;60:907–915. doi: 10.1104/pp.60.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna KR, Sastri DC. Stigma-surface esterase activity and stigma receptivity in some taxa characterized by wet stigmas. Annals of Botany. 1981;47:53–64. [Google Scholar]
- Stejskal J, Griga M. Comparative analysis of some isozymes and proteins in somatic and zygotic embryos of soybean (Glycine max L. Merr.) Journal of Plant Physiology. 1995;146:497–502. [Google Scholar]
- Swanson R, Edlund AF, Preuss D. Species specificity in pollen–pistil interactions. Annual Review of Genetics. 2004;38:793–818. doi: 10.1146/annurev.genet.38.072902.092356. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Shimada T, Kondo M, et al. Ectopic expression of an esterase, which is candidate for the unidentified plant cutinase, causes cuticular defects in Arabidopsis thaliana. Plant and Cell Physiology. 2010;51:123–131. doi: 10.1093/pcp/pcp173. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Akita I, Yoshino N, Suzuki Y. Regulation of self-incompatibility by acetylcholine and cAMP in Lilium longiflorum. Journal of Plant Physiology. 2007;164:878–885. doi: 10.1016/j.jplph.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Webb, EC. Enzyme nomenclature 1992: recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the nomenclature and classification of enzymes. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Zienkiewicz K, Castro AJ, Alché JD, Zienkiewicz A, Suarez C, Rodríguez-García MI. Identification and localization of a caleosin in olive (Olea europaea L.) pollen during in vitro germination. Journal of Experimental Botany. 2010;61:1537–1546. doi: 10.1093/jxb/erq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienkiewicz K, Zienkiewicz A, Rodríguez-García MI, Castro AJ. Characterization of a caleosin expressed during olive (Olea europaea L.) pollen ontogeny. BMC Plant Biology. 2011;11(122.) doi: 10.1186/1471-2229-11-122. http://dx.doi.org/10.1186/1471-2229-11-122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.