Abstract
There is mounting evidence that tumors are initiated by a rare subset of cells called cancer stem cells (CSCs). CSCs are generally quiescent, self-renew, form tumors at low numbers, and give rise to the heterogeneous cell types found within a tumor. CSCs isolated from multiple tumor types differentiate both in vivo and in vitro when cultured in serum, yet the factors responsible for their differentiation have not yet been identified. Here we show that vitronectin is the component of human serum driving stem cell differentiation through an integrin αVβ3-dependent mechanism. CSCs cultured on vitronectin result in downregulation of stem cell genes, modulation of differentiation markers, and loss of β-catenin nuclear localization. Blocking integrin αVβ3 inhibits differentiation and subsequently tumor formation. Thus, CSCs must be engaged by one or more extracellular signals to differentiate and initiate tumor formation, defining a new axis for future novel therapies aimed at both the extrinsic and intracellular pathways.
Keywords: Prostate cancer, Breast cancer, Tumor-initiating, Vitronectin, Arginine-glycine-aspartic acid peptide, Integrin alphaVbeta3
Introduction
Cancer stem cells (CSCs) are a subset of cells capable of giving rise to both hematopoietic [1, 2] and solid tumors [3–8]. These tumor-initiating cells have been named CSCs because they retain many properties of normal stem cells, including the ability to self-renew [8, 9] and differentiate into the heterogeneous cells present in tumors [3, 8, 10]. Furthermore, they express genes (such as BMI1, Oct-3/4, and Nanog) [10, 11] and utilize signaling pathways, including Hedgehog [12–14], Notch [15, 16] and Wnt/β-catenin [17, 18], known to be important in the maintenance and self-renewal of both embryonic and hematopoietic stem cells.
Prostate CSCs have been identified through different approaches, including flow cytometric sorting using various markers such as CD44 [7, 10], integrin α2β1 [19], and CD133 [20], and by isolating cells that form spheres in low-attachment culture conditions [21, 22], whereas breast CSCs have been identified as CD44+CD24− cells [3]. Isolated CSCs maintained in vitro retain CSC properties as long as they are cultured in conditions that favor anchorage independence and do not include serum [10, 23, 24]. The addition of serum or growth of the cells in a xenograft model results in these cells shifting their phenotype away from CSCs to the more differentiated cells found within the tumors [7, 10, 23]. Therefore, an extrinsic factor present in the microenvironment of CSCs is responsible for inducing differentiation.
The tumor microenvironment, including the extracellular matrix (ECM), is an important factor contributing to both the maintenance of tissue homeostasis and the promotion of tumorigenesis [25, 26]. Major components of the ECM include collagens, laminin, fibronectin, and vitronectin (VN), and signaling by the ECM largely occurs through the integrin family of proteins [17]. Integrins are composed of an α and β chain leading to a large set of family signaling molecules involved in a diverse set of cellular processes such as adhesion, migration, polarity, cell survival, apoptosis, and proliferation [17]. Integrins have important roles in stem cell fate as well. Embryonic stem cells differentiate when grown on either fibronectin or laminin [27]. VN, and its main receptor integrin αVβ3, can support the differentiation of the endoderm from the inner cell mass in mouse development [28], and contact with VN alone is sufficient to induce the osteogenic differentiation of mesenchymal stem cells [29]. Additionally, several CSCs express integrins that bind ECM components, including integrin αV, on prostate CSCs [30–32]. However, their role in CSC biology is unknown.
To determine what component present in serum was driving differentiation of the CSCs, we fractionated human serum and performed microcapillary reversed-phase liquid chromatography-tandem mass spectrometry analysis on the fraction that retained the capacity to differentiate the CSCs. We identified VN as the human serum component regulating differentiation of both prostate and breast CSCs. VN mediates its effects predominantly through integrin αVβ3 and results in altered β-catenin localization. Moreover, we determined that blocking vitronectin not only blocks differentiation of CSCs by inhibiting signaling of its receptor, integrin αVβ3, but it also blocks tumor formation.
Materials and Methods
Isolation of CSCs
CSCs were obtained from LNCaP and MCF7 by flow cytometry sorting CD44+CD24− cells as previously described [10]. Prostate cancer stem cells (PCSC2 and PCSC3) were purchased from CelProgen (San Pedro, CA, http://www.celprogen.com) and are short-term cultures of primary prostate stem cells collected from resected prostates. Following resection, the prostate tumors were disaggregated, selected on the basis of CD133 expression, single-cell cloned, and further tested for tumorigenesis [33]. PCSCs are maintained using proprietary maintenance media and plates (CelProgen) as recommended by the supplier. PCSCs are further enriched for CSCs by plating in conditions that favor nonadherent sphere formation (SCM+1% KnockOut Serum Replacement media; see below), as demonstrated for many prostate cell lines and primary samples [7, 10, 21, 22, 34].
Culture of Isolated CSCs
CSCs were maintained as nonadherent spheres in stem cell media (stem cell medium (SCM); F12:Dulbecco’s modified Eagle’s medium supplemented 10 ng/ml bFGF, 20 ng/ml EGF, 5 μg/ml insulin, and 0.4% bovine serum albumin) as previously described [10]. For comparison during differentiation studies, PCSCs were maintained in SCM supplemented with 1% knockout serum (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) where they grow as spheres. Human serum (Gemini Bio-Products, West Sacramento, CA, http://www.gembio.com/index.cfm?page=926756) was added as indicated. Antibodies and peptides were added at the same time as human serum when indicated. CSCs were plated at a density of 100 cells per well in 96-well plates for differentiation purposes.
Quantitative Real Time RT-PCR
Gene expression was measured by quantitative real time polymerase chain reaction (qRT-PCR) using cDNA generated from cell lysates obtained from 1,000 to 3,000 cells using Ambion’s Cells-to-Ct kit (Applied BioSystems, Foster City, CA, http://www.applied-biosystems.com) following the manufacturer’s recommendations. Quantification of mRNA levels was obtained using Assays-on-Demand Taqman primers/probes and Gene Expression Master mix (Applied Biosystems) and read on a StepOne real-time detector (Applied Bioystems). Expression was normalized to the reference gene, 18S.
Microarray Analysis
Total RNA was isolated using Trizol (Invitrogen) from the indicated cells and amplified using Ambion’s MessageAMP II aRNA amplification kit (Applied Bio-systems). Agilent 4 × 44k whole genome arrays (Agilent, Santa Clara, CA, http://www.home.agilent.com/agilent/home.jspx?cc=US&lc=eng) were performed as previously described [33].
Western Blots and In-Cell Westerns
For western blots, cells were lysed in RadioImmuno Precipitation Assay (RIPA) buffer and 30 μg were loaded on 4–20% gradient gels (Invitrogen). IRDye (LI-COR, Lincoln, NE, http://www.licor.com/) secondary antibodies were used and the blots were imaged on Licor’s Odysey Infrared scanner. For LNCaP and MCF-7 in-cell westerns, 1,000 CD44+CD24− cells were plated per well in a 384-well plate, and for PCSC2 and PCSC3, 10,000 cells were plated per well in a 96-well tissue culture plate. Human serum (1%) and peptides were added as indicated followed by an in-cell western as previously described [35].
Beta-Catenin Immunocytochemistry
Cells were plated (100,000 cells per well) on a four-well Lab-Tek chamber slide in either SCM or SCM supplemented with 1% KnockOut Serum Replacement and allowed to recover overnight. Cells were fixed with 4% paraformaldehyde, washed, and permeabilized with 0.5% saponin.
Mouse Xenograft Studies
NCI-Frederick is accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals [36]. Animals were randomized into three treatment groups: a control group that received 1:10 dimethyl sulfoxide (DMSO):saline mixture and the RAD (arginine-alanine-aspartic acid peptide) and RGD (arginine-glycine-aspartic acid) peptide groups that received peptides in 1:10 DMSO:saline at a dose of 15 mg/kg three times weekly.
Results
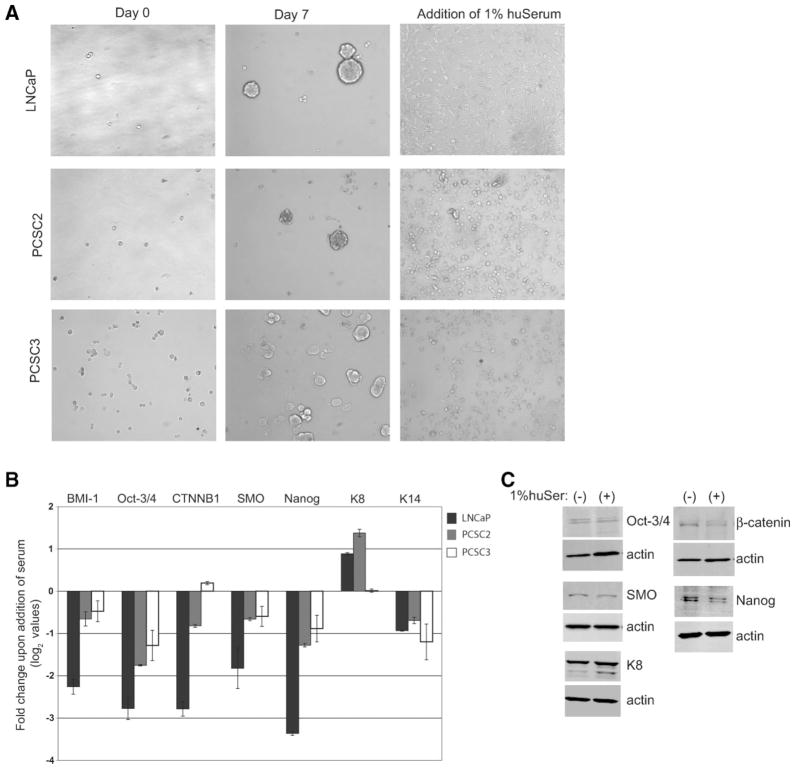
Since many CSCs can differentiate in vitro in the presence of serum [9, 10, 24, 37], we first determined whether CSCs derived from prostate cancer patients could also undergo differentiation. CCSs from both LNCaP and short-term cultures of primary patients (PCSCs) were isolated as indicated in Materials and Methods. Indeed, upon addition of serum, CSCs isolated from both LNCaP (CD44+CD24− cells) and PCSCs (nonadherent spheres) no longer grew as spheres, which is characteristic of prostate CSCs [7, 10, 21, 22, 34], but became an adherent monolayer that is a marker of differentiation [21, 38] (Fig. 1A). This dramatic shift in phenotype was accompanied by downregulation of several genes implicated in CSC self-renewal (Fig. 1B). The adherent cells also decreased expression of the basal cell maker cytokeratin 14 (K14) and increased the expression of the luminal marker cytokeratin 8 (K8), consistent with the differentiation of the cells from a basal to a more luminal-like cell type (Fig. 1B). There was a corresponding change in protein levels for PCSC2 (Fig. 1C). Taken together, these data demonstrate that prostate CSCs isolated from either established cell lines or from short-term cultures of primary patients differentiate when exposed to serum.
Figure 1.
Prostate cancer stem cells (CSCs) differentiate in culture with human serum. (A): Morphology of CSCs isolated from LNCaP and patient samples (PCSC2 and PCSC3) grown in serum-replacement media (left panels) and in 1% human serum (right panels). (B): Log2 values for fold-change of stem cell genes and cytokeratins of CSCs grown in 1% human serum compared with serum-replacement media. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was done in triplicate, normalized to 18S with standard error shown. (C): Western blots of CSCs isolated from PCSC2 grown in SCM+1% KnockOut serum replacement in the absence and presence of 1% human serum for 24 hours. Abbreviations: CSC, cancer stem cell; huSerum, human serum.
To elucidate the component in serum that drives CSCs to differentiate, we separated serum using weak anion-exchange chromotography, pooled several fractions together, and tested for their ability to induce differentiation of CSCs isolated from LNCaP (data not shown). The fraction retaining CSC differentiation activity was further separated, activity was again assessed (Supporting Fig. 1), and mass spectrometry was performed to identify the proteins within each fraction. Vitronectin (VN) was among the list of proteins identified (Supporting Table 1) and, because of its known role in differentiation, it represented a good candidate to investigate for its role in differentiation of prostate CSCs. VN and its main receptor, integrin αVβ3, can support the differentiation of the endoderm from the inner cell mass in mouse development [28], and contact with VN alone is sufficient to induce the osteogenic differentiation of mesenchymal stem cells [29]. Additionally, several CSCs express integrins that bind VN, including integrin αV, on prostate CSCs [30–32]. Therefore, we tested the ability of VN to induce the differentiation of CSCs.
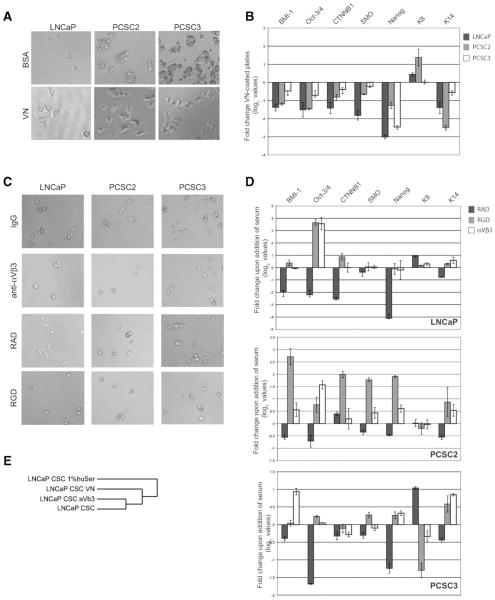
Culturing CSCs on VN-coated plates resulted in an adherent monolayer (Fig. 2A) reminiscent of cells grown in the presence of serum (Fig. 1A). This was true for CSCs isolated from both LNCaP (CD44+CD24−) and the primary patient samples (spheres), even though the LNCaP cell line as a total population has minimal binding to VN [39–41]. However, the percentage of CD44+CD24− cells in LNCaP (0.04%; [10] is consistent with the low level of binding to VN seen when the total cell line is used [40]. Furthermore, prostate CSCs grown on VN decreased expression of stem cell genes in comparison with cells grown on bovine serum albumin (BSA)-coated plates (Fig. 2B). Moreover, there was an increase in K8 and a decrease in K14 expression consistent with the differentiation of these cells (Fig. 2B). A blocking antibody against integrin αVβ3 and, to a much lesser extent, integrin αVβ5, but not integrin β1, inhibited the morphologic change induced by serum (Fig. 2C and Supporting Fig. 2) and attenuated the down-regulation of stem cell genes and the changes observed in the expression of certain cytokeratins (Fig. 2D). Furthermore, a cyclic RGD peptide (cyclicRGDfK), specific for the αVβ3 and αVβ5 integrins [42], but not the control RAD peptide, was also able to block these changes (Fig. 2C, 2D). Moreover, global gene expression profiling revealed that LNCaP CSCs grown on VN-coated plates and those grown in 1% human serum shared a common gene expression pattern (Fig. 2E). We observed similar results with CSCs (CD44+CD24−) [23, 44] isolated from the breast cancer cell line MCF7 (Supporting Fig. 3A, 3B). Thus, VN is sufficient to induce differentiation of both prostate and breast CSCs. Moreover, integrin αVβ3 appears to be the main receptor for VN in these cells, although a blocking antibody to integrin αVβ5 was able to partially abrogate the change in phenotype upon the addition of serum.
Figure 2.
Growth on vitronectin (VN) alone is sufficient to induce differentiation of prostate cancer stem cells (CSCs) in an integrin αVβ3–dependent mechanism. (A): Morphology of CSCs grown on either bovine serum albumim-coated (top) or VN-coated (bottom) plates. (B): Quantitative real time polymerase chain reaction (log2 values) for stem cell genes and cytokeratins of cells grown on VN-coated plates compared with BSA-coated plates. qRT-PCR was analyzed in the same manner as Figure 1B. (C): Growth of prostate CSCs in the presence of 1% human serum with the following additions, as indicated: preimmune IgG, anti-αVβ3 (10 μg/ml), RAD peptide (10 μg/ml) or RGD peptide (10 μg/ml). (D): Quantitative real time polymerase chain reaction (qRT-PCR) for stem cell genes and cytokeratins for cells grown as in Figure 2C. Log2 values are shown for qRT-PCR performed in triplicate, normalized to 18S and shown relative to the respective CSCs grown in 1% human serum. (E): Dendogram depicting the genomic relationship of LNCaP CSCs grown on either VN-coated plates or in 1% serum ± anti-αVβ3 based on gene expression of 694 genes showing twofold differences between CSCs grown in the absence and presence of 1% human serum. Abbreviations: BSA, bovine serum albumin; CSC, cancer stem cell; RAD, arginine-alanine-aspartic acid peptide; RGD, arginine-glycine-aspartic acid peptide; VN, vitronectin.
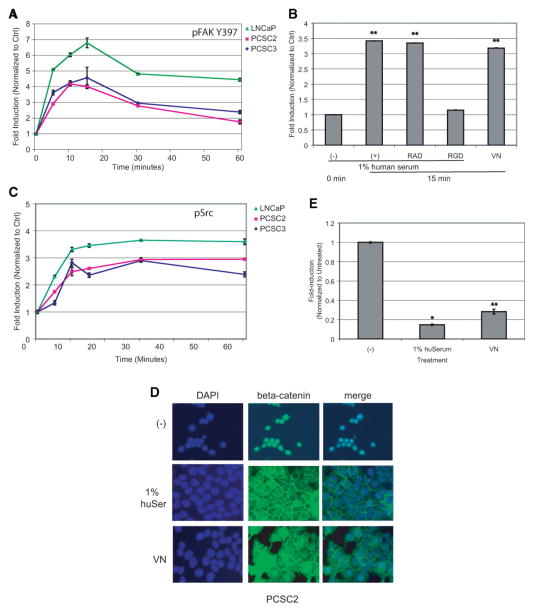
After engagement of most integrins, focal adhesion kinase (FAK) is phosphorylated mediating migration, proliferation, survival, and differentiation of many cell types [44]. Using an in-cell western, we determined that FAK is phosphorylated within 5 minutes and maximally at 15 minutes in prostate CSCs following the addition of serum (Fig. 3A). This increase in serum-induced FAK Y397 phosphorylation was blocked by the RGD peptide, indicating that integrin αVβ3 engagement activates FAK. Moreover, activation of FAK was seen when the cells were cultured in the absence of serum on VN-coated plates (Fig. 3B). Phosphorylation of FAK at Y397 reveals an SH2 domain for the association of Src [44]. We also observed a rapid phosphorylation of Src following addition of serum (Fig. 3C). Again, similar results were obtained for breast CSCs (Supporting Fig. 3C). However, both the Src inhibitor PP2 and short hairpin RNA (shRNA) knockdown of either FAK or Src were insufficient to prevent serum-induced differentiation of LNCaP cells (Supporting Fig. 4). This result may indicate that the level of knockdown achieved was not sufficient to effectively inhibit differentiation, or that there are other key factors involved in the differentiation and inhibition of either Fak or Src by itself is insufficient.
Figure 3.
Growth in 1% serum or on vitronectin (VN) results in phosphorylation of focal adhesion kinase (FAK) and Src and results in a redistribution of β-catenin. (A): Quantitation of an in-cell western showing a time course for the phosphorylation of FAK in cancer stem cells (CSCs) isolated from the indicated cell lines after the addition 1% human serum. (B): Quantitation of an in-cell western of CSCs isolated from PCSC2. Phosphorylation of FAK also occurs when cells are cultured on VN-coated plates and is blocked by the RGD peptide. Data are normalized to the 0-minute control where two asterisks represent Student’s t test p < .005. (C): Quantitation of an in-cell western showing a time course for the phosphorylation of Src in CSCs isolated from the indicated cell lines after the addition of 1% human serum. (D): Immunofluorescence of β-catenin in PCSC2 grown as indicated for 48 hours. (E): Luciferase assay of TOP-FLASH in LNCaP CSCs. Assay was performed in triplicate with standard error shown, where an asterisk represents Student’s t test p < .05 and two asterisks represent p < .005. Abbreviations: Ctrl, control; DAPI, 4′6-diamidino-2-phenylindole; huSer, human serum; RAD, arginine-alanine-aspartic acid peptide; RGD, arginine-glycine-aspartic acid peptide; VN, vitronectin.
Since activation of the FAK-Src complex can result in the activation of many downstream pathways [45], we performed a transcription factor analysis of genes downregulated by serum addition to LNCaP, PCSC2, and PCSC3 using the Broad Institutes’ molecular signatures database. There was a statistically significant over-representation of genes with binding sites for TCF3, LEF1, and c-Myc (Supporting Table 2). The transcription factors TCF and LEF can bind in a complex with the coregulator β-catenin, a known regulator of stem cell maintenance. Furthermore, c-Myc is a downstream target of the TCF/LEF/β-catenin pathway. The downregulation of TCF/LEF target genes suggests the loss of nuclear β-catenin. Immunofluorescence of β-catenin with spheres isolated from PCSC2 revealed that in the CSCs, β-catenin is nuclear, whereas β-catenin is plasma membrane-associated following the addition of serum or when cells are cultured on VN-coated plates (Fig. 3D). Similar results were obtained using spheres isolated from PCSC3 (data not shown). Furthermore, TCF/LEF transcriptional activity is reduced in CSCs isolated from LNCaP upon the addition of serum or when they are grown on VN-coated plates (Fig. 3E). Although there is evidence linking FAK activation and the localization of β-catenin at the plasma membrane during fibroblast adhesion [46] and v-Src transfected cells showed β-catenin present in cadherin-catenin complexes [47], the direct association of these events in the differentiation of prostate CSCs requires further investigation.
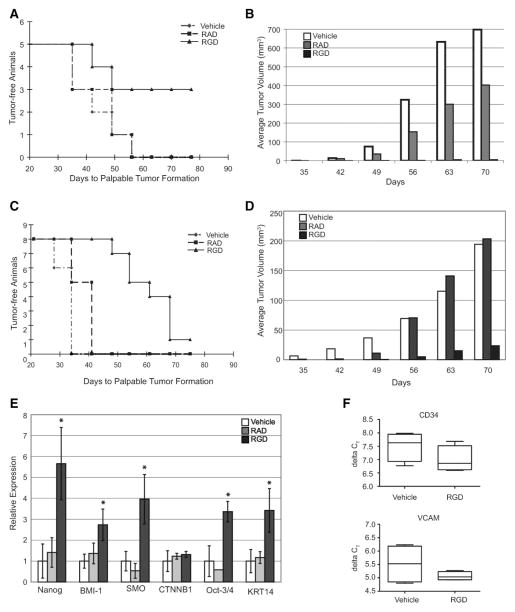
CSC differentiation into the more proliferative transient amplifying (TA) cells may drive tumorigenesis. Therefore, we hypothesized that blocking CSCs from differentiating would result in dormant CSCs, thus blocking CSC-driven tumorigenesis. Administration of RGD peptide (15 mg/kg, three times weekly) was able to reduce tumor incidence from 100% (5 of 5) in both the vehicle and RAD-treated mice to 40% (2 of 5) (Fig. 4A). Although two of the mice receiving RGD developed palpable tumors, the tumors did not grow and remained at 6 mm3 for 4 weeks (Fig. 4B). In contrast, mice receiving either vehicle or RAD had tumors that continued to grow. On day 78, injections of the peptides were halted, and in two of three animals that did not previously have palpable tumors, tumors began to grow (data not shown), indicating that the CSCs were still present and that the RGD peptide had not killed the CSCs, but had, instead, prevented outgrowth of the tumor. We also tested the ability of the RGD peptide to block tumorigenesis of patient-derived CSCs. The RGD peptide treated PCSC2-injected mice showed similar results (Fig. 4C, 4D). Moreover, the small tumors that resulted from RGD-treated animals injected with PCSC2 CSCs retained higher expression of stem cell genes (Fig. 4E). Since certain RGD peptides can reduce angiogenesis [48, 49], we tested the expression of both CD34 and vascular cell adhesion molecule (VCAM), markers of angiogenesis, in the tumors isolated from vehicle- and RGD-treated animals (Fig. 4F). There was no difference in the expression of these markers of angiogenesis between the RGD- and vehicle-treated animals, suggesting that angiogenesis was not impaired. Moreover, integrin β3 knockout mice do not exhibit defects in neovascularization [50], therefore, the ability of the cyclic-RGD peptide to reduce tumor formation suggests that blockade of CSC differentiation may be a viable cotreatment option.
Figure 4.
Tumor growth of prostate cancer stem cells (CSCs) is blocked by inhibiting integrin αVβ3. (A): Kaplan-Meier plot of palpable tumor formation in NOD/SCID mice injected with 2000 CSCs isolated from LNCaP and injected with vehicle, RAD or RGD peptide (15 mg kg−1) three times weekly. Mantel-Cox logrank test of RGD-treated and vehicle-treated data p < .05. (B): Volume of resulting LNCaP tumors, Student’s t test of vehicle- and RGD-treated samples p < .005. (C): Kaplan-Meier plot of palpable tumor formation in NOD/SCID mice injected with 100 CSCs isolated from PCSC2 and injected with vehicle, RAD, or RGD peptide (15 mg kg−1) three times weekly. Mantel-Cox logrank test of RGD-treated and vehicle-treated data p < .001. (D): Volume of tumors resulting from PCSC2 injections, Student’s t test of vehicle- and RGD-treated samples p < .001. (E): Quantitative real time polymerase chain reaction results for tumors removed from RAD-treated (n = 2) and RGD-treated (n = 4) mice. Values represent fold over the average expression of untreated animals (n = 4) and are normalized to GAPDH expression. Standard error is shown and an asterisk represent p < .05 for RGD-treated compared with vehicle-treated animals. (F): Measurement of murine CD34 and vascular cell adhesion molecules present in tumors from mice injected with CSCs isolated from PCSC2 and treated with either vehicle (n = 4) or RGD (n = 4). There is no statistically significant difference (CD34, t test p = .21 and F test p = .87; vascular cell adhesion molecules t test p = .49 and F test p = .079). Abbreviations: RAD, RAD peptide; RGD, RGD peptide; VCAM, vascular cell adhesion molecules. CSC, cancer stem cells; NOD/SCID, non-obese diabetic/severe combined immunodeficiency; RAD, arginine-alanine-aspartic acid peptide, RGD, arginine-glycine-aspartic acid peptide.
Discussion
We have identified VN as a component of serum driving the differentiation of both breast and prostate CSCs. Engagement of integrin αVβ3 by VN resulted in loss of expression of several genes implicated in stem cell maintenance and induced a cytokeratin profile more consistent with transit amplifying cells [51]. Both FAK and Src were phosphorylated and there was a loss of nuclear β-catenin after growing cells on VN-coated plates. Moreover, prostate CSC-driven tumor formation was blocked by inhibiting integrin αVβ3 in a xenograft model. Taken together, these data indicate that CSCs differentiate in response to extracellular VN, and that while CSCs are the only cells capable of giving rise to tumors, the tumorigenesis process itself requires CSC differentiation. To our knowledge, this is the first demonstration that CSCs must differentiate to form a tumor and that this process is controlled by an extrinsic signal.
VN and its receptor, integrin αVβ3, are implicated in the pathogenesis of several tumor types, including the brain, breast, prostate, where CSCs have been identified and characterized. In the glioblastoma, inhibition of integrin-linked kinase led to a decrease in soft-agar colony formation and invasion of glioblastoma cells lines [52] and downregulation of integrin αVβ3 led to an increase in sensitization to radiotherapy [53]. This would be consistent with our findings that engagement of integrin αVβ3 results in the loss of CSC characteristics. Moreover, in prostate cancer, integrin αVβ3 is expressed on primary prostate cancer cells, but not normal epithelium [40] and is involved mediating migration and metastasis, particularly to the bone [54]. Both resistance to radiotherapy and metastasis have been attributed to CSCs. Therefore, expression of integrin αVβ3 in CSCs may confer not only tumorigenic properties, but also metastatic capabilites through engagement of the extracellular matrix.
Although CSC-driven tumorigenesis results in tumors with heterogeneous phenotypes, the mechanisms and initiation factors required had not previously been determined. We have demonstrated that CSCs differentiate in response to vitronectin through engagement of integrin αVβ3 and redistribution of β-catenin from the nucleus to the cytoplasm. Moreover, this is a necessary step for tumorigenesis since blockade of the RGD-integrin αVβ3 interaction attenuates tumor formation. Therefore, although CSCs are the cells responsible for tumor formation, they must receive extrinsic cues, and this identifies a unique axis for the development of targeted therapies.
Supplementary Material
Acknowledgments
This research has been funded in part with federal funds from the National Cancer Institute, NIH, under contract No. N01-CO-12400. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Disclosure of Potential Conflicts of interest
The authors indicate no potential conflicts of interest.
Author contributions: E.H.: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; K.C.: Conception and design, collection and assembly of data, manuscript writing; M.A.D.S.: Provision of study materials, collection of data; S.T.: Collection of data; T.V.: Conception and design, financial support; W.F.: Conception and design, financial support, final approval of manuscript.
See www.StemCells.com for supporting information available online.
References
- 1.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dontu G, Al-Hajj M, Abdallah WM, et al. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 7.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 8.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 9.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 10.Hurt EM, Kawasaki BT, Klarmann GJ, et al. CD44(+)CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka H, Nakamura M, Kameda C, et al. The Hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24−/low subpopulation and the side population of breast cancer cells. Anti-cancer Res. 2009;29:2147–2157. [PubMed] [Google Scholar]
- 13.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Q, Yuan X, Liu G, et al. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–3026. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XP, Zheng G, Zou L, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008;307:101–108. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- 16.Sansone P, Storci G, Giovannini C, et al. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- 17.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi-Ihara N, Murohashi I, Nara N, et al. Promotion of the self-renewal capacity of human acute leukemia cells by Wnt3A. Anti-cancer Res. 2008;28:2701–2704. [PubMed] [Google Scholar]
- 19.Collins AT, Habib FK, Maitland NJ, et al. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 20.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 21.Shi X, Gipp J, Bushman W. Anchorage-independent culture maintains prostate stem cells. Dev Biol. 2007;312:396–406. doi: 10.1016/j.ydbio.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Stupack DG. The biology of integrins. Oncology (Williston Park) 2007;21:6–12. [PubMed] [Google Scholar]
- 26.Moschos SJ, Drogowski LM, Reppert SL, et al. Integrins and cancer. Oncology (Williston Park) 2007;21:13–20. [PubMed] [Google Scholar]
- 27.Hayashi Y, Furue MK, Okamoto T, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, He X, Corbett SA, et al. Integrins are required for the differentiation of visceral endoderm. J Cell Sci. 2009;122:233–242. doi: 10.1242/jcs.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salasznyk RM, Williams WA, Boskey A, et al. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 31.Kouros-Mehr H, Bechis SK, Slorach EM, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birnie R, Bryce SD, Roome C, et al. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9:R83. doi: 10.1186/gb-2008-9-5-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klarmann GJ, Hurt EM, Mathews LA, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulholland DJ, Xin L, Morim A, et al. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki BT, Hurt EM, Kalathur M, et al. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: An integrated molecular profiling approach. Prostate. 2009;69:827–837. doi: 10.1002/pros.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 37.Hodge DR, Peng B, Cherry JC, et al. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65:4673–4682. doi: 10.1158/0008-5472.CAN-04-3589. [DOI] [PubMed] [Google Scholar]
- 38.Mokry J, Karbanova J, Filip S, et al. Phenotypic and morphological characterization of in vitro oligodendrogliogenesis. Stem Cells Dev. 2008;17:333–341. doi: 10.1089/scd.2007.0091. [DOI] [PubMed] [Google Scholar]
- 39.Witkowski CM, Rabinovitz I, Nagle RB, et al. Characterization of integrin subunits, cellular adhesion and tumorgenicity of four human prostate cell lines. J Cancer Res Clinoncol. 1993;119:637–644. doi: 10.1007/BF01215981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng DQ, Woodard AS, Fornaro M, et al. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 41.Zheng DQ, Woodard AS, Tallini G, et al. Substrate specificity of alpha (v) beta(3) integrin-mediated cell migration, phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 42.Pfaff M, Tangemann K, Muller B, et al. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 43.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Hua ZC. FAK expression regulation and therapeutic potential. Adv Cancer Res. 2008;101:45–61. doi: 10.1016/S0065-230X(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 45.McLean GW, Carragher NO, Avizienyte E, et al. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 46.Ho AT, Voura EB, Soloway PD, et al. MMP inhibitors augment fibroblast adhesion through stabilization of focal adhesion contacts and up-regulation of cadherin function. J Biol Chem. 2001;276:40215–40224. doi: 10.1074/jbc.M101647200. [DOI] [PubMed] [Google Scholar]
- 47.Behrens J, Vakaet L, Friis R, et al. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saiki I, Murata J, Makabe T, et al. Inhibition of tumor angiogenesis by a synthetic cell-adhesive polypeptide containing the Arg-Gly-Asp (RGD) sequence of fibronectin, poly(RGD) Jpn J Cancer Res. 1990;81:668–675. doi: 10.1111/j.1349-7006.1990.tb02625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grant DS, Kleinman HK, Martin GR. The role of basement membranes in vascular development. Ann N Y Acad Sci. 1990;588:61–72. doi: 10.1111/j.1749-6632.1990.tb13197.x. [DOI] [PubMed] [Google Scholar]
- 50.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzo S, Attard G, Hudson DL. Prostate epithelial stem cells. Cell Prolif. 2005;38:363–374. doi: 10.1111/j.1365-2184.2005.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koul D, Shen R, Bergh S, et al. Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol Cancer Ther. 2005;4:1681–1688. doi: 10.1158/1535-7163.MCT-05-0258. [DOI] [PubMed] [Google Scholar]
- 53.Monferran S, Skuli N, Delmas C, et al. Alphavbeta3 and alphavbeta5 integrins control glioma cell response to ionising radiation through ILK and RhoB. Int J Cancer. 2008;123:357–364. doi: 10.1002/ijc.23498. [DOI] [PubMed] [Google Scholar]
- 54.McCabe NP, De S, Vasanji A, et al. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.