Abstract
The metabolic syndrome is characterized by a state of metabolic dysfunction resulting in the development of several chronic diseases that are potentially deadly. These metabolic deregulations are complex and intertwined and it has been observed that many of the mechanisms and pathways responsible for diseases characterizing the metabolic syndrome such as type 2 diabetes and cardiovascular disease are linked with cancer development as well. Identification of molecular pathways common to these diverse diseases may prove to be a critical factor in disease prevention and development of potential targets for therapeutic treatments. This review focuses on several molecular pathways, including AMPK, PPARs and FASN that interconnect cancer development, type 2 diabetes and cardiovascular disease. AMPK, PPARs and FASN are crucial regulators involved in the maintenance of key metabolic processes necessary for proper homeostasis. It is critical to recognize and identify common pathways deregulated in interrelated diseases as it may provide further information and a much more global picture in regards to disease development and prevention. Thus, this review focuses on three key metabolic regulators, AMPK, PPARs and FASN, that may potentially serve as therapeutic targets.
Keywords: Cancer, cancer stem cells, cardiovascular, diabetes, metabolic syndrome
INTRODUCTION
Metabolic syndrome describes a state of metabolic dysfunction in a subgroup of patients sharing risk for several chronic diseases such as obesity, diabetes, hypertension, stroke, hyperinsulinemia, atherosclerosis and other disorders [1]. The major components involved in the diagnosis of metabolic syndrome include insulin resistance, obesity, dyslipidemia and endothelial dysfunction (reviewed in [2, 3]). According to the definition proposed by the World Health Organization (WHO), insulin resistance is a requirement for a diagnosis of metabolic syndrome [4], The underlying causes associated with these potentially deadly metabolic deregulations are complex and intertwined. Recently, it has been observed that many of the mechanisms and pathways deemed responsible for development and progression of diseases are also linked with cancer development. These commonalities suggest that the metabolic deregulation observed in individuals suffering from diseases such as obesity, type 2 diabetes and cardiovascular disease may be associated with a predisposition to cancer. Identification of molecular pathways common to these seemingly diverse diseases may prove to be a nexus of focus for preventions on a broader scale of human health.
Cancer, which is characterized by a cell’s ability to undergo uncontrollable proliferation, is a leading cause of death among both men and women worldwide [5]. In 2009, it was estimated that there would be a total of 1,479,350 new cancer cases and 562,340 deaths from cancer in the United States alone, which corresponds to more than 1,500 deaths per day [5]. The dominant characteristic of cancer is its ability to grow uncontrollably and facilitate the division of abnormal cells. The events which lead to dysregulation of critical homeostatic signaling pathways are key to understanding the biology of cancer and its development. To date, there is evidence supporting the idea that a major factor responsible for cancer initiation and tumor formation appears to be a subset of biologically distinct clonogenic cells termed cancer stem cells which have been identified in a variety of cancers [6–12]. Cancer stem cells are defined by their ability to self-renew, initiate tumor formation and differentiate into the heterogeneous populations of cells present within the parental tumors [13–16]. Additionally, it is hypothesized that this subset of cancer cells is responsible for chemotherapeutic resistance often seen among cancer patients, thereby contributing to relapse [16].
Currently in the United States alone, more than one third of all adults are obese [17]. Obesity has been recognized as a risk factor for the development of type 2 diabetes, cardiovascular disease and cancer based malignancies such as cancers of the endometrium, kidney, liver, pancreas, prostate, breast and of hematopoietic tissues like non-Hodgkin’s lymphoma, multiple myeloma and leukemia [18–21]. The excess adipose tissue found in an obese individual is thought to influence the development of obesity related diseases as it is an active metabolic organ responsible for the production of adipokines that function as pro-inflammatory molecules [21]. Type 2 diabetes is often associated with obesity and develops as a result of insulin resistance and reduced secretion of insulin by pancreatic (β-cells [3, 22, 23]. Based on the definition provided by the WHO, insulin resistance is an absolute requirement for a metabolic syndrome diagnosis [4]. The metabolic and epidemiological links found between obese individuals diagnosed with type 2 diabetes and cancer incidence, specifically in the case of aggressive cancers such as colorectal, liver and pancreatic cancers, are vast and must be further investigated [24–30].
Cardiometabolic diseases, characterized by complications such as hypertension and atherosclerosis, are associated with metabolic syndrome and in individuals suffering from both obesity and type 2 diabetes [20, 31, 32]. The interrelationship between vascular dysfunction, obesity and type 2 diabetes is based on common deregulations in homeostatic pathways, mainly involved in metabolic signaling, that are necessary for the maintenance of a healthy physiological state. The co-occurrence of these diseases in individuals implies there is common ground and direct links which must be further investigated. Recently, the links between these metabolic disturbances have also been tied to the promotion and development of cancer as well [2, 33–35]. It is imperative to understand the biology of these abnormalities and identify the common mediators involved in these conditions to properly prevent, target and treat damages which are inflicted on the individual at a cellular level.
This emerging link between cancer development, obesity, diabetes and cardiovascular disease is currently under investigation. In this article, we review several molecular pathways that interconnect cancer development, type 2 diabetes and cardiovascular disease. We also discuss potential targets that share the trait of dysfunction between these malignancies, revealing possible targets for therapeutic treatments.
THE LINKS BETWEEN CANCER, TYPE 2 DIABETES AND CARDIOVASCULAR DISEASE
1. AMPK and its Role in Human Disease
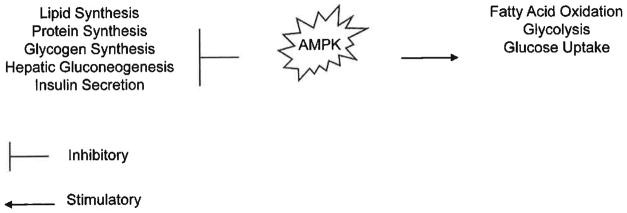
The adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) is a critical factor in the regulation of energy generating pathways. It is becoming apparent that AMPK is a central factor involved in the development of many diseases associated with metabolic syndrome such as type 2 diabetes and cardiovascular dysfunctions such as cardiac ischemia, arrhythmias and cardiac cellular growth [36–38]. Furthermore, AMPK is a known contributor to cancer development [1, 34, 39, 40]. AMPK, a serine/threonine protein kinase, is a heterotrimeric complex consisting of a catalytic α subunit and regulatory (β and γ subunits, encoded by multiple genes [37–43]. AMPK is activated, regulated by and sensitive to a rise in the AMP/ATP ratio, which is a characteristic response to metabolic stress. AMPK in an activated state is responsible for the inhibition of anabolic processes including lipid, protein and glycogen synthesis and the activation of catabolic processes such as fatty acid oxidation and glycolysis (Fig. 1) [1, 34, 37, 38, 40, 41, 43]. Inactivation of AMPK has been implicated in a variety of human metabolic disorders and is reflective of its importance as a therapeutic target.
Fig. 1. AMPK activation.
AMPK activation is responsible for both the inhibition and stimulation of critical homeostatic pathways. AMPK activation results in the inhibition of processes such as lipid, protein and glycogen synthesis, hepatic gluconeogenesis and insulin secretion, AMPK activation results in the activation of processes such as fatty acid oxidation, glycolysis and glucose uptake.
1.1. AMPK as a Major Regulator of Cancer Development
The mechanism(s) by which deregulation of homeostatic signaling pathways prompt malignant cellular changes are under intense investigation. The ability of AMPK to regulate cellular energy pathways; coupled with its ability to regulate processes significant in cancer development such as protein synthesis, glycolysis and hypoxia implicate AMPK as a potential therapeutic target [1]. Normally, AMPK inhibits tumorigenesis by controlling key regulators downstream which are involved in cellular proliferation, cell cycle progression and cellular survival [1, 34, 40]. These key regulators include well known cancer associated signaling pathways such as PI3K, mTOR and p53 signaling [1, 40]. It has previously been demonstrated that AMPK activation can result in inhibition of several cancers including glial [44], breast [45], lung [46], liver [47], stomach [48] and prostate [44, 49] cancers (Table 1). AMPK activation is dependent upon phosphorylation by the tumor suppressor serine threonine 11 (STK11/LKB1), which is often associated with cancer development. LKB1 loss is often associated with sporadic cancers including neuroendocrine lung, papillary breast, non-small cell lung, endometrial, pancreatic and testicular cancers [50]. LKB1 inactivation is most commonly linked with Peutz-Jeghers cancer syndrome, an autosomal dominant disorder characterized by an increased risk of epithelial tumors and benign hamartomas [1, 34, 39, 42]. LKB1, a kinase upstream of AMPK, is stimulated by AMP binding to AMPK which in turn activates AMPK via phosphorylation on Thr172 [1, 34, 39, 42]. Additionally, the calmodulin-dependent kinase kinase-β (CAMKKβ) is an upstream activator of AMPK as well. CAMKKβ is activated in response to an intracellular increase of Ca2+, a general second messenger responsible and necessary for a variety of cellular processes, including cellular proliferation and regulation of gene transcription [51]. Calmodulin regulates the cell cycle and tumorigenesis [51, 52] and calmodulin agonists can inhibit breast cancer cell proliferation [53]. Additionally, calmodulin can bind to the androgen receptor (AR) in prostate cancer cells possibly contributing to AR-responsive prostate cancer cell proliferation, which implies that calmodulin agonists may inhibit prostate cancer cell proliferation [54]. Activation of AMPK by CAMKKβ differs in comparison to LKB1-dependent activation as CAMKKβ can activate AMPK independent of changes in the AMP/ATP ratio [55–57]. Regardless of the upstream mechanism used to activate AMPK, activation is necessary in cancer prevention as downstream targets of AMPK are involved in the regulation of protein metabolism, cellular growth and apoptosis. Inactivation of AMPK as a result of mutation or deletion of LKB1 [58] or the increased activation of CAMKKβ, which has been reported in some cancer types such as breast cancer [52], can result in deregulation of AMPK activity thereby contributing to cancer development.
Table 1.
AMPK and Human Disease
| Pathological State | AMPK | Result | Reference |
|---|---|---|---|
| Cancer | |||
| Lung | Activation | Inhibition of cellular growth | [46] |
| Prostate | Activation | Inhibition of cellular growth | [49] |
| Glial | Activation | Inhibition of cellular growth | [44] |
| Gastric | Activation | Inhibition of cellular growth | [48] |
| Liver | Activation | Inhibition of cellular growth | [47] |
| Breast | Activation | Inhibition of cellular growth | [45] |
| Type 2 Diabetes | |||
| Type 2 Diabetes | Inactivation | High glucose levels | [50] |
| Cardiovascular Disease | |||
| Cardiac hypertrophy | Activation | Functions in prevention and regression | [36] |
| Ischemia | Activation | Lesser cardiac injury | [36] |
| Cardiovascular maintenance (i.e. platelet aggregation, vascular smooth muscle cell proliferation and vascular tone) | Activation | Regulation of cardiovascular function | [36] |
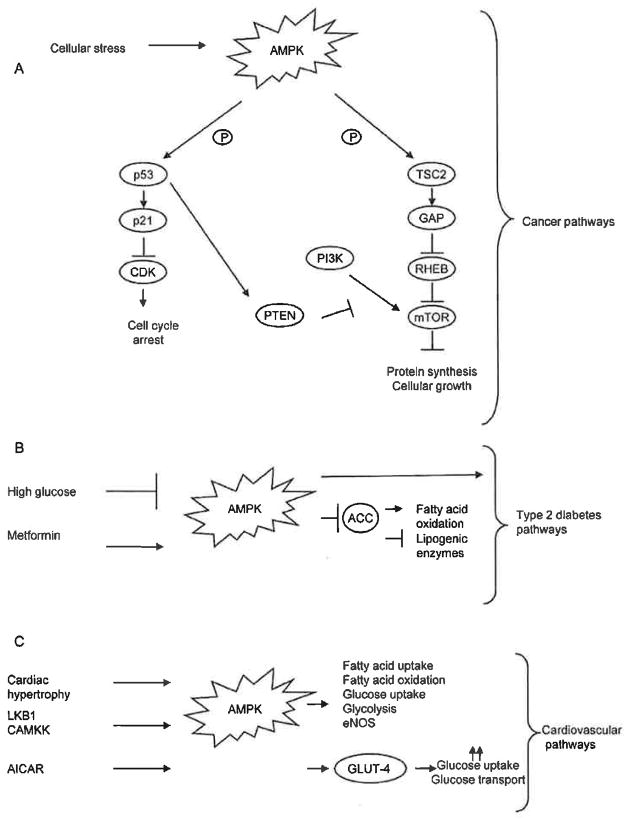
Downstream targets of AMPK regulate protein metabolism, cellular growth and apoptotic pathways which are known to be involved in cancer development. The mammalian target of rapamycin (mTOR), a well-studied regulatory pathway involved in the control of cellular growth by monitoring cellular energy levels and mitogenic signals, is a major downstream target of AMPK activation. Tuberous sclerosis 1 and 2 (TSC1 and TSC2) are two tumor suppressors downstream of AMPK which are involved in the development of tuberous sclerosis complex (TSC), a disorder characterized by the development of benign tumors in multiple organs [40]. Activation of AMPK results in the phosphorylation and activation of TSC2 which in turn activates GTPase-activation protein (GAP), resulting in the inactivation of Ras homolog enriched in brain (RHEB), a Ras family GTPase involved in activation of the mTOR complex [39, 40]. The inactivation of RHEB results in an inhibition of mTOR, which is necessary to control protein synthesis in a stressed cellular environment (Fig. 2A). Inactivation of AMPK leads to a constant activation of mTOR, resulting in continued proliferation as well as providing the necessary environment needed for survival. It is well-established that overactive mTOR signaling is a shared characteristic in a variety of cancers, including prostate, breast, lung, bladder, melanoma, renal, endometrial, thyroid, glial and chronic myeloid leukemia (CML) [59].
Fig. 2. AMPK activation in human disease.
(A) AMPK in cancer. Upon cellular stress, the activation of AMPK results in the phosphorylation of both p53 and TSC2. p53 activation results in activation of p21 which then results in inhibition of CDK leading to cell cycle arrest. Additionally, p53 activation also results in activation of PTEN which functions as an antagonist of the PI3K pathway, leading to mTOR activation, resulting in the inhibition of protein synthesis and cellular growth. TSC2 activation by AMPK results in GAP activation leading to the inactivation of RHEB. RHEB inactivation leads to the inhibition of the mTOR pathway resulting in the inhibition of protein synthesis and cellular growth as well. (B) AMPK in type 2 diabetes. High glucose levels result in the inhibition of AMPK activation leading to the development of type 2 diabetes. The anti-hyperglycemic agent metformin leads to AMPK activation resulting in inhibition of ACC leading to either activation of fatty acid oxidation or inhibition of lipogenic enzymes. (C) AMPK in cardiovascular disease. Cardiac hypertrophy, LKB1 and CAMKK lead to AMPK activation resulting in the activation of critical pathways involved in cardiovascular disease. AICAR activates AMPK resulting in GLUT4 translocation and results in an increase in glucose uptake and glycolysis which is cardioprotective.
An additional downstream target of AMPK is the well-known tumor suppressor p53. p53 is the most frequently identified tumor suppressor and functions in defense mechanisms against cellular damage or stress by inducing apoptosis or growth arrest (reviewed in [60–62]). The regulation of p53 by AMPK results in a mechanism that regulates the cell cycle. AMPK activation results in phosphorylation and activation of p53 resulting in the regulation of p53 downstream targets p21 and cyclin dependent kinase (CDK). The activation of p21 inhibits CDK activity which results in cell cycle arrest (Fig. 2A) [39, 40].
This cellular stress sensed by AMPK results in cell cycle regulation. Additionally, the ability of p53 to regulate the transcription of a tumor suppressor such as the phosphatase and tensin homolog (PTEN), a negative regulator of mTOR signaling and antagonist of the phosphoinositol-3 kinase (PI3K) signaling pathway, demonstrates the importance of proper p53 regulation [60]. However, inactivation of AMPK may result in improper cellular proliferation contributing to malignant cell development as p53 may not be activated in response to cellular stress or inappropriate mitogenic stimuli.
It is established that the processes associated with AMPK activation/inactivation are critical in maintaining homeostatic cellular metabolism and that loss of these functions contribute to tumor development. An additional function of AMPK is to ensure proper chromosomal segregation during mitosis (reviewed in [63]). It is speculated that the inability of AMPK to regulate proper cell cycle progression can result in abnormal cellular proliferation thus, contributing to malignancy [63]. Furthermore, there are specific signaling pathways such as the Wnt/β-catenin, Notch or Hedgehog pathways that if deregulated may provide advantages to a specific subset of cells that represent a tumor-initiating population [64, 65]. As previously mentioned, it is recognized that there is a population of cells within a tumor which are responsible for tumor formation, these cells are termed cancer stem cells (CSC) or tumor-initiating cells [6–12]. CSCs are defined by their ability to self-renew, initiate tumor formation and differentiate into the heterogeneous populations of cells present within the parental tumors [13–16]. The aforementioned pathways are implicated in the regulation of self-renewal and maintenance of CSCs. The exact mechanism(s) by which tumor-initiating cells develop is currently under investigation. In relation to AMPK, it is plausible to speculate that deregulation of AMPK may provide the key factor(s) necessary to give rise to a population of cells with tumor-initiating abilities [63]. For example, it has been demonstrated that the maternal embryonic leucine zipper kinase (MELK), an AMPK-related kinase activated by autophosphorylation [66], has been shown to regulate neural stem cell self-renewal through control of the cell cycle [67]. MELK expression has also been shown to correlate with high-grade glioma and can regulate the survival and proliferation of neural progenitor cells [67, 68]. MELK is an AMPK-related kinase that shares sequence homology with the protein kinase domain of the AMPK-α catalytic subunit [66]. Although there has been no direct correlation made between AMPK signaling and cancer stem cells, the ability of an AMPK-related kinase such as MELK to regulate stem cell self-renewal implies that AMPK may have a possible role in self-renewal as well, and thus, it will be interesting to investigate the role of AMPK.
1.2. AMPK, Type 2 Diabetes and Clinical Modulation
Since AMPK is a major metabolic regulator and is associated with the metabolic syndrome, the potential role it plays in the development of type 2 diabetes is an area of investigation as well. Type 2 diabetes is a major worldwide epidemic characterized by both insulin resistance and reduced secretion of insulin by pancreatic β-cells [3, 22, 23]. The events leading to type 2 diabetes development are complex and appear to be multi-causal. Recently, it has come to light that pharmacological agents often used to target type 2 diabetes act upon the AMPK pathway as well.
AMPK plays a critical role in maintaining glucose homeostasis and insulin antagonizes activation of AMPK [50]. High glucose levels lead to a decrease of AMPK activity in both skeletal and β-cells and low glucose levels in fact improve AMPK activity [50]. As previously discussed, inhibition of AMPK activity can have detrimental effects on downstream targets involved in critical cellular processes. Additionally, the evidence demonstrating that high glucose levels inhibit AMPK activity further show that those individuals diagnosed with type 2 diabetes have an increased risk of cancer development (Table 1).
Although there is evidence demonstrating that individuals with type 2 diabetes or increased insulin serum levels are at high risk of developing human malignancies such as cancer [69–71], there are numerous observational studies arguing that patients with type 2 diabetes appear to have a decreased incidence of cancer, specifically prostate cancer [72, 73]. Recently, this decrease of cancer incidence has been linked to the therapeutic agent metformin. In individuals diagnosed with type 2 diabetes, the drug metformin is often used as an antihyperglycemic agent that inhibits hepatic gluconeogenesis and directly inhibits complex 1 of the mitochondrial respiratory chain [74, 75]. Metformin has been identified as an activator of the AMPK pathway in hepatocytes, myocytes [76, 77] and skeletal muscle cells [78]. This activation of the AMPK pathway, specifically in hepatocytes, decreases acetyl-CoA carboxylase (ACC) activity, curtails the expression of lipogenic enzymes and induces fatty oxidation as well (Fig. 2B) [78]. The ability of metformin to indirectly activate AMPK in hepatocytes is dependent upon LKB1 [79]. Additionally, it has been demonstrated that metformin can suppress p53-deficient tumor cell growth [80]. Studies have demonstrated that metformin use is associated with a decrease risk of cancers of the colon or pancreas and can also be used as an anticancer agent and is a candidate for adjuvant therapy, specifically in the case of breast cancer [81–85]. It was recently demonstrated that metformin is capable of selectively targeting and killing breast cancer stem cells and it was further shown that in combination with doxorubicin, both the cancer stem cell and non-cancer stem cell population were killed [86]. However, the exact mechanism(s) by which this occurs is unknown. This study provides further support to the idea that patients with type 2 diabetes treated with metformin have lower incidence of cancer development. It is reasonable to speculate that diabetic patients specifically using the anti-diabetic drug metformin have lower incidence of cancer because these drugs exhibit the capability of targeting the small subpopulation of cells driving tumor formation and cancer initiation. Additionally, the use of metformin as an anti-diabetic agent as well as its role as an AMPK activator and its demonstrated role in cancer development, demonstrates the critical role of AMPK in human disease and shows a relationship between the pathways regulating type 2 diabetes and cancer.
1.3. AMPK and Incidence of Cardiometabolic Disease
The incidence of cardiometabolic diseases including hypertension or atherosclerosis is higher among patients diagnosed with metabolic syndrome [1, 87]. The prevalence of cardiometabolic disease is also increased in individuals suffering from both obesity and type 2 diabetes, common traits among those diagnosed with metabolic syndrome [20, 31, 32].
AMPK is expressed in many tissues including heart muscle and specifically, cardiomyocytes contain large quantities of AMPK [36]. Both LKB1 and CAMKK are responsible for AMPK activation in the heart [88] and during cardiovascular complications such as cardiac hypertrophy and ischemia, there is an activation of AMPK (Fig. 2C). However, during normal cardiac function there is an abundance of ATP and an inactivation of AMPK [88]. Normal smooth muscle cells reside in a G0 phase cell cycle and numerous events such as a growth-stimulating event or serum stimulation must occur to activate a proliferation. Interestingly, it has been reported that AMPK activation in serum stimulated smooth muscle cells results in cell cycle arrest at the G1 phase and inhibits cells proliferation [34, 89]. To date, it is disputed if AMPK activation during ischemia is indeed harmful or beneficial, but it appears that AMPK activation is beneficial (Table 1). AMPK activation increases fatty acid uptake, oxidation, and increases glucose uptake and glycolysis [36] which implicates it is a major regulator in the cardiovascular system (Fig. 2C).
AMPK is up-regulated in response to an increased AMP/ATP ratio which is increased in a stressed cellular state. It has been shown that 5-amino-4-imidazole-carboxamide riboside-1-β-D-ribofuranoside (AICAR), an AMPK activator, increases glucose uptake in an in vitro rat model via glucose transporter type 4 (GLUT-4) translocation independent of the PI3K signaling pathway [90]. GLUT-4 translocates to the plasma membrane in response to insulin and in circumstances of cellular stress, such as hypoxia or ischemia this translocation facilitates an increase in rapid glucose uptake and glycolysis [91]. This increase in both glucose uptake and glucose transport in cardiac myocytes has a cardioprotective effect in animal models and cell culture [90, 91]. The connection between AMPK activation and GLUT-4 translocation further supports the role of AMPK signaling as a critical regulator of cardiovascular homeostasis.
AMPK activation also results in an increase in the activity of endothelial nitric oxide synthase (eNOS) that is responsible for promotion of vasodilation, inhibition of platelet aggregation and proliferation of vascular smooth muscle [92]. The ability to perform these actions is critical in the regulation and maintenance of proper cardiovascular function. AMPK activation by AICAR results in nitric oxide (NO) production in endothelial cells thereby, resulting in vascular tone maintenance [92]. Metformin has also been demonstrated to decrease myocardial injury in both diabetic and non-diabetic mice via AMPK-eNOS activation [93]. This evidence supports a cardioprotective role for AMPK activation which is necessary to ensure proper cardiovascular function in response to cellular stress (Table 1).
Inactivation of AMPK results in the deregulation of fundamental processes such as the PI3K, mTOR and eNOS signaling pathways that are deemed necessary for cellular homeostasis and are interconnected across several diseases [36, 38, 40, 41]. There is substantial evidence that implicates inactivated AMPK as a critical factor in human disease development including cancer, type 2 diabetes and cardiovascular disease which demonstrates the potential of AMPK to function as a therapeutic target. The beneficial effects of AMPK activation must be further investigated to aid in the development of therapeutics for treatment and prevention of the diseases discussed here. Interestingly, AMPK is only one of several common links which connect cancer, type 2 diabetes and cardiometabolic disease.
2. PPAR and Human Disease Development
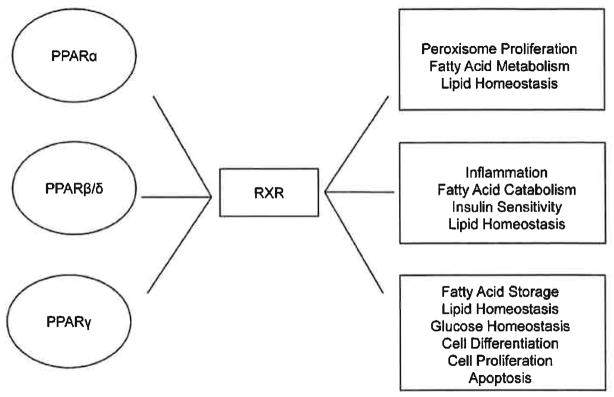
Peroxisome proliferator-activated receptors (PPARs) are members of a nuclear hormone receptor family responsible for the regulation of a wide variety of genes involved in cellular and metabolic processes such as fatty acid metabolism, lipid metabolism, glucose and insulin homeostasis. The distinct subtypes of PPARs which have been identified to date include PPARα, PPARβ/δ and PPARγ [94–97]. PPARs display broad range tissue distribution and receptor function appears to be specific to each subtype. PPAR activity can be modulated by a variety of ligands including endogenous compounds such as fatty acids, eicosanoids and is also a receptor for a variety of drugs used in treatment of human disease [94, 95]. Activation of PPARs is followed by the formation of heterodimers with the retinoid X receptor (RXR) which, in turn, recognizes DNA at sequence specific regions on target gene promoters or PPAR response elements (PPRE); thus, allowing activation or repression of gene transcription (Fig. 3) [94, 96, 98, 99]. PPARs regulate critical metabolic processes that function in maintaining normal homeostasis and deregulation of these processes may contribute to the development of metabolic syndrome as explained in further detail below (Fig. 3) [98]. The central role of PPAR in metabolic processes implicates PPAR in human diseases such as cancer, diabetes and cardiovascular disease. The exact function PPAR has in disease development is currently under investigation.
Fig. 3. PPARs.
PPAR α, PPAR β/δ and PPAR γ must form a heterodimers with RXR to function in controlling metabolic processes needed to maintain critical regulatory processes. PPARα controls peroxisome proliferation, fatty acid metabolism and lipid homeostasis. PPARβ/δ controls inflammation, fatty acid catabolism, insulin sensitivity and lipid homeostasis. PPARγ controls fatty acid storage, lipid and glucose homeostasis, cellular differentiation, cellular proliferation and apoptosis.
2.1. PPAR and its Function in Cancer Development
The role PPARs play in specific cancer types and oncogenesis is important to further elucidate the mechanism(s) which function in the promotion of cancer development. PPAR activation can result in antiproliferative, proapoptotic, prodifferentiation and antiangiogenic effects in cancer cells [96, 97, 100, 101]. Most recently, PPARβ/δ and PPARγ have been considered as potential therapeutic targets in cancer prevention.
PPARβ/δ is expressed in most tissues and functions in various physiological processes including inflammation, fatty acid catabolism and insulin sensitivity [97, 100]. Interestingly, there is controversial evidence demonstrating a role for PPARβ/δ in cellular proliferation in various cancer models including colon, breast and lung cancers. In the case of colon cancer, the most studied cancer with regard to the role of PPARβ/δ, there are conflicting reports suggesting a role for PPARβ/δ in cellular proliferation [101]. As there is high expression of PPARβ/δ in both the small and large intestine, it is known that this receptor plays a critical role in gastrointestinal tract maintenance. However, the controversy lies in the conflicting evidence which shows that PPARβ/δ activation can either inhibit or promote colon tumorigenesis (extensively reviewed in [101]). In the case of breast cancer development, PPARβ/δ has both proliferative and anti-apoptotic effects in estrogen receptor-positive cells [102]. Lung cancer cell models have shown that PPARβ/δ is expressed in primary airway epithelial cells [103]; however, the exact result of PPARβ/δ activation is unclear as it can either result in the inhibition or activation of lung cancer cell growth (reviewed in [97]). Although the exact role of PPARβ/δ in cancer development remains controversial due to the discrepancy in the published data sets, it is clear that PPARβ/δ ligand activation has some importance in cancer development and requires further investigation (Table 2).
Table 2.
PPARs and Human Disease
| Pathological State | PPARs | Result | Reference |
|---|---|---|---|
| Cancer | |||
| Colon | PPARβ/δ activation PPARγ overexpression |
Inhibits and promotes | [97,101,106] |
| Breast | PPARβ/δ activation PPARγ overexpression |
Proliferative and anti-apoptotic | [97,102,106] |
| Lung | PPARβ/δ activation | Inhibits and promotes | [97,103] |
| Type 2 Diabetes | |||
| Insulin resistance | PPARα activation | Reduces insulin resistance | [116] |
| Hyperinsulinemia | PPARγ activation | Reverses hyperinsulinemia | [115] |
| Cardiovascular Disease | |||
| Vascular health | PPARα activation | Promotes vascular health | [98] |
PPARγ expression, which is largely limited to adipose tissue and stimulates fatty acid storage, is involved in both lipid and glucose homeostasis and is a major receptor for the thiazolidinedione (TZD) class of insulin-sensitizing drugs [104, 105]. Similar to the other PPARs, PPARγ functions in cell differentiation, proliferation and apoptosis. PPARγ is expressed in a wide variety of cancers including colon, breast, bladder, lung and gastric cancers [100, 106] (Table 2) and it is speculated that it may function as a tumor suppressor by inhibiting replication of malignant cells (extensively reviewed in [100, 106]). PPARγ can exert antiproliferative effects in many of these cancers and is currently under investigation as a target in drug development.
Interestingly, PPARγ agonists have been shown to interact with pathways involved and implicated in stem cell signaling such as the Wnt signaling pathway which functions in the self-renewal of both embryonic and hematopoietic stem cells [107–109]. β-catenin, a nucleocytoplasmic protein, is stabilized by the binding of a Wnt signal to its receptor Frizzled (Fz). This binding results in the recruitment of disheveled (DSH) to the membrane which in turn inhibits GSK3 activation, thereby, resulting in activation of β-catenin and its translocation to the nucleus [108]. The translocation of β-catenin to the nucleus results in binding to the TCF/LEF family of transcriptional regulators leading to activation of gene transcription [108]. It has been demonstrated that both β-catenin-dependent and -independent Wnt signaling is involved in the inhibition or stimulation of adipogenesis, which is controlled by PPARβ [110, 111]. Activation of PPARγ results in an induction of β-catenin degradation, thereby resulting in suppression of β-catenin activity. It is established that aberrant β-catenin activity is linked to cancer development and contributes to malignancy as well [112, 113]. More specifically, the link between PPARγ and β-catenin has been demonstrated in breast cancer development [112]. Jiang et al demonstrated that both PPARγ and β-catenin are expressed in patient-derived human breast tumors and were able to correlate PPARγ deregulation with abnormal β-catenin signaling by investigating both protein level expression and their molecular interaction [112]. Interestingly, aberrant Wnt signaling and deregulated β-catenin activity have been implicated as key players involved in the self-renewal capability of cancer stem cells including prostate, colon and breast [112–114]. The ability of PPARγ to control β-catenin activity, an important player in stem cell development, in combination with its known expression in a wide variety of cancers, leads one to speculate that abnormal PPARγ expression may play a role in cancer stem cell development. This hypothesis must be tested and it must be determined if this would serve as a useful target in developing pharmacological targets aimed at cancer stem cells.
2.2. PPAR and its Role in the Development of Type 2 Diabetes
PPARs have emerged as critical transcriptional regulators of metabolism. The role of PPARs in development of the metabolic syndrome, specifically with a focus on obesity and type 2 diabetes, is of increasing interest especially with regards to identifying insulin-sensitizing drugs for diabetic treatment. Specifically, there has been focus on PPARα and PPARγ with regard to type 2 diabetes.
The most studied PPAR in relation to type 2 diabetes is PPARγ as it is essential for proper glucose homeostasis. It is well established in a clinical setting that the class of insulin-sensitizing drugs, TZDs, specifically rosiglitazone and pioglitazone express high affinity for PPARγ and function in the treatment of type 2 diabetes [115]. Activation of PPARγ in adipose tissue can result in suppression of hepatic glucose production and in the reversal of hyperinsulinemia, further supporting the link between PPARγ, obesity and the development of type 2 diabetes (Table 2) [115].
The activation of PPARα results in the transcriptional regulation of several key enzymes involved in lipid transport [100]. PPARα agonists have been shown to function in lowering both triglycerides and low density lipoprotein metabolism, specifically via β-oxidation stimulation [98]. In terms of PPARα and insulin sensitivity, the ability of PPARα activators, such as fibrates, to reduce both triglyceride levels and adiposity result in a reduction of insulin resistance in mice [116] (Table 2). Fibrates are PPARα agonists commonly used in the treatment of dyslipidemia, which often occurs in conjunction with type 2 diabetes. Interestingly, Guerre-Millo et al demonstrated that in two models of diet-induced and genetic obesity-linked insulin resistance, PPARα activators, such as fibrates, are capable of increasing insulin action on glucose utilization resulting in a decrease of elevated serum glucose and insulin concentrations [116]. As type 2 diabetes is characterized by an increase in glucose blood levels, the ability to decrease glucose levels by PPARα agonists implicates PPARα activation as a potential mechanism in the treatment of insulin resistance.
2.3. PPAR and its Role in the Prevention of Cardiovascular Disease
In addition to the role of PPARs in both cancer and type 2 diabetes, there is overwhelming evidence indicating that PPAR agonists may be useful therapeutic targets in the prevention or treatment of cardiovascular disease. PPARs have emerged as potential targets in clinical use in relation to cardiovascular disease because of their ability to regulate homeostatic processes such as fatty acid oxidation, lipid metabolism, inflammatory and vascular responses [98]. Proper regulation of these processes is essential to maintaining normal and healthy vascular function.
PPARα activation through its direct effect on both glucose and lipid homeostasis, is capable of indirectly influencing both inflammation and atherosclerosis. PPARα activation results in the regulation of a variety of pathways which function in the promotion of vascular health [98] (Table 2). The ability of PPARα to regulate lipid metabolism allows for the decrease of atherogenic dense LDL-cholesterol, the prevention of foam cell formation, an increase in cholesterol efflux, a decrease in both vasoconstriction and thrombosis and an increase in plaque stability (extensively reviewed in [98]). The ability of specific drugs which trigger PPARα activation, such as fibrates, can function in decreasing atherosclerosis risk which further highlights the potential of PPARα as a pharmacological target in cardiovascular risk prevention.
The link existing between the activation of PPARs, obesity, insulin resistance and cancer implies that PPAR agonists may serve as useful therapeutics having the ability to target a wide variety of pathways interconnected between these diseases. PPARs display broad range tissue distribution and have the ability to regulate a large variety of genes involved in processes that ensure proper metabolic homeostasis. The ability of PPARs to function in common signaling pathways that intertwine these diseases makes it a strong candidate for pharmacological agents.
3. Fatty Acid Synthase and its Role in Human Disease
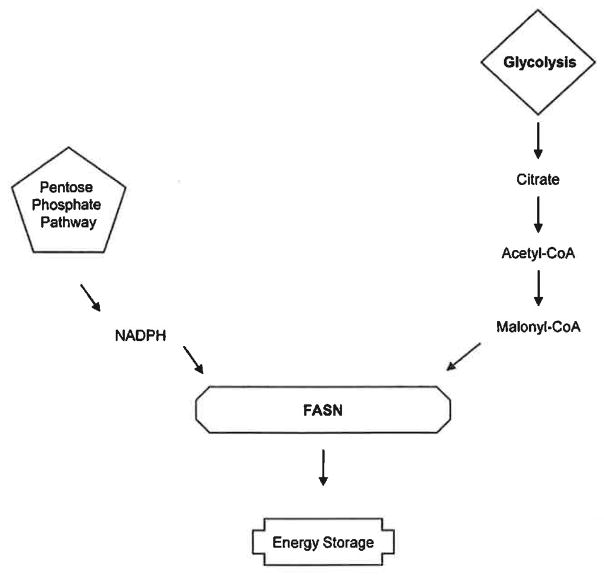
Fatty acids are major sources of energy and comprise critical components of membrane lipids functioning as cellular signaling molecules. Fatty acids are derived from food sources or from the synthesis of acetyl-coenzyme A (acetyl-CoA) via reactions such as glycolysis and the citric acid cycle [117]. The process responsible for the metabolism of glucose to fatty acids is extensive, however, the major players include acetyl-CoA, the major rate-limiting enzyme responsible for pathway regulation, citrate lyase, the pentose phosphate pathway, NADPH and fatty acid synthase (pathway reviewed in [118, 119]). The major function of fatty acid synthesis in normal humans is to store excess energy ingested in carbohydrates, convert it to fatty acids which then undergo esterification to store triacylglycerols. However, it has recently been shown that fatty acids are potential targets in the regulation of human disease such as diabetes, cancer and cardiovascular disease.
3.1. Fatty Acid Synthase and its Role in Cancer Metabolism
The ability of cancer cells to utilize anaerobic metabolism as a means of survival is known as the “Warburg Effect” which is further defined by a shift from oxidative phosphorylation to glycolysis, a trait thought to be a requirement of all cancer cells [119]. The relationship between fatty acids and cancer development was further supported by studies performed in human cancer patients which demonstrated that overall these patients had an increase in metabolic rate and an increase in free fatty acid turnover and oxidation [120, 121]. Specifically, there has been a focus on the role of fatty acid synthase (FASN), a vital enzyme involved in fatty acid synthesis, and its abundance in many human carcinomas [118]. FASN expression is highly expressed in human cancers including carcinomas of the breast, prostate, colon, ovary, endometrium and thyroid [118, 119] (Table 3). As FASN has a role in the synthesis of membrane lipids which function as cellular signaling molecules, it is plausible to expect that overexpression of FASN may contribute to deregulation of a variety of cellular signaling pathways (Fig. 4). As a means of survival, cancer cells have the ability to manipulate normal homeostatic processes for cellular growth. Specifically, the ability of a cancer cell to overexpress FASN is advantageous to its growth and survival as it provides a protective mechanism against hypoxic conditions and harsh microenvironments often seen in tumors. It is also hypothesized that the overexpression and synthesis of fatty acids is necessary for the demand of rapidly proliferating tumor cells.
Table 3.
FASN and Human Disease
| Pathological State | FASN | Result | Reference |
|---|---|---|---|
| Cancer | |||
| Breast | Overexpression | Cell survival | [118,119] |
| Prostate | Overexpression | Cell survival | [118,119] |
| Ovary | Overexpression | Cell survival | [118,119] |
| Thyroid | Overexpression | Cell survival | [118,119] |
| Endometrium | Overexpression | Cell survival | [118,119] |
| Colon | Overexpression | Cell survival | [118,119] |
| Type 2 Diabetes | |||
| Insulin-related disorders | Overexpression | Control of glucose and insulin signaling pathways – exact mechanism(s) unknown | [129] |
Fig. 4. FASN.
FASN is a critical enzyme that functions downstream of both the pentose phosphate and glycolysis pathways. FASN is responsible for energy storage by functioning as the main enzyme in the fatty acid synthesis pathway.
FASN is typically expressed at minimal levels in normal human tissue and the overexpression or upregulation of FASN in tumor cell lines and tumors has been interconnected with various other pathways and processes often deregulated in a variety of cancers. FASN inhibition results in a decrease in DNA synthesis and inhibition of cell cycle progression, specifically halting progress into the S-phase [122], Additionally, inhibition of FASN synthesis induces programmed cell death in human breast cancer cells [123] and delays progression of ovarian cancer in a xenograft model [124]. Activation of the phosphatidylinositol 3′-kinase (PI3K) pathway, established as a critical pathway typically involved in human prostate cancer development, results in an increase in FASN transcription [125]. Expression of the tumor suppressor PTEN, an antagonist of the PI3K pathway and the most commonly mutated tumor suppressor in prostate cancer, is inversely correlated with FASN levels and targeting of both FASN and the PI3K pathway results in enhanced tumor cell death [126].
Additionally, there has been a significant link made between FASN and the Wnt/β-catenin pathway as palmitic acid, a product of FASN synthesis, is responsible for palmitoylation, a specific type of lipid modification resulting in activation of the Wnt/β-catenin pathway [127, 128]. Activation and stabilization of the Wnt/β-catenin pathway as a result of an increase in palmitoylation due to FASN overexpression has been shown to occur, specifically in prostate cancer cells [127]. Additionally, knockdown of FASN results in a decrease of (β-catenin activation [127], further demonstrating that overexpression of FASN effects the Wnt/ β-catenin pathway. This data suggested a possible oncogenic role for FASN in the prostate as well. As the Wnt/β-catenin pathway plays a significant role in the maintenance and self-renewing capability of cancer stem cells [64], it is plausible to speculate that the stabilization of this pathway due to FASN overexpression may contribute to aggressiveness and ability of cancer stem cells to initiate tumor formation. It will be interesting to examine whether there is a direct connection between these two critical pathways specifically in the cancer stem cell population. The connection between FASN and the Wnt/β-catenin pathway has been demonstrated specifically in the case of prostate cancer however, it is unclear if this holds true in other cancer subtypes and will be interesting to investigate as well.
The abundance of FASN in a variety of human cancers reflects the importance of this enzyme in cancer cell survival. FASN overexpression is necessary to manipulate metabolic processes, such as fatty acid synthesis, which provides a source of energy to cancer cells [129], FASN overexpression provides cancer cells with advantages of both growth and survival mechanisms; therefore setting the stage for dominance. The ability of cancer cells to utilize anaerobic metabolism as a means of survival and the common overexpression of fatty acid synthase in human carcinomas, implies that development of therapeutics targeting FASN overexpression would be beneficial.
3.2. Fatty Acid Synthase Overexpression and Type 2 Diabetes Regulation
Insulin resistance is an absolute requirement for a diagnosis of metabolic syndrome. The factors and mechanism(s) that are involved in the switch from insulin resistance to type 2 diabetes are still unclear. However, as of recent, FASN overexpression has been shown regulate the expression of several genes involved in insulin-related disorders [129].
The association that exists between insulin resistance and a high level of fat in tissues among individuals suffering from obesity is widely accepted. FASN transcription is often induced among those individuals with diets high in carbohydrates [129] and there is a positive correlation among obese individuals diagnosed with type 2 diabetes and high fat diets. FASN is required for fatty acid synthesis and it comes as no surprise that FASN levels are often overexpressed in these cases. The expression of FASN at the transcriptional level is controlled by key transcription factors which respond to various growth factors including steroid hormone receptors such as the estrogen (ER), androgen (AR) and progesterone (PR) receptors [130], and growth factors receptors such as the epidermal growth factor receptor (EGFR) and HER2 [130]. The activation of these receptors results in activation of downstream pathways such as PI3K and the mitogen-activated protein kinase (MAPK) which mediates FASN expression. FASN expression is mediated by these pathways via the sterol regulatory element-binding protein 1c (SREBP-1c) [130]. Most importantly, FASN expression is regulated in response to both glucose and insulin levels via sterol response-element binding proteins (SREBP-1 and SREBP-2) and carbohydrate-responsive element-binding proteins (ChREBP) [117]. Interestingly, FASN expression can modulate critical genes which are involved in the glucose and insulin signaling pathways (Table 3) (reviewed in [129]). To date, the exact mechanism(s) which connect FASN overexpression and insulin resistance leading to type 2 diabetes is unknown. However, there is evidence demonstrating a strong correlation between FASN overexpression and insulin-resistant disorders [129]. FASN is a critical factor involved in energy storage and it may be beneficial to further investigate how FASN overexpression influences a variety of cellular signaling pathways contributing to metabolic disorder.
3.3. Fatty Acid Synthase Overexpression and Cardiovascular Disease
The development of cardiometabolic diseases, as previously mentioned, is characteristic of individuals diagnosed with metabolic syndrome. The increase in cardiovascular disease among individuals suffering from both obesity and type 2 diabetes appears to be a result of the deregulation of various signaling pathways coupled together. FASN overexpression is strongly associated with cancer development and appears to be a key factor in the development of insulin resistance resulting in type 2 diabetes. The strong correlation which exists between FASN overexpression and diseases such as obesity and type 2 diabetes where there is a significant increase in cardiometabolic disorder prognosis leads us to believe FASN overexpression may directly influence cardiovascular disease as well.
As discussed, FASN is a key player in maintaining whole body homeostasis by regulating lipid metabolism. Often times, in obesity-related cardiovascular disease, there are atypical quantities of specific hormones and cytokines released by adipose tissue such as tumor necrosis alpha (TNF-α), interleukin-6 (IL-6), leptin, adiponectin and plasminogen activator inhibitor-1 (PAI-1) [131]. The primary function of adipose tissue is to store and release free fatty acids as an energy source [22], FASN, one of the key enzymes involved in de novo fatty acid synthesis, is responsible for the synthesis of palmitate, a fatty acid which plays a role not only in post-translational modification but in triglyceride synthesis as well [132]. Palmitate may also induce programmed cell death in human cardiomyocytes [133] and breast cancer cells [134]. Cardiovascular diseases are often characterized by loss of cardiomyocytes due to apoptosis or programmed cell death, increased storage of triglycerides and accumulation of toxic lipids. Thus, it is plausible to speculate that FASN overexpression or deregulation may result in unnecessary or aberrant apoptosis of cardiomyocytes thereby contributing to cardiovascular disease development. The role which FASN overexpression may possibly play in cardiovascular disease development should be investigated.
FASN is a common link which exists between cancer development, type 2 diabetes and cardiovascular disease. The role of fatty acid synthase as a key player in fatty acid metabolism demonstrates that strict regulation of this enzyme is necessary for normal homeostasis. Improper regulation of FASN results in human malignancy and its involvement in critical metabolic processes make it a potential target for therapeutics.
CONCLUSION
Human diseases such as cancer, type 2 diabetes and cardiovascular disease are often the result of severe deregulations which are interlinked. Metabolic syndrome is a disease which is characterized by a diagnosis of several chronic diseases such as obesity, diabetes, hypertension and cardiovascular disease [1]. The underlying causes associated with these metabolic deregulations are complex and multicausal. However, individuals plagued by these diseases are at an increased risk for cancer development. This common link comes to no surprise as many of the pathways often deemed responsible for cancer development and progression are also associated with many of the diseases which characterize metabolic syndrome such as type 2 diabetes and cardiovascular disease. In this review, we have analyzed three common links which are often deregulated in these diseases: the AMPK signaling pathway, PPARs and FASN (Fig. 5). The common thread between them is the regulation of key metabolic processes necessary for proper homeostasis. The ability to identify common pathways which can be potential targets for treatment of several diseases is necessary as often times metabolic dysfunction presents itself with different faces. The key to understanding disease development from cancer, type 2 diabetes and cardiovascular disease is to unveil the commonalities which contribute to the deterioration and disruption of healthy normal physiological states. The ability to step back and reveal a more global picture in regards to disease development will be a major step in the development of pharmacological agents which can target multiple interlinked diseases.
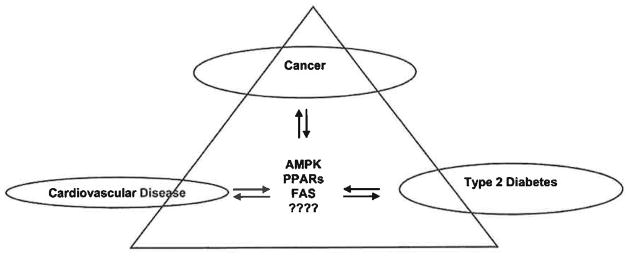
Fig. 5. The links between cancer, cardiovascular disease and type 2 diabetes.
AMPK, PPARs and FASN are some of the links interconnecting cancer, cardiovascular disease and type 2 diabetes. These diseases are linked via these signaling pathways that are responsible for proper regulation of critical homeostatic processes as described. It must be further investigated if there are other essential pathways which interlink these diseases as well.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institute of Health, under contract No. N01-CO-12400. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26(2):69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–7. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hemandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98(4):756–65. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 10.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 12.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- 13.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15(9):1010–2. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 14.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 15.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 16.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anticancer therapy. FASEB J. 2007;21(14):3777–85. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh WA, Law RE. Cardiovascular risk continuum: implications of insulin resistance and diabetes. Am J Med. 1998;105(1A):4S–14S. doi: 10.1016/s0002-9343(98)00205-8. [DOI] [PubMed] [Google Scholar]
- 19.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 20.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 21.Murthy NS, Mukherjee S, Ray G, Ray A. Dietary factors and cancer chemoprevention: an overview of obesity-related malignancies. J Postgrad Med. 2009;55(1):45–54. doi: 10.4103/0022-3859.43549. [DOI] [PubMed] [Google Scholar]
- 22.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–71. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 23.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86(3):S867–71. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 24.Adami H-O, Chow W-H, Nyren O, et al. Excess Risk of Primary Liver Cancer in Patients With Diabetes Mellitus. J Natl Cancer Inst. 1996;88(20):1472–7. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 25.Adami H-O, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2(5):307–14. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Murphy TK, Rodriguez C, Thun MJ, Heath CW. Diabetes mellitus and pancreaticcancer mortality in a prospectivecohort of United States adults. Cancer Causes Control. 1998;9(4):403–10. doi: 10.1023/a:1008819701485. [DOI] [PubMed] [Google Scholar]
- 27.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 28.Vecchia CL, Negri E, Decarli A, Franceschi S. Diabetes mellitus and the risk of primary liver cancer. Int J Cancer. 1997;73(2):204–7. doi: 10.1002/(sici)1097-0215(19971009)73:2<204::aid-ijc7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89(18):1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 30.Will JC, Galuska DA, Vinicor F, Calle EE. Colorectal Cancer: Another Complication of Diabetes Mellitus? Am J Epidemiol. 1998;147(9):816–25. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 31.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 32.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 33.Gregory JW. Metabolic disorders. Endocr Dev. 2009;15:59–76. doi: 10.1159/000207610. [DOI] [PubMed] [Google Scholar]
- 34.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574(Pt 1):63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276(20):5738–46. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116(8):607–20. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582(1):81–9. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–16. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Fay JR, Steele V, Crowell JA. Energy homeostasis and cancer prevention: the AMP-activated protein kinase. Cancer Prev Res (Phila Pa) 2009;2(4):301–9. doi: 10.1158/1940-6207.CAPR-08-0166. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol (Oxf) 2009;196(1):55–63. doi: 10.1111/j.1748-1716.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89(3):1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 42.Sanz P. AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci. 2008;9(5):478–92. doi: 10.2174/138920308785915254. [DOI] [PubMed] [Google Scholar]
- 43.Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33(6):395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-{beta}-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24(2):479–87. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swinnen JV, Beckers A, Brusselmans K, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65(6):2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Jiang P, Robinson M, Lawrence TS, Sun Y. AMPK- {beta}1 subunit is a p53-independent stress responsive protein that inhibits tumor cell growth upon forced expression. Carcinogenesis. 2003;24(5):827–34. doi: 10.1093/carcin/bgg032. [DOI] [PubMed] [Google Scholar]
- 47.Meisse D, Van de Casteele M, Beauloye C, et al. Sustained activation of AMP-activated protein kinase induces c-Jun N- terminal kinase activation and apoptosis in liver cells. FEBS Letters. 2002;526(1–3):38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- 48.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67(10):2005–11. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321(1):161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 50.Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17(5):205–15. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev. 2003;24(6):719–36. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Mora O, LaHair MM, Howe CJ, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent protein kinases as potential targets in cancer therapy. Expert Opin Ther Targets. 2005;9(4):791–808. doi: 10.1517/14728222.9.4.791. [DOI] [PubMed] [Google Scholar]
- 53.Wei JW, Hickie RA, Klaassen DJ. Inhibition of human breast cancer colony formation by anticalmodulin agents: trifluoperazine, W-7, and W-13. Cancer Chemother Pharmacol. 1983;11(2):86–90. doi: 10.1007/BF00254251. [DOI] [PubMed] [Google Scholar]
- 54.Cifuentes E, Mataraza JM, Yoshida BA, et al. Physical and functional interaction of androgen receptor with calmodulin in prostate cancer cells. Proc Natl Acad Sci USA. 2004;101(2):464–9. doi: 10.1073/pnas.0307161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32 (Suppl 4):S55–9. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 56.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-[beta] is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabolism. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Witters LA, Kemp BE, Means AR. Chutes and Ladders: the search for protein kinases that act on AMPK. Trends Biochem Sci. 2006;31(1):13–6. doi: 10.1016/j.tibs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27(55):6908–19. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 59.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11(8):353–61. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9(10):691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 61.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–22. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hupp TR, Lane DP, Ball KL. Strategies for manipulating the p53 pathway in the treatment of human cancer. Biochem J. 2000;352(Pt 1):1–17. [PMC free article] [PubMed] [Google Scholar]
- 63.Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Menendez JA. AMPK: Evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle. 2009;8(22):3679–83. doi: 10.4161/cc.8.22.9905. [DOI] [PubMed] [Google Scholar]
- 64.Hurt EM, Chan K, Serrat MAD, Thomas SB, Veenstra TD, Farrar WL. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells. 2010;28(3):390–8. doi: 10.1002/stem.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 66.Bright NJ, Thornton C, Carling D. The regulation and function of mammalian AMPK-related kinases. Acta Physiol. 2009;196(1):15–26. doi: 10.1111/j.1748-1716.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 67.Nakano I, Paucar AA, Bajpai R, et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. J Cell Biol. 2005;170(3):413–27. doi: 10.1083/jcb.200412115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakano I, Masterman-Smith M, Saigusa K, et al. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res. 2008;86(1):48–60. doi: 10.1002/jnr.21471. [DOI] [PubMed] [Google Scholar]
- 69.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006;169(5):1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89(18):1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 71.Hsing AW, Chua S, Jr, Gao YT, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001;93(10):783–9. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 72.Fukui M, Tanaka M, Kadono M, et al. Serum Prostate-Specific Antigen Levels in Men With Type 2 Diabetes. Diabetes Care. 2008;31(5):930–1. doi: 10.2337/dc07-1962. [DOI] [PubMed] [Google Scholar]
- 73.Velicer CM, Dublin S, White E. Diabetes and the risk of prostate cancer: the role of diabetes treatment and complications. Prostate Cancer Prostatic Dis. 2006;10(1):46–51. doi: 10.1038/sj.pcan.4500914. [DOI] [PubMed] [Google Scholar]
- 74.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 75.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293(1):H457–66. doi: 10.1152/ajpheart.00002.2007. [DOI] [PubMed] [Google Scholar]
- 77.Yang J, Holman GD. Long-term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology. 2006;147(6):2728–36. doi: 10.1210/en.2005-1433. [DOI] [PubMed] [Google Scholar]
- 78.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 81.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 82.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Currie C, Poole C, Gale E. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 84.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 200;8(6):909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 85.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8(13):2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 86.Hirsch HA, lliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69(19):7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 88.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100(4):474–88. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 89.Igata M, Motoshima H, Tsuruzoe K, et al. Adenosine Monophosphate-Activated Protein Kinase Suppresses Vascular Smooth Muscle Cell Proliferation Through the Inhibition of Cell Cycle Progression. Circ Res. 2005;97(8):837–44. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 90.Russell RR, 3rd, Bergeron R, Shulman Gl, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277(2 Pt 2):H643–9. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 91.Brosius FC. How glucose and glucose transporters protect cardiac myocytes. Journal of Molecular and Cellular Cardiology. 2003;35(10):1187–9. doi: 10.1016/s0022-2828(03)00242-6. [DOI] [PubMed] [Google Scholar]
- 92.Morrow VA, Foufelle F, Connell JMC, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278(34):31629–39. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 93.Calvert JW, Gundewar S, Jha S, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 94.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 95.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol Genet Metab. 2004;83(1–2):93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 96.Karpe F, Ehrenborg EE. PPARdelta in humans: genetic and pharmacological evidence for a significant metabolic function. Curr Opin Lipidol. 2009;20(4):333–6. doi: 10.1097/MOL.0b013e32832dd4b1. [DOI] [PubMed] [Google Scholar]
- 97.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796(2):230–41. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fruchart J-C. Peroxisome proliferator-activated receptor-alpha (PPAR[alpha]): At the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205(1):1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 99.Jeninga EH, Gurnell M, Kalkhoven E. Functional implications of genetic variation in human PPAR[gamma] Trends Endocrinol Metab. 2009;20(8):380–7. doi: 10.1016/j.tem.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. J Cell Physiol. 2007;212(1):1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 101.Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor beta/delta (PPARbeta/delta) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115(4):107–27. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Conzen SD. Minireview: Nuclear Receptors and Breast Cancer. Mol Endocrinol. 2008;22(10):2215–28. doi: 10.1210/me.2007-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Denning GM, Stoll LL. Peroxisome proliferator-activated receptors: potential therapeutic targets in lung disease? Pediatr Pulmonol. 2006;41(1):23–34. doi: 10.1002/ppul.20338. [DOI] [PubMed] [Google Scholar]
- 104.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 105.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276(41):37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 106.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: implications for chemoprevention. Clin Cancer Res. 2009;15(1):2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]
- 107.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 108.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13(14):4042–5. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 109.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6(10):e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Tienen FH, Laeremans H, van der Kallen CJ, Smeets HJ. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun. 2009;387(1):207–11. doi: 10.1016/j.bbrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Kennell JA, MacDougaid OA. Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J Biol Chem. 2005;280(25):24004–10. doi: 10.1074/jbc.M501080200. [DOI] [PubMed] [Google Scholar]
- 112.Jiang Y, Zou L, Zhang C, et al. PPARγ and Wnt/β-Catenin pathway in human breast cancer: expression pattern, molecular interaction and clinical/prognostic correlations. J Cancer Res Clin Oncol. 2009;135(11):1551–9. doi: 10.1007/s00432-009-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19(6):683–97. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 114.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 115.Sugii S, Olson P, Sears DD, et al. PPAR{gamma} activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci USA. 2009;106(52):22504–9. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guerre-Millo Ml, Gervois P, Raspé E, et al. Peroxisome Proliferator-activated Receptor α Activators Improve Insulin Sensitivity and Reduce Adiposity. J Biol Chem. 2000;275(22):16638–42. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- 117.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50 (Suppl):S138–43. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66(12):5977–80. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 119.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–8. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 120.Legaspi A, Jeevanandam M, Starnes HF, Jr, Brennan MF. Whole body lipid and energy metabolism in the cancer patient. Metabolism. 1987;36(10):958–63. doi: 10.1016/0026-0495(87)90132-6. [DOI] [PubMed] [Google Scholar]
- 121.Hyltander A, Drott C, Korner U, Sandstrom R, Lundholm K. Elevated energy expenditure in cancer patients with solid tumours. Eur J Cancer. 1991;27(1):9–15. doi: 10.1016/0277-5379(91)90050-n. [DOI] [PubMed] [Google Scholar]
- 122.Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58(20):4611–5. [PubMed] [Google Scholar]
- 123.Pizer ES, Jackisch C, Wood FD, Pastemack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56(12):2745–7. [PubMed] [Google Scholar]
- 124.Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56(6):1189–93. [PubMed] [Google Scholar]
- 125.Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 2002;62(3):642–6. [PubMed] [Google Scholar]
- 126.Bandyopadhyay S, Pai SK, Watabe M, et al. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24(34):5389–95. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- 127.Fiorentino M, Zadra G, Palescandolo E, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of [beta]-catenin in prostate cancer. Lab Invest. 2008;88(12):1340–8. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402(3):515–23. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM. Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem. 2009;55(3):425–38. doi: 10.1373/clinchem.2008.115352. [DOI] [PubMed] [Google Scholar]
- 130.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100(9):1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wnkler G, Kiss S, Keszthelyi L, et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur J Endocrinol. 2003;149(2):129–35. doi: 10.1530/eje.0.1490129. [DOI] [PubMed] [Google Scholar]
- 132.Kridel SJ, Lowther WT, Pemble CWt. Fatty acid synthase inhibitors: new directions for oncology. Expert Opin Investig Drugs. 2007;16(11):1817–29. doi: 10.1517/13543784.16.11.1817. [DOI] [PubMed] [Google Scholar]
- 133.Listenberger LL, Schaffer JE. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc Med. 2002;12(3):134–8. doi: 10.1016/s1050-1738(02)00152-4. [DOI] [PubMed] [Google Scholar]
- 134.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects1. Cancer Res. 2000;60(22):6353–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.