Abstract
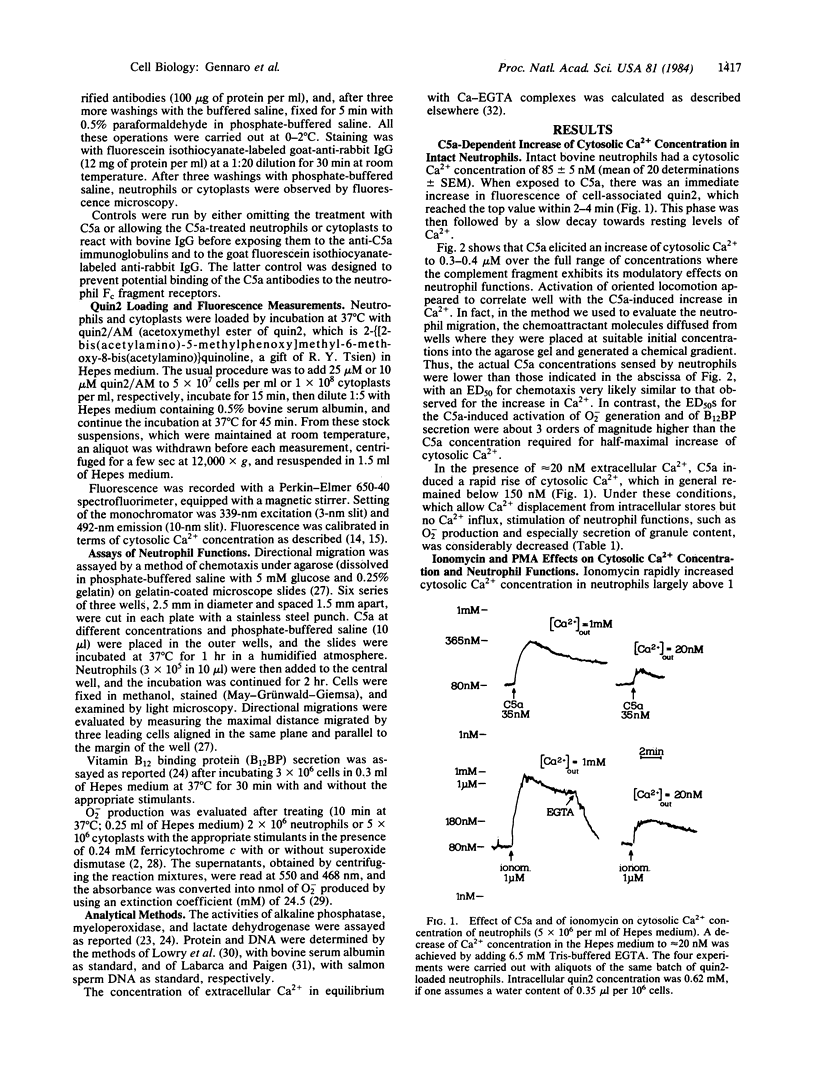
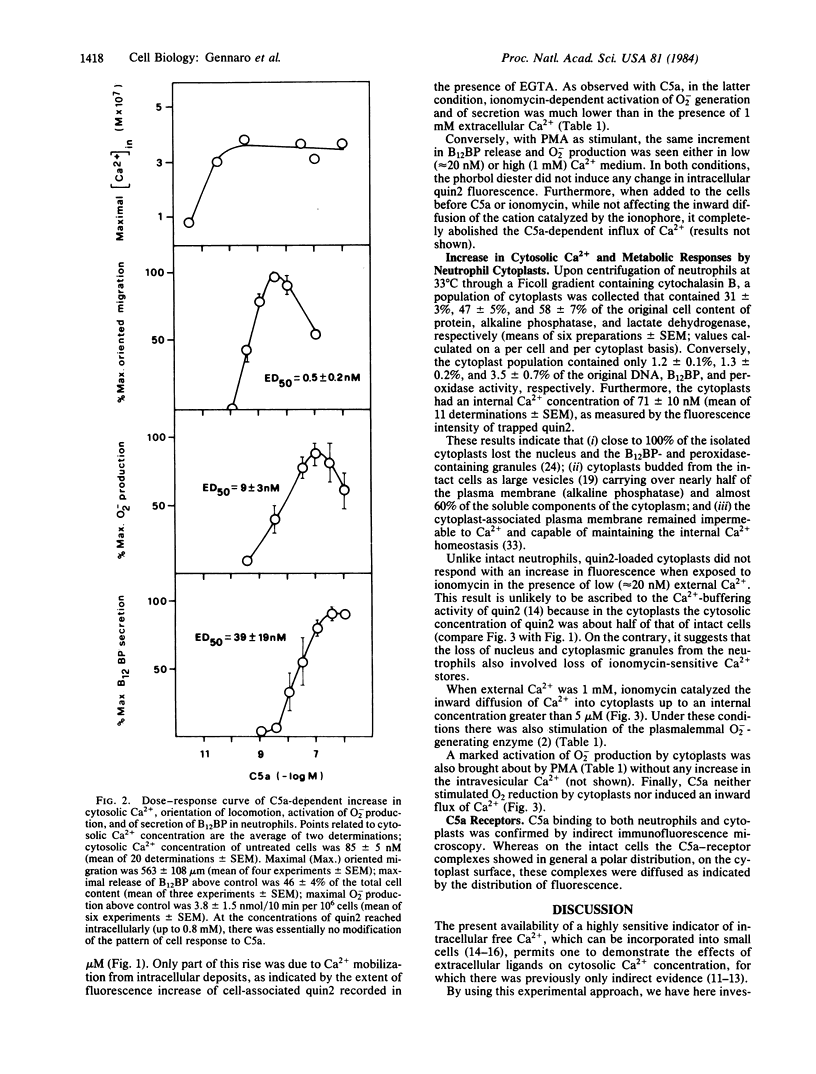
The cytosolic concentration of free Ca2+ in bovine neutrophils was monitored by using the intracellular Ca2+ indicator quin2, 2-[[2-bis(acetylamino)-5-methylphenoxy]methyl-6-methoxy-8- bis(acetylamino)]quinoline. Neutrophils at rest have a cytosolic Ca2+ concentration of 85 +/- 5 nM, which in 2-4 min increases to 300-400 nM upon interaction with the complement fragment C5a in a concentration range of 35 pM to 1.2 microM. In the same concentration range, C5a also sequentially activates neutrophil directional migration (ED50 less than 0.5 nM), O-2 production (ED50 = 9 nM), and secretion of the contents of specific granules (ED50 = 39 nM). The selective Ca2+ ionophore ionomycin also increases cytosolic Ca2+ concentration above 1 microM under conditions where it stimulates neutrophil functions. Conversely, phorbol 12-myristate 13-acetate markedly activates secretion and O-2 production without modifying the average cytosolic Ca2+ concentration. In the presence of EGTA (Ca2+out approximately equal to 20 nM), with both C5a and ionomycin, cytosolic Ca2+ increases to less than 200 nM, and functional responses are greatly decreased. Nucleus- and granule-free neutrophil cytoplasts accumulate Ca2+ and produce O-2 when exposed to ionomycin but not to C5a. These results and other considerations suggest that (i) activation of neutrophil functions may occur after cytosolic Ca2+ has exceeded the apparent threshold level of 200 nM; (ii) C5a receptor-mediated activation of Ca2+ influx may require cooperation between the neutrophil surface and some cytoplasmic organelle and/or redistribution of the C5a-receptor complexes on the cell surface; and (iii) the phorbol diester stimulates Ca2+-dependent pathways presumably by directly activating other mechanisms such as protein phosphorylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellavite P., Dri P., Della Bianca V., Serra M. C. The measurement of superoxide anion production by granulocytes In whole blood. A clinical test for the evaluation of phagocyte function and serum opsonic capacity. Eur J Clin Invest. 1983 Aug;13(4):363–368. doi: 10.1111/j.1365-2362.1983.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Human C5a and C5a analogs as probes of the neutrophil C5a receptor. Mol Immunol. 1980 Feb;17(2):151–161. doi: 10.1016/0161-5890(80)90067-x. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. Stimulus-secretion coupling in rabbit neutrophils is not mediated by phosphatidylinositol breakdown. Nature. 1980 Nov 20;288(5788):275–277. doi: 10.1038/288275a0. [DOI] [PubMed] [Google Scholar]
- Deleers M., Malaisse W. J. Binding of tumor-promoting and biologically inactive phorbol esters to artificial membranes. Cancer Lett. 1982 Nov-Dec;17(2):135–140. doi: 10.1016/0304-3835(82)90025-8. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Gennaro R., Dewald B., Horisberger U., Gubler H. U., Baggiolini M. A novel type of cytoplasmic granule in bovine neutrophils. J Cell Biol. 1983 Jun;96(6):1651–1661. doi: 10.1083/jcb.96.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Mottola C., Schneider C., Romeo D. Ca2+-dependent ATPase activity of alveolar macrophage plasma membrane. Biochim Biophys Acta. 1979 Mar 16;567(1):238–246. doi: 10.1016/0005-2744(79)90190-6. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Schneider C., de Nicola G., Cian F., Romeo D. Biochemical properties of bovine granulocytes. Proc Soc Exp Biol Med. 1978 Mar;157(3):342–347. doi: 10.3181/00379727-157-40050. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Appelbaum B. D., Vogler W. R., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase and its substrates in human neutrophils. Biochem Biophys Res Commun. 1983 Mar 29;111(3):847–853. doi: 10.1016/0006-291x(83)91376-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Mottola C., Romeo D. Calcium movement and membrane potential changes in the early phase of neutrophil activation by phorbol myristate acetate: a study with ion-selective electrodes. J Cell Biol. 1982 Apr;93(1):129–134. doi: 10.1083/jcb.93.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Miani N., Rossi F. Ion movement across leukocyte plasma membrane and excitation of their metabolism. Nature. 1975 Feb 13;253(5492):542–544. doi: 10.1038/253542a0. [DOI] [PubMed] [Google Scholar]
- Roos D., Voetman A. A., Meerhof L. J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983 Aug;97(2):368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E. Leukocyte chemotaxis. Annu Rev Physiol. 1982;44:553–568. doi: 10.1146/annurev.ph.44.030182.003005. [DOI] [PubMed] [Google Scholar]
- Schneider C., Zanetti M., Romeo D. Surface-reactive stimuli selectively increase protein phosphorylation in human neutrophils. FEBS Lett. 1981 May 5;127(1):4–8. doi: 10.1016/0014-5793(81)80327-4. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Phorbol myristate acetate-induced release of granule enzymes from human neutrophils: inhibition by the calcium antagonist, 8-(N,N-diethylamino)-octyl 3,4,5-trimethoxybenzoate hydrochloride. Biochem Biophys Res Commun. 1979 Nov 14;91(1):263–271. doi: 10.1016/0006-291x(79)90612-0. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Goetzl E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Nabi Z. F., Tatscheck B., Minakami S. Release of calcium from membranes and its relation to phagocytotic metabolic changes: a fluorescence study on leukocytes loaded with chlortetracycline. Biochem Biophys Res Commun. 1980 Jul 16;95(1):410–415. doi: 10.1016/0006-291x(80)90753-6. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Korchak H. M., Perez H. D., Smolen J. E., Goldstein I. M., Hoffstein S. T. The secretory code of the neutrophil. J Reticuloendothel Soc. 1979 Dec;26(Suppl):687–700. [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C. Leucocyte locomotion and chemotaxis. The influence of divalent cations and cation ionophores. Exp Cell Res. 1975 Jul;93(2):420–426. doi: 10.1016/0014-4827(75)90468-1. [DOI] [PubMed] [Google Scholar]



