Abstract
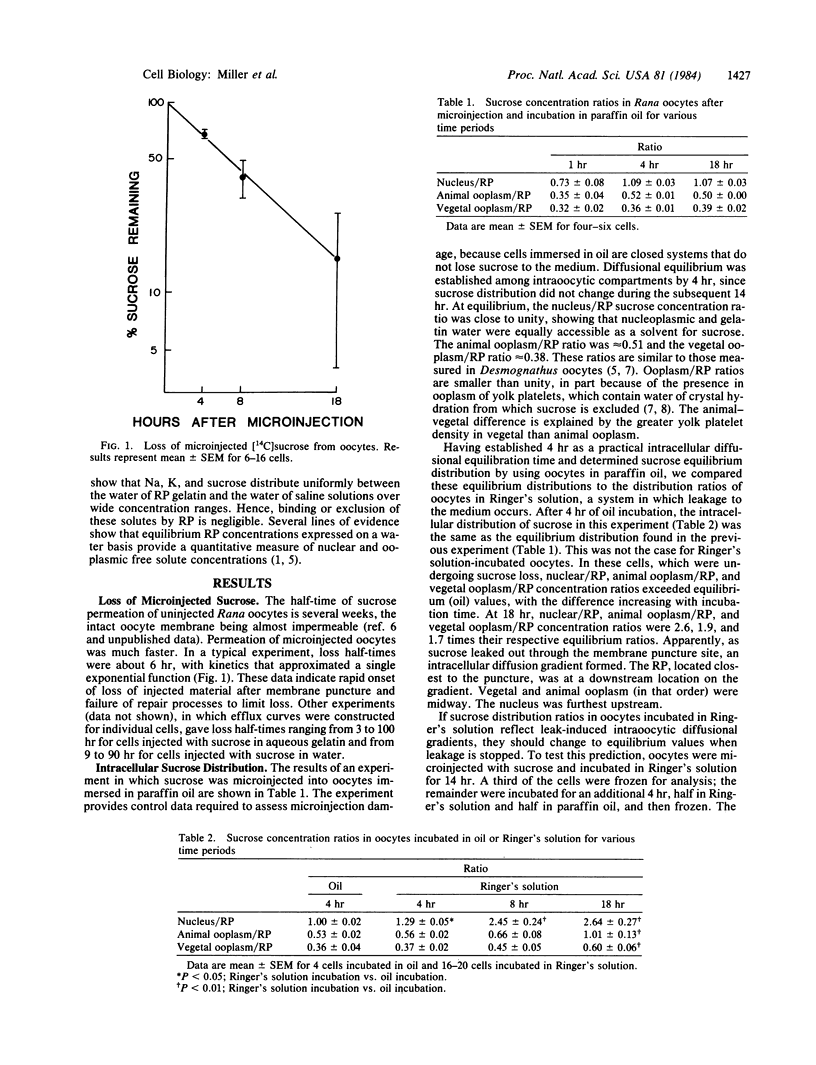
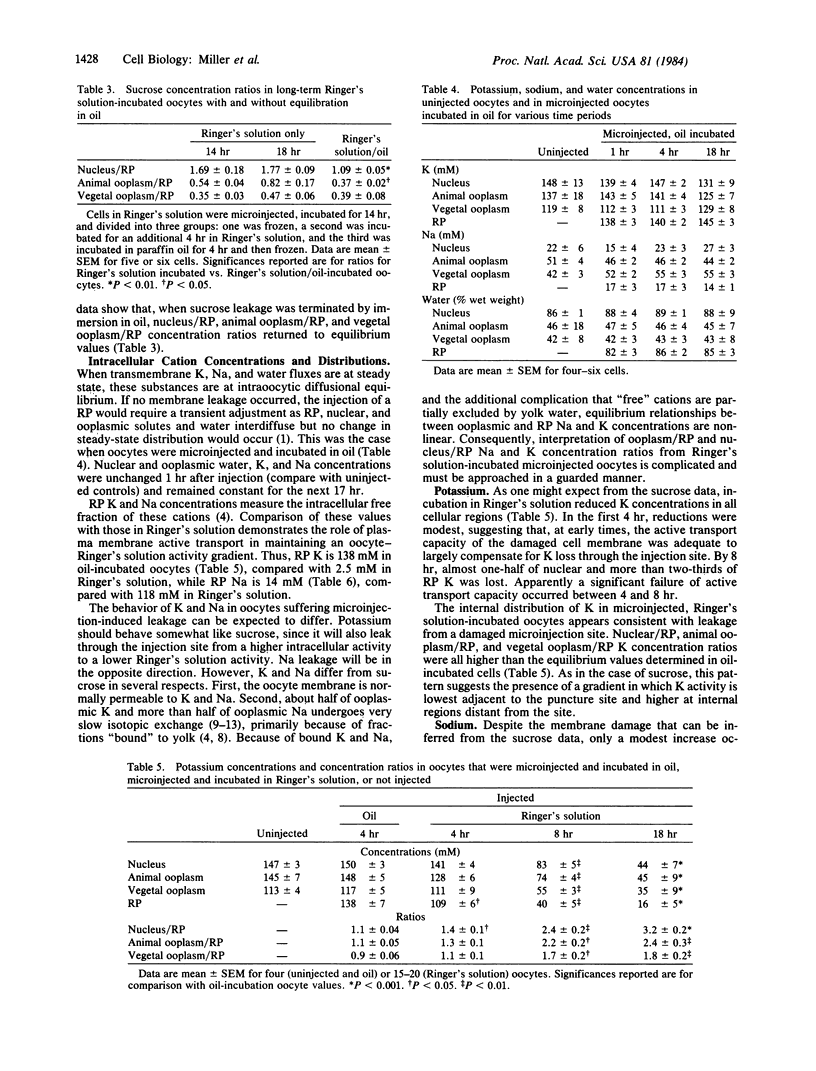
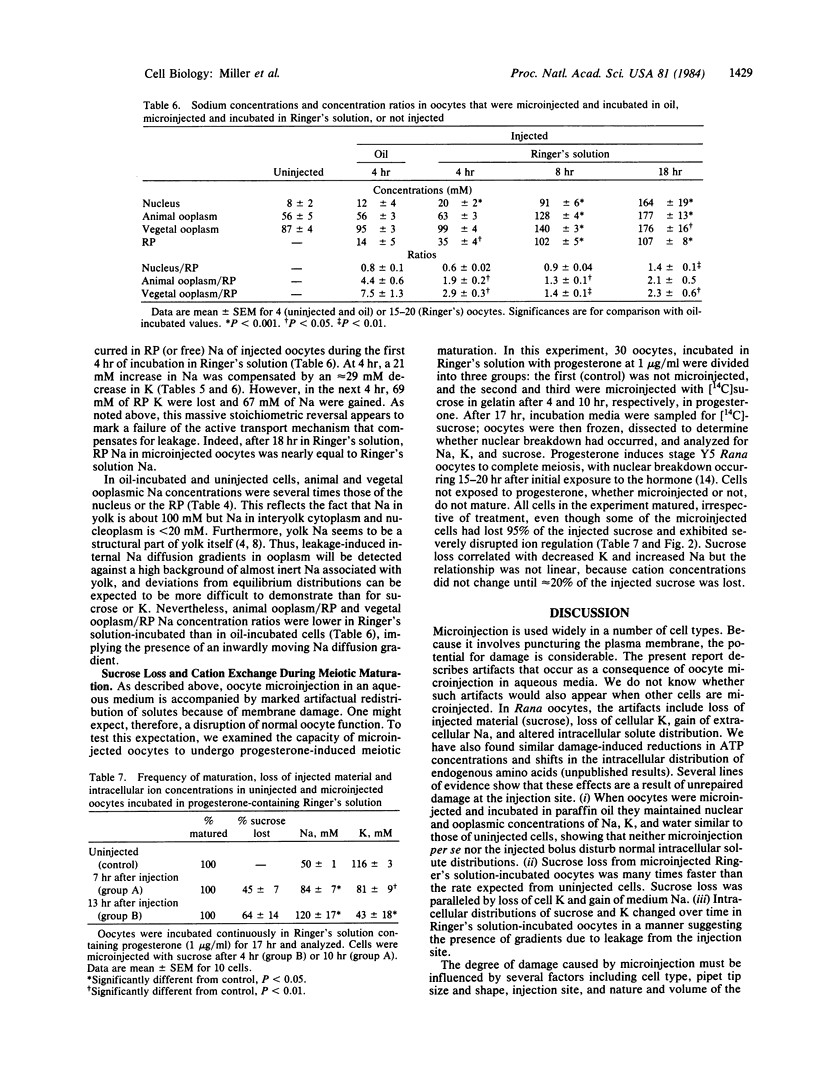
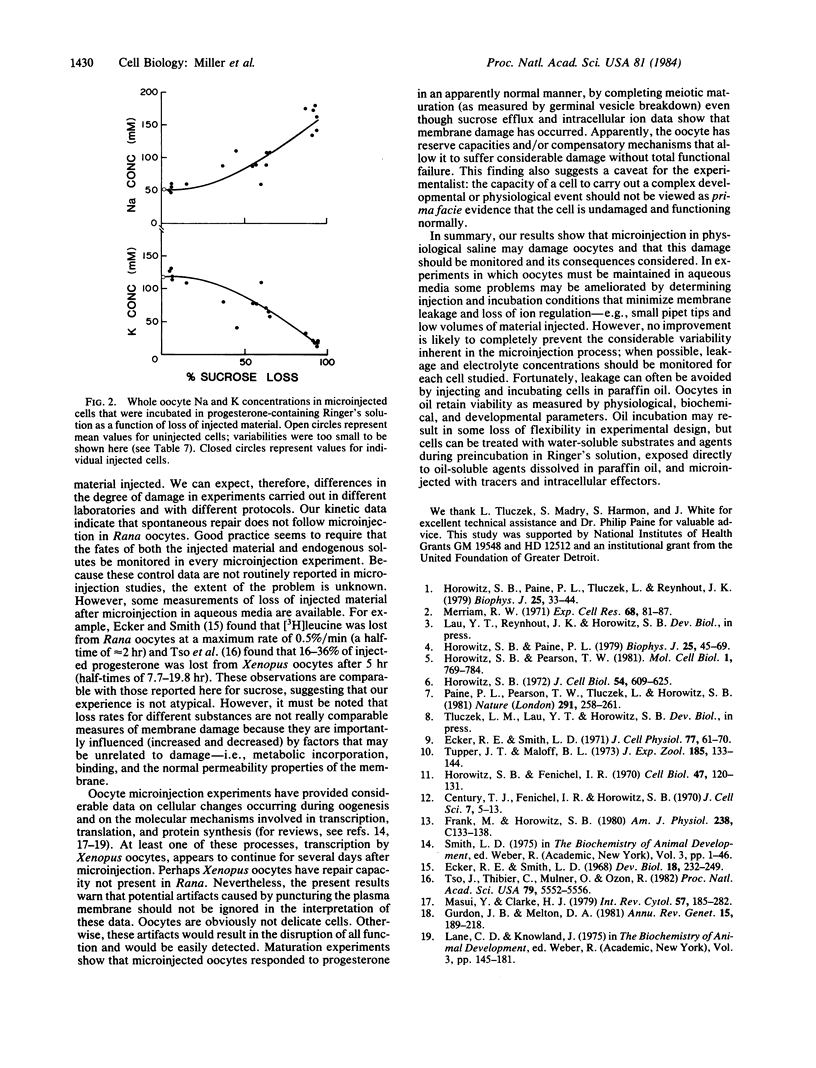
The effects of microinjection on Rana pipiens oocytes were determined using cryomicrodissection to measure Na, K, water, and injected radiolabeled sucrose (in gelatin) in the nucleus, animal, and vegetal ooplasm and injected bolus (reference phase, RP). The results point to potential problems in the interpretation of microinjection experiments. When oocytes were injected and incubated in Ringer's solution, nucleus, ooplasm, and RP lost K and sucrose and gained Na. Patterns of loss and gain were complex but were consistent with continuous solute leakage at the injection site causing artifactual intracellular diffusion gradients. In spite of leakage, oocytes completed scheduled meiotic maturation when exposed to progesterone. When oocytes were microinjected and incubated in paraffin oil (a medium in which polar solutes cannot exchange), nuclear and ooplasmic Na, K, and water concentrations remained identical to those in uninjected cells. Neither microinjection per se nor the injected bolus affected intraoocytic solute distributions. These findings imply that, after microinjection in aqueous media, metabolites are lost from and redistribute in cells, and that these artifactual changes are inadequately reflected in the ability of the cell to carry out a complex process. They also show that injection artifacts can be avoided by injecting and incubating cells under paraffin oil.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Century T. J., Fenichel I. R., Horowitz S. B. The concentrations of water, sodium and potassium in the nucleus and cytoplasm of amphibian oocytes. J Cell Sci. 1970 Jul;7(1):5–13. doi: 10.1242/jcs.7.1.5. [DOI] [PubMed] [Google Scholar]
- Ecker R. E., Smith L. D. Influence of exogenous ions on the events of maturation in Rana pipiens oocytes. J Cell Physiol. 1971 Feb;77(1):61–70. doi: 10.1002/jcp.1040770108. [DOI] [PubMed] [Google Scholar]
- Ecker R. E., Smith L. D. Protein synthesis in amphibian oocytes and early embryos. Dev Biol. 1968 Sep;18(3):232–249. doi: 10.1016/0012-1606(68)90034-1. [DOI] [PubMed] [Google Scholar]
- Frank M., Horowitz S. B. Potassium exchange in the whole cell, cytoplasm, and nucleus of amphibian oocytes. Am J Physiol. 1980 Mar;238(3):C133–C138. doi: 10.1152/ajpcell.1980.238.3.C133. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Fenichel I. R. Analysis of sodium transport in the amphibian oocyte by extractive and radioautographic techniques. J Cell Biol. 1970 Oct;47(1):120–131. doi: 10.1083/jcb.47.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. B., Paine P. L. Reference phase analysis of free and bound intracellular solutes. II. Isothermal and isotopic studies of cytoplasmic sodium, potassium, and water. Biophys J. 1979 Jan;25(1):45–62. doi: 10.1016/S0006-3495(79)85277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. B., Paine P. L., Tluczek L., Reynhout J. K. Reference phase analysis of free and bound intracellular solutes. I. Sodium and potassium in amphibian oocytes. Biophys J. 1979 Jan;25(1):33–44. doi: 10.1016/S0006-3495(79)85276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. B., Pearson T. W. Intracellular monosaccharide and amino acid concentrations and activities and the mechanisms of insulin action. Mol Cell Biol. 1981 Sep;1(9):769–784. doi: 10.1128/mcb.1.9.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. B. The permeability of the amphibian oocyte nucleus, in situ. J Cell Biol. 1972 Sep;54(3):609–625. doi: 10.1083/jcb.54.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Merriam R. W. Progesterone-induced maturational events in oocytes of Xenopus laevis. II. Change in intracellular calcium and magnesium distribution at germinal vesicle breakdown. Exp Cell Res. 1971 Sep;68(1):81–87. doi: 10.1016/0014-4827(71)90589-1. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Pearson T. W., Tluczek L. J., Horowitz S. B. Nuclear sodium and potassium. Nature. 1981 May 21;291(5812):258–259. doi: 10.1038/291258a0. [DOI] [PubMed] [Google Scholar]
- Tso J., Thibier C., Mulner O., Ozon R. Microinjected progesterone reinitiates meiotic maturation of Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5552–5556. doi: 10.1073/pnas.79.18.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Maloff B. L. The ionic permeability of the amphibian oocyte in the presence or absence of external calcium. J Exp Zool. 1973 Jul;185(1):133–144. doi: 10.1002/jez.1401850113. [DOI] [PubMed] [Google Scholar]



