Abstract
We hypothesized that treatment with testosterone (T) and recombinant human growth hormone (rhGH) would increase lean mass (LM) and muscle strength proportionally and an in a linear manner over 16 weeks. This was a multicenter, randomized, controlled, double-masked investigation of T and rhGH supplementation in older (71 ± 4 years) community-dwelling men. Participants received transdermal T at either 5 or 10 g/day as well as rhGH at 0, 3.0 or 5.0 µg/kg/day for 16 weeks. Body composition was determined by dual-energy X-ray absorptiometry (DEXA) and muscle performance by composite one-repetition maximum (1-RM) strength and strength per unit of lean mass (muscle quality, MQ) for five major muscle groups (upper and lower body) at baseline, week 8 and 17. The average change in total LM at study week 8 compared with baseline was 1.50 ± 1.54 kg (P < 0.0001) in the T only group and 2.64 ± 1.7 (P < 0.0001) in the T + rhGH group and at week 17 was 1.46 ± 1.48 kg (P < 0.0001) in the T only group and 2.14 ± 1.96 kg (P < 0.0001) in the T + rhGH group. 1-RM strength improved modestly in both groups combined (12.0 ± 23.9%, P < 0.0001) at week 8 but at week 17 these changes were twofold greater (24.7 ± 31.0%, P < 0.0001). MQ did not significantly change from baseline to week 8 but increased for the entire cohort, T only, and T + rhGH groups by week 17 (P < 0.001). Despite sizeable increases in LM measurements at week 8, tests of muscle performance did not show substantive improvements at this time point.
Keywords: Anabolic hormones, Androgen supplementation, Muscle quality, HORMA study
Introduction
With an increase in life expectancy, older persons are faced with the challenge of maintaining health and physical function while their anabolic hormone levels decline during the aging process. Approximately 25–30% of men over 60 years of age have serum levels of testosterone that may be diagnosed as hypogonadal (Harman et al. 2001). In addition, GH status may decline as much as 70% by the eighth decade of life (Corpas et al. 1992; Iranmanesh et al. 1991). It is possible that the declines in testosterone and GH are associated with the age-related loss of muscle mass (sarcopenia), skeletal muscle strength, and physical function (Baumgartner et al. 1998; Frontera et al. 2000). Sarcopenia may increase the risk of frailty, dependency, and depression (Dutta 1997; Penninx et al. 1998; Rantanen et al. 2000). Loss of muscle mass may be associated with decrements in muscle performance, including power to climb stairs or generate gait speed (Bassey et al. 1988) and eventually advancing to severe loss of skeletal muscle strength resulting in overt frailty and impaired activities of daily living. Limited data support the potential value of treatment with replacement doses of testosterone to improve lean mass in older persons (Orwoll et al. 2006; Page et al. 2005). However, increases in lean mass in this population will likely not be meaningful unless there are improvements in skeletal muscle strength and performance.
An increase in muscle strength relative to lean mass (muscle quality) (Lynch et al. 1999) is an important means to quantify relative change in muscle performance. To date, studies have suggested that testosterone supplementation does not improve muscle quality, even though doses sufficient to substantially increase serum testosterone levels may enhance muscle mass and strength (Bhasin et al. 2001; Schroeder et al. 2003). However, it is uncertain how early in the course of treatment that clinical enhancements in muscle mass and performance can be achieved with testosterone supplementation alone or in combination with rhGH.
In the HORMA (Hormonal Regulators of Muscle and Metabolism During Aging) study (Sattler et al. 2009), we hypothesized that testosterone and rhGH supplementation affect myofibrillar proteins by different but complimentary mechanisms and together might enhance not only voluntary muscle strength but other measures of muscle performance, and perhaps muscle quality also. Although there were dose-dependent effects of these anabolic hormones on muscle mass and performance at the completion of 16 weeks of treatment, we hypothesized that the effects were linear over time and any sizable improvements in lean mass would be associated with proportional changes in muscle performance. We herein report the findings of our secondary analysis to test this hypothesis by assessing whether changes in muscle mass and function were together proportionally greater shortly after completion of study therapy compared to mid way through treatment.
Methods
Study design
Methods are described elsewhere (Sattler et al. 2009; Schroeder et al. 2007a) and briefly summarized here. This was a multicenter [University of Southern California (USC), Tufts University, and Washington University] controlled, double-masked investigation of supplementation with testosterone and rhGH at physiologic doses in older community-dwelling men. Eligible participants were randomized to either 5 or 10 g/day transdermal testosterone (Androgel, Solvay Pharmaceuticals Inc) and all participants received a Leydig cell clamp to suppress endogenous testosterone, which could confound dose–response relationships. Participants were also randomized to placebo rhGH (0 µg/kg/day) or one of two doses of rhGH (3.0 or 5.0 µg/kg/day) (Nutropin, Genentech Inc). Treatment duration was 16 weeks with post intervention outcomes determined at week 17. In our previous intent to treat report, there was sizable heterogeneity in outcomes within the treatment assignment groups. Although changes in serum testosterone and IGF-1 levels were also heterogeneous in the treatment groups, they were directly related to changes in LBM, which were necessary to enhance muscle performance (Sattler et al. 2011). Therefore, in this secondary analysis we sought to determine if the effects of testosterone alone and testosterone plus rhGH on lean mass and strength at 8 and 16 weeks were corroborated by changes in muscle performance.
Study participants
All participants provided informed consent approved by the institutional review boards of USC, Tufts University, and Washington University. Eligibility required that men 65–90 years of age had serum IGF-1 in the lower tertile for adults (<167 ng/dl) and morning serum testosterone in the lower portion (150–550 ng/dl) of the adult male range. Other eligibility criteria included prostate-specific antigen (PSA) <4.0 ng/ml, hematocrit <50%, and fasting blood glucose <126 mg/dl. Volunteers were excluded if they participated in regular exercise in the previous 6 months and participants were instructed not to perform any structured exercise during the study.
Body composition
Whole-body DEXA scans were performed at baseline and study weeks 8 and 17 to quantify total lean mass, appendicular lean mass, and fat mass. One experienced technician (blinded to treatment) performed and analyzed the scans at the USC Reading Center. To corroborate that changes in lean mass represented changes in muscle mass, total-body skeletal muscle (total-body SM) was estimated, according to the formula total-body SM = (1.13 × appendicular lean mass)−(0.02 × age) + (0.61 × gender) + 0.97; where gender = 1 for men. This strategy may better reflect changes in whole body muscle mass than measures of appendicular lean mass (Kim et al. 2002).
Skeletal muscle strength
Maximal voluntary strength was assessed (baseline, weeks 8 and 17) using the one-repetition maximum (1-RM) method (Schroeder et al. 2007b) for the bilateral leg press, leg extension, leg flexion, latissimus pull-down, and chest press exercises on Keiser pneumatic equipment at USC and on weight stack resistance machines at Tufts and Washington universities. The highest of the respective 1-RM values assessed at pre-entry or baseline were used as the pre-treatment values. To normalize and consolidate whole-body strength assessments from multiple testing sites, results are presented as percentage change from baseline for the composite sum of 1-RM values for the five exercises.
Muscle quality assessment
Muscle quality was calculated as the maximal composite strength score in units of kilograms divided by DEXA total lean mass in units of kilograms (Inaba et al. 2010; Lynch et al. 1999; Newman et al. 2006). An optimal change in muscle quality would be greater increases in strength relative to lean mass so that the ratio values increase. Because some of the resistance training equipment used for assessing maximal strength was pneumatic with units of measure in pounds per square inch (PSI), we converted all units of measure to kilograms to determine the numerator in the ratio of strength to lean mass. Both absolute and relative changes in composite strength relative to total lean mass are presented in the “Results”.
Lower leg edema
Lower leg edema was assessed for pitting (none, trace, 1+ to 4+) at baseline, study week 8, and study week 16 by the same study physician at one testing site. For this analysis, we determined if new or worse (at least two grade increase, e.g. from 1+ to 3+) lower leg edema was present or absent at weeks 8 and 16.
Statistical analysis
A total of 88 participants had complete data for muscle quality at all three visits (baseline, week 8, and week 17). Since the baseline demographic, week 8 and week 17 variables were similar between the 88 subjects and the original cohort (N = 112), the main analyses were conducted on these 88 participants only and are referred to as the entire cohort in this paper. Descriptive statistics are presented for the demographic and baseline characteristics for all 88 subjects, the T only group, and the T + rhGH group. Continuous variables were summarized using mean and standard deviation (SD). Discrete variables were described by frequency counts and percentages.
Each body composition parameter (total and appendicular lean mass, and total-body skeletal mass) was compared between the two groups using t test for baseline, week 8, and week 17 visit. In addition, within the entire cohort as well as each group, the mixed effect model was fitted to the repeated body composition parameter measures across the three visits. Composite strength change at week 8 and week 17 relative to baseline was compared between groups using t test. Paired t tests were applied to compare the composite strength changes at these two time points for all 88 participants and each group.
The mixed effect model was also fitted to the absolute composite strength/total lean mass ratio measures across baseline, week 8, and week 17 visit within each group and for the entire cohort. The overall and group specific changes (baseline vs. week 8, baseline vs. week 17, and week 8 vs. week 17) were compared using the paired t test.
Side by side bar plots for mean changes from week 8 and week 17 to baseline were presented for the entire cohort and for each group for changes in total lean mass, changes in total-body skeletal mass, changes in composite strength, and changes in composite strength/total lean mass ratio.
Results
Of the 242 subjects consented and who completed screening visits, 122 eligible participants were randomized to study treatments. One hundred twelve participants completed 16 weeks of the intervention and were evaluated at week 17. Of the 112 participants, 88 completed each test of 1-RM strength and DEXA scans at baseline, study week 8, and study week 17. The most common reason for participants not having a 1-RM test of a specific muscle group at any study visit was due to shoulder or knee discomfort (usually due to osteoarthritis and fluid retention) and therefore if one of five tests could not be completed, this precluded calculation of composite strength for that visit. These 88 participants with complete composite (five different 1-RM tests) strength tests were similar to the entire population of 112 (data not shown). A detailed description of baseline characteristics for this study population has been published (Sattler et al. 2009).
Because there were no differences across study sites for the variables of interest, the data were collapsed and presented as an entire cohort N = 88, testosterone (T) N = 29, and testosterone plus rhGH (T + rhGH) N = 59. Table 1 shows the baseline characteristics of these two cohorts. Baseline testosterone levels were similar between the T and T + rhGH groups. Although baseline IGF-1, an indirect measure of GH status, was significantly lower in the T only group (102 ± 27 ng/ml) at baseline than the T + rhGH group (118 ± 30 ng/ml), the meaning of this small difference is unclear.
Table 1.
Descriptive characteristics
| Characteristics | Entire cohort N = 88 |
T onlya N = 29 |
T + rhGHa N = 59 |
P valueg |
|---|---|---|---|---|
| Age (years) | 70.6 ± 4.2b | 71.2 ± 5.1 | 70.2 ± 3.7 | 0.34 |
| BMI (kg/m2) | 27.3 ± 3.3 | 27.3 ± 3.6 | 27.2 ± 3.1 | 0.90 |
| Non-hispanic Caucasian # (%) | 77 (88%) | 22 (76%) | 55 (93%) | 0.02 |
| Total LBM (kg) | 58.3 ± 7.3 | 57.1 ± 5.8 | 58.9 ± 7.9 | 0.27 |
| Appendicular LBM (kg) | 25.5 ± 3.5 | 25.0 ± 2.8 | 25.8 ± 3.8 | 0.33 |
| Total body fat (%) | 29.0 ± 4.0 | 28.4 ± 3.2 | 29.3 ± 4.3 | 0.32 |
| Total testosterone (ng/dL)c,d | 358 ± 98 | 377 ± 106 | 348 ± 93 | 0.20 |
| IGF-1 (ng/mL)e | 113 ± 30 | 102 ± 27 | 118 ± 30 | 0.01 |
| Albumin (g/dL) | 4.11 ± 0.31 | 4.06 ± 0.29 | 4.14 ± 0.32 | 0.27 |
| Hemoglobin (%) | 14.7 ± 1.0 | 14.5 ± 1.3 | 14.8 ± 0.7 | 0.37 |
| Creatinine (mg/dL) | 1.04 ± 0.16 | 1.04 ± 0.15 | 1.03 ± 0.17 | 0.70 |
| VO2 peak test (mL/kg/min) | 24.7 ± 4.8 | 25.1 ± 7.1 | 24.5 ± 3.3 | 0.71 |
| PASEf | 143 ± 61 | 150 ± 73 | 140 ± 54 | 0.51 |
T = Testosterone, rhGH = recombinant human growth hormone
Mean ± standard deviation
By automated platform immunoassays in local university clinical laboratories for study screening
Conversion to nmol/L = ng/dL × 0.03467
Conversion to nmol/L = ng/mL × 0.13
Physical Activity Scale for the Elderly
Two-sample t test comparing T only group with T + rhGH group
Changes in body composition
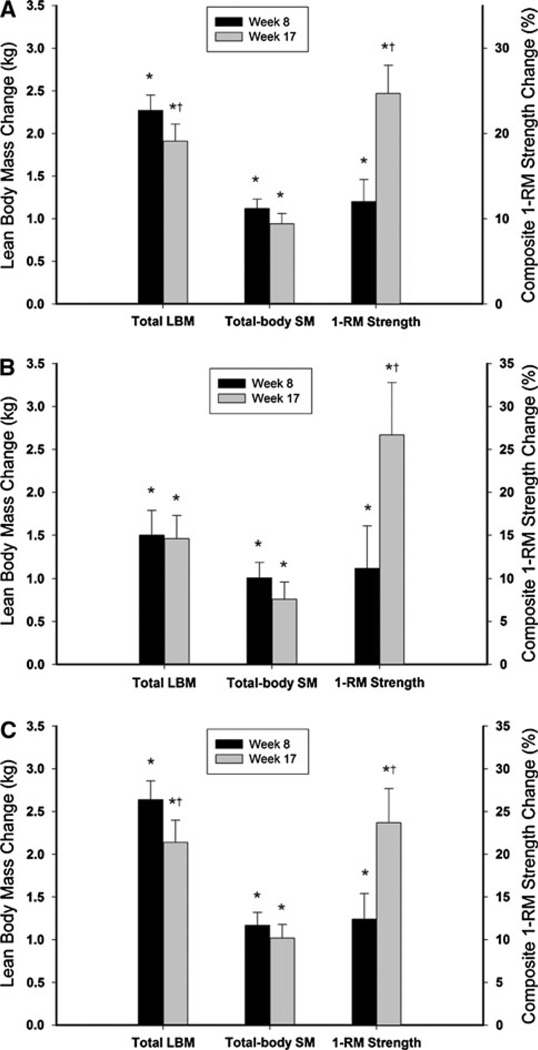
Total and appendicular lean mass and total-body SM significantly increased at study week 8 and at study week 17. These changes were significantly different from baseline with the greatest changes occurring at study week 8 for the entire cohort and the T + rhGH group but not for the T only group (Table 2; Fig. 1).
Table 2.
Lean mass, strength, and muscle quality by time point
| N | Baseline | Week 8 | Week 17 | P valueb | |
|---|---|---|---|---|---|
| Total body lean (kg) | |||||
| T only | 29 | 57.1 ± 5.8a | 58.6 ± 5.7* | 58.5 ± 5.6* | <0.0001 |
| T + GH | 59 | 58.9 ± 7.9 | 61.5 ± 8.1* | 61.0 ± 8.4* | <0.0001 |
| P valuec | 0.27 | 0.052 | 0.10 | ||
| Appendicular lean (kg) | |||||
| T only | 29 | 25.0 ± 2.8 | 26.0 ± 2.6* | 25.8 ± 2.5* | <0.0001 |
| T + GH | 59 | 25.8 ± 3.8 | 27.0 ± 3.8* | 26.8 ± 4.0* | <0.0001 |
| P value | 0.33 | 0.18 | 0.13 | ||
| Total-body SMd (kg) | |||||
| T only | 29 | 28.4 ± 3.2 | 29.5 ± 3.0* | 29.3 ± 2.9* | <0.0001 |
| T + GH | 59 | 29.3 ± 4.3 | 30.6 ± 4.4* | 30.5 ± 4.5* | <0.0001 |
| P value | 0.33 | 0.18 | 0.13 | ||
| Composite strengthe (%) | |||||
| T only | 29 | N/A | 11.2 ± 26.5 | 26.7 ± 32.8 | 0.0006 |
| T + GH | 59 | N/A | 12.4 ± 22.8 | 23.7 ± 30.3 | 0.0002 |
| P value | N/A | 0.83 | 0.67 | ||
| Muscle qualityf | |||||
| T only | 29 | 6.48 ± 0.94 | 6.40 ± 0.84 | 6.62 ± 0.87† | 0.002 |
| T + GH | 59 | 6.44 ± 0.91 | 6.31 ± 0.89* | 6.48 ± 0.92† | 0.0004 |
| P value | 0.86 | 0.68 | 0.50 |
P < 0.05 compared with baseline
P < 0.05 compared with week 8
Mean ± standard deviation
Mixed effects model comparing mean scores across visits
Two-sample t test
Total-body skeletal muscle = 1.13 × appendicular lean mass−0.02 × age + 0.61 × gender + 0.97 (gender = 1 for males)
Sum of percentage change of maximal voluntary strength for the five exercises
Muscle quality = composite strength (kg)/total lean body mass (kg)
Fig. 1.
a Change in total lean body mass (LBM), total-body skeletal mass, and composite one repetition maximum strength for the entire cohort of 88 subjects. b Change in total lean body mass (LBM), total-body skeletal mass, and composite one repetition maximum strength for the subjects that received testosterone only, N = 29. c Change in total lean body mass (LBM), total-body skeletal mass, and composite one repetition maximum strength for the subjects that received testosterone plus rhGH, N = 59. The left y axis shows the absolute change in kilograms for LBM and the right y axis shows the relative (%) change for composite strength. The black bars represent the mean change at study week 8 and the gray bars represent the mean change at study week 17. The whiskers represent the standard error. *P < 0.0001 compared with Baseline. †P ≤ 0.003 comparing study week 8 to week 17
Changes in maximal voluntary muscle strength
Composite strength improved for the entire cohort (12.0%), T only group (11.2%), and the T + rhGH group (12.4%) by week 8 (Table 2; Fig. 1). However, by study week 17 composite strength increased further by approximately twofold for the entire cohort (24.7%, P < 0.0001), the T only group (26.7%, P = 0.0006), and the T + rhGH group (23.7%, P = 0.0002) compared with the changes achieved at week 8 (Table 2).
Changes in muscle quality
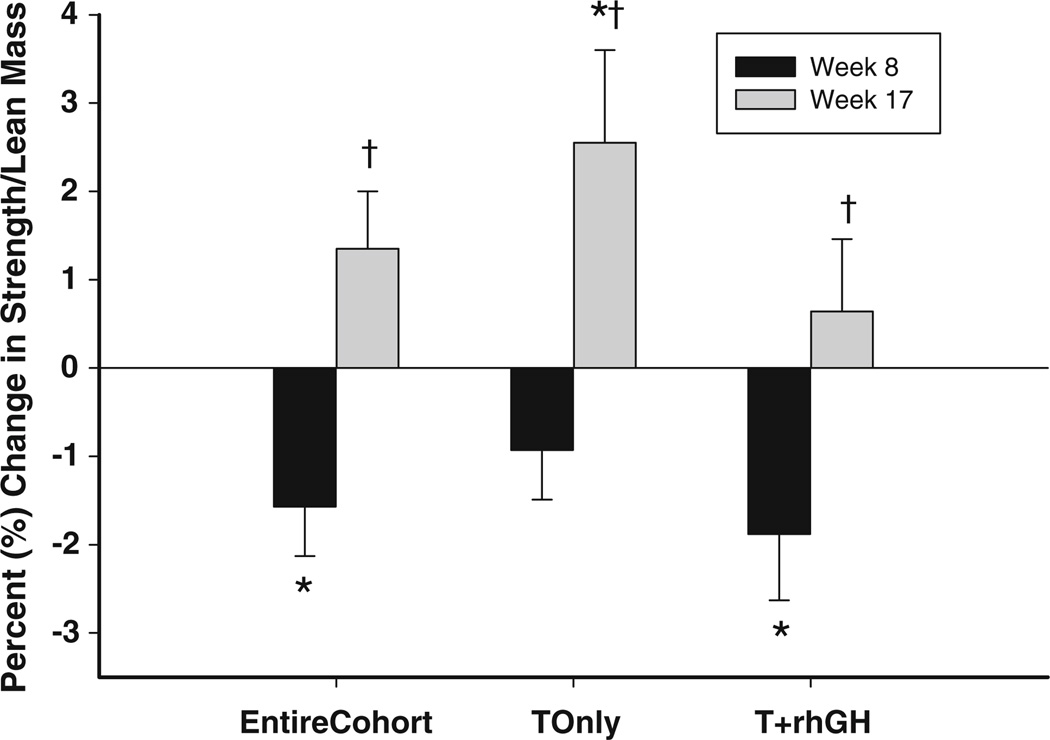
There were small but statistically significant decreases in muscle quality from baseline to study week 8 for the entire cohort and the T + rhGH group (Table 2; Fig. 2). In the T only group, MQ did not significantly change from baseline to study week 8. However, muscle quality improved from study week 8 to 17 for the entire cohort, the T only group, and the T + rhGH group (Fig. 2).
Fig. 2.
Relative percent change in muscle quality (composite strength/total lean mass) for the entire cohort, N = 88, the testosterone only group, N = 29, and the testosterone plus rhGH group, N = 59. The black bars represent the mean change at study week 8 and the gray bars represent the mean change at study week 17. The whiskers represent the standard error. *P ≤ 0.01 compared with Baseline. †P ≤ 0.002 comparing study week 8 to week 17
Changes in lower leg edema
New pre-tibial or ankle edema was evaluated carefully at one study site and occurred more commonly (23 cases) at week 8 compared with week 16 (16 cases). However, this frequency was not statistically significant (P = 0.25) by McNemar’s test.
Discussion
In the primary study (Sattler et al. 2009), we showed for the first time that testosterone supplementation with and without the addition of rhGH in older men substantially augmented average total lean mass ~1.5 kg; for the high-dose combination, average total lean mass increased ~3.0 kg, and the maximum increase was 7.5 kg after 17 weeks. There were parallel improvements in composite maximal voluntary strength of ~24% (~35% in highest dose combination; maximum of 117%) (Sattler et al. 2009), unlike prior studies evaluating treatment with the combination of these potent anabolic hormones (Blackman et al. 2002; Giannoulis et al. 2006; Meinhardt et al. 2010). Surprisingly, in this secondary analysis, improvements in lean mass at study week 8 were significantly greater than the changes at study week 17 for the entire cohort and T + rhGH group but not for the T only group. However, the converse was true of changes in maximal voluntary muscle strength (1-RM) and muscle quality which were greater at study week 17 than week 8. The twofold greater increases in composite strength of the major muscle groups in the upper and lower body at week 17 suggest that improvements in lean mass at this time point were likely a result of contractile myofibrillar protein accumulation.
The most convincing evidence to corroborate that changes in lean mass are due to the accretion of substantive amounts of contractile myofibrillar protein is the demonstration of significant improvements in skeletal muscle strength and performance. The modest increases in composite strength and decreases in muscle quality at study week 8 suggest that these changes were not due to meaningful accretion of functional myofibrillar proteins despite significant gains in both total and regional lean mass during therapy with testosterone and rhGH. We can only conjecture that the changes in lean mass could have been due to an increase in extracellular water as may occur with either anabolic hormone treatment (Johannsson et al. 2005; Meinhardt et al. 2010) or intramyocellular accumulation of structural, mitochondrial or other proteins, which could later be used as building blocks for contractile proteins. The observation that lower extremity edema tended to be greater at week 8 than at the end of study therapies supports the possibility that some portion of the lean tissue mass change was due to accumulation of extracellular fluid. That lean mass values at study week 17 were statistically smaller for the entire cohort and T + rhGH group than the increases achieved at study week 8 did not support our hypothesis for this secondary analysis that lean mass would increase linearly over time. Because the T only group did not have significantly different lean tissue values at study week 8 compared with study week 17, it is likely that the combined treatment with rhGH was responsible for the elevated lean tissue measurements at study week 8. Further, the overall gains in strength and the improved muscle quality at week 17 indicated that there were some gains in muscle performance at week 8 but these were in the 5–10% range and may be of questionable clinical importance, whereas gains in skeletal muscle mass and maximal voluntary strength at week 17 were more substantial and indicated that there had been meaningful increases in functional components of lean mass needed to enhance muscle performance by the end of treatment.
Earlier studies of testosterone therapy (Gruenewald and Matsumoto 2003; Wald et al. 2006) reported increases of total lean mass in the range of 1–2 kg but failed to show improvements in skeletal muscle strength (Clague et al. 1999; Emmelot-Vonk et al. 2008; Kenny et al. 2001; Snyder et al. 1999). In these studies, gains in total lean mass may not have been sizable enough to sufficiently augment myofibrillar proteins necessary to improve maximal voluntary muscle strength. Indeed, we have recently shown that T and rhGH-induced increases of approximately 1.5 kg of total lean mass or about 0.8 kg of appendicular lean mass are necessary to increase muscle performance (Sattler et al. 2011). In the earlier studies cited above, the outcomes may have been too heterogeneous to demonstrate overall significant increases in muscle strength despite average increases that were in the range of the critical thresholds that we have shown important to improve 1-RM strength (Sattler et al. 2011). The benefits of rhGH administration on muscle mass and strength in healthy older adults remains unclear and is an area of research that needs further study, particularly the influence of rhGH isoforms that likely have different physiologic significance than that of the 22 kD form (Kraemer et al. 2010). Although our rhGH dose was small relative to other studies, our data support the contention that rhGH may be associated with fluid retention and likely does not contribute to improvements in maximal voluntary muscle strength or muscle mass that would translate into enhanced muscle performance even when combined with testosterone supplementation.
Describing muscle quality is an important measure of muscle performance (Tracy et al. 1999), but previous androgen administration studies have failed to show improvement in this measure since increases in muscle strength have been proportional to increases in muscle mass (Bhasin et al. 2001; Schroeder et al. 2003). Conversely, in our current study, we report significant decreases muscle quality at week 8 followed by further improvements from week 8 to 17 with testosterone and rhGH supplementation. These findings were not anticipated since improvement in muscle quality often requires disproportional increases in muscle strength relative to muscle mass that is usually the result of resistance training-induced neural adaptations (Schroeder et al. 2003; Tracy et al. 1999). Further studies, including pharmacologic therapies with potent combinations or even different promyogenic agents, e.g. SARMs, anti-myostatin strategies, etc. may confirm or deny our findings.
The study findings do not support our hypothesis that the gains in lean mass and muscle strength at week 8 would be less than the gains achieved by week 17. Indeed, in a prior report from members of our group, treatment of older men with oxandrolone resulted in 90% of the gains in lean mass, reductions in adipose tissue, and improvements in muscle strength being achieved by 6 weeks in a 12-week treatment study (Schroeder et al. 2005). Likewise, in the present study significant reductions in total and trunk fat (data not shown) occurred at week 8 and the changes were greater at week 17 supporting a time-dependent effect of the anabolic hormone combination on other tissues. Why increases in lean mass did not follow a more time-dependent linear change is unclear. It may be that fluid accumulation or other constituents of lean mass (e.g. non-contractile proteins or amino acids which also are associated with hydration effects) masked any early gains in lean mass at week 8 that had yet to be translated to improved muscle performance. Indeed, the accumulation of tissue water is measured as lean mass by various testing modalities such as DEXA, computed tomography, and magnetic resonance imaging (St-Onge et al. 2004; Wang et al. 1999). Regardless of the mechanism, it is difficult to compare studies testing different anabolic treatments because of their duration, route of administration or other factors such as age of the study population, their physical activity level, etc.
There are several limitations. First, as indicated above, we used DEXA measures of lean mass changes which are influenced by hydration status or changes in body water that can introduce measurement variability over serial time points. However, other muscle imaging procedures are affected by the same limitation. Further, our participants were treated with leuprolide acetate to suppress endogenous testosterone production, which is also associated with fluid retention and weight gain that could have confounded the week 8 measurements when participants were still acclimating to the study interventions and changes in total body water. Last, our multicenter study included resistance exercise machines that provided units of measure that were different at each testing site. Therefore, we reported relative change in composite strength to standardize the measurements. Regardless, the relative changes in 1-RM strength at week 8 and 17 were determined for each participant relative to baseline on the same exercise equipment and independently validate important accrual of functional myofibrillar proteins at week 17.
In conclusion, the discordant results at week 8 between changes in muscle strength and muscle quality compared with lean mass suggest that apparent gains in lean mass measured by DEXA were due to either extracellular water or non-contractile muscle proteins. The important finding is that regardless of treatment strategy the large increases in lean mass at study week 8 were not supported by large increases in muscle function. Our findings emphasize the importance of muscle performance testing when evaluating promyogenic agents, to validate that increases in lean mass translate to improvements in muscle function. This is especially important in studies of older sarcopenic adults. Understanding the timing and functional benefits of short-term administration of promyogenic agents for older adults will be important in designing future studies of pharmacologic strategies to treat sarcopenia or frailty.
Acknowledgments
We gratefully acknowledge the volunteer participants who committed substantial time and efforts to make this study successful. Support for this trial was provided in part from the National Institutes of Health R01 AG18169 and local NCRR GCRC M0I RR000043 at USC, the US Department of Agriculture (USDA) ARS Cooperative Agreement 58-1950-9-001, the NCRR GCRC grant M01 RR000054 at Tufts University, where any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA, and the NCRR GCRC at Washington University School of Medicine (M01 RR000036), the Mass Spectrometry Research Resource at Washington University (NIH RR000954, DK020579, and DK056341), and NIH grants U01AG14369 and 1R01DK70534 at Boston Medical Center, Boston University School of Medicine. Study therapies were provided by Solvay Pharmaceuticals Inc, Genentech Inc, and TAP Pharmaceutical Products Inc; industry sponsors provided no monetary support.
Contributor Information
E. Todd Schroeder, Email: eschroed@usc.edu, Division of Biokinesiology and PT, University of Southern California, 1540 East Alcazar St. CHP-155, Los Angeles, CA 90033, USA; Department of Medicine, University of Southern California, Los Angeles, CA, USA.
Jiaxiu He, Department of Preventive Medicine, University of Southern California, Los Angeles, CA, USA.
Kevin E. Yarasheski, Department of Medicine, Washington University, St. Louis, MO, USA
Ellen F. Binder, Department of Medicine, Washington University, St. Louis, MO, USA
Carmen Castaneda-Sceppa, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Shalender Bhasin, Section of Endocrinology, Diabetes, and Nutrition, Boston University, Boston, MA, USA.
Christina M. Dieli-Conwright, Division of Biokinesiology and PT, University of Southern California, 1540 East Alcazar St. CHP-155, Los Angeles, CA 90033, USA
Miwa Kawakubo, Department of Preventive Medicine, University of Southern California, Los Angeles, CA, USA.
Ronenn Roubenoff, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Stanley P. Azen, Department of Preventive Medicine, University of Southern California, Los Angeles, CA, USA
Fred R. Sattler, Division of Biokinesiology and PT, University of Southern California, 1540 East Alcazar St. CHP-155, Los Angeles, CA 90033, USA Department of Medicine, University of Southern California, Los Angeles, CA, USA.
References
- Bassey EJ, Bendall MJ, Pearson M. Muscle strength in the triceps surae and objectively measured customary walking activity in men and women over 65 years of age. Clin Sci (Lond) 1988;74:85–89. doi: 10.1042/cs0740085. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol. 2001;170:27–38. doi: 10.1677/joe.0.1700027. [DOI] [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- Clague JE, Wu FC, Horan MA. Difficulties in measuring the effect of testosterone replacement therapy on muscle function in older men. Int J Androl. 1999;22:261–265. doi: 10.1046/j.1365-2605.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Pineyro MA, Roberson R, Blackman MR. Growth hormone (GH)-releasing hormone-(1–29) twice daily reverses the decreased GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metab. 1992;75:530–535. doi: 10.1210/jcem.75.2.1379256. [DOI] [PubMed] [Google Scholar]
- Dutta C. Significance of sarcopenia in the elderly. J Nutr. 1997;127:992S–993S. doi: 10.1093/jn/127.5.992S. [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. discussion 115. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Inaba M, Kurajoh M, Okuno S, Imanishi Y, Yamada S, Mori K, Ishimura E, Yamakawa T, Nishizawa Y. Poor muscle quality rather than reduced lean body mass is responsible for the lower serum creatinine level in hemodialysis patients with diabetes mellitus. Clin Nephrol. 2010;74:266–272. [PubMed] [Google Scholar]
- Iranmanesh A, Lizarralde G, Johnson ML, Veldhuis JD. Nature of altered growth hormone secretion in hyperthyroidism. J Clin Endocrinol Metab. 1991;72:108–115. doi: 10.1210/jcem-72-1-108. [DOI] [PubMed] [Google Scholar]
- Johannsson G, Gibney J, Wolthers T, Leung KC, Ho KK. Independent and combined effects of testosterone and growth hormone on extracellular water in hypopituitary men. J Clin Endocrinol Metab. 2005;90:3989–3994. doi: 10.1210/jc.2005-0553. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Dunn-Lewis C, Comstock BA, Thomas GA, Clark JE, Nindl BC. Growth hormone, exercise, and athletic performance: a continued evolution of complexity. Curr Sports Med Rep. 2010;9:242–252. doi: 10.1249/JSR.0b013e3181e976df. [DOI] [PubMed] [Google Scholar]
- Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- Meinhardt U, Nelson AE, Hansen JL, Birzniece V, Clifford D, Leung KC, Graham K, Ho KK. The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Ann Intern Med. 2010;152:568–577. doi: 10.7326/0003-4819-152-9-201005040-00007. [DOI] [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings SR. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166:2124–2131. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Penninx BW, Masaki K, Lintunen T, Foley D, Guralnik JM. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc. 2000;48:613–617. doi: 10.1111/j.1532-5415.2000.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler F, Bhasin S, He J, Chou CP, Castaneda-Sceppa C, Yarasheski K, Binder E, Schroeder ET, Kawakubo M, Zhang A, Roubenoff R, Azen S. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66:122–129. doi: 10.1093/gerona/glq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder ET, Terk M, Sattler FR. Androgen therapy improves muscle mass and strength but not muscle quality: results from two studies. Am J Physiol Endocrinol Metab. 2003;285:E16–E24. doi: 10.1152/ajpendo.00032.2003. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Vallejo AF, Zheng L, Stewart Y, Flores C, Nakao S, Martinez C, Sattler FR. Six-week improvements in muscle mass and strength during androgen therapy in older men. J Gerontol A Biol Sci Med Sci. 2005;60:1586–1592. doi: 10.1093/gerona/60.12.1586. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Castaneda-Sceppa C, Wang Y, Binder EF, Kawakubo M, Stewart Y, Storer T, Roubenoff R, Bhasin S, Yarasheski KE, Sattler FR, Azen SP. Hormonal regulators of muscle and metabolism in aging (HORMA): design and conduct of a complex, double masked multicenter trial. Clin Trials. 2007a;4:560–571. doi: 10.1177/1740774507083569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder ET, Wang Y, Castaneda-Sceppa C, Cloutier G, Vallejo AF, Kawakubo M, Jensky NE, Coomber S, Azen SP, Sattler FR. Reliability of maximal voluntary muscle strength and power testing in older men. J Gerontol A Biol Sci Med Sci. 2007b;62:543–549. doi: 10.1093/gerona/62.5.543. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, Wang Z, Horlick M, Wang J, Heymsfield SB. Dual-energy X-ray absorptiometry lean soft tissue hydration: independent contributions of intra- and extracellular water. Am J Physiol Endocrinol Metab. 2004;287:E842–E847. doi: 10.1152/ajpendo.00361.2003. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, Metter EJ, Fozard JL, Fleg JL, Hurley BF. Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol. 1999;86:195–201. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- Wald M, Meacham RB, Ross LS, Niederberger CS. Testosterone replacement therapy for older men. J Androl. 2006;27:126–132. doi: 10.2164/jandrol.05036. [DOI] [PubMed] [Google Scholar]
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol. 1999;276:E995–E1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]