Abstract
Little is known about quality-of-life (QOL) differences over time between incident ductal carcinoma in situ (DCIS) and early-stage invasive breast cancer (EIBC) cases as compared with same-aged women without breast cancer (controls). We prospectively recruited and interviewed 1096 women (16.8% DCIS, 33.3% EIBC [25.7% Stage I, 7.6% Stage IIA], 49.9% controls; mean age 58; 23.7% non-white) a mean 6.7 weeks (T1), and 6.2 (T2), 12.3 (T3), and 24.4 months (T4) after surgery (patients) or screening mammogram (controls). We tested two hypotheses: (1) DCIS patients would report lower levels of QOL compared with controls but would report similar QOL compared with EIBC patients at baseline; and (2) DCIS patients’ QOL would improve during 2-year follow-up and approach levels similar to that of controls faster than EIBC patients. We tested Hypothesis 1 using separate general linear regression models for each of the eight subscales on the RAND 36-item Health Survey, controlling for variables associated with at least one subscale at T1. Both DCIS and EIBC patients reported lower QOL at T1 than controls on all subscales (each p < .05). We tested Hypothesis 2 using generalized estimating equations to examine change in each QOL subscale over time across the three diagnostic groups adjusting for covariates. By T3, physical functioning, role limitations due to physical problems, energy/fatigue, and general health each differed significantly by diagnostic group at P < 0.05, due to larger differences between EIBC patients and controls; but DCIS patients no longer differed significantly from controls on any of the QOL subscales. At T4, EIBC patients still reported worse physical functioning (P = 0.0001) and general health (P = 0.0017) than controls, possibly due to lingering treatment effects. DCIS patients’ QOL was similar to that of controls two years after diagnosis, but some aspects of EIBC patients’ QOL remained lower.
Keywords: Breast cancer, ductal carcinoma in situ (DCIS), early-stage invasive breast cancer, quality of life
Introduction
Ductal carcinoma in situ (DCIS) is a noninvasive breast cancer diagnosed with greater frequency due to more widespread use of screening mammograms [1] and accounts for nearly 25% of breast cancer cases in the United States [2]. Despite increased incidence, few studies have addressed the quality of life (QOL) of women with DCIS [3–6]. Since women diagnosed with DCIS and early-stage invasive breast cancer (EIBC) have similar treatment options (i.e., mastectomy or breast-conserving surgery and radiation therapy, each with or without hormone therapy, as indicated) [7], DCIS and EIBC patients may be similar in their QOL experiences following surgery. However, since women diagnosed with DCIS have an excellent prognosis and this diagnosis is clinically distinct from EIBC [8–12], QOL in women with DCIS might be more similar to QOL in women without a history of breast cancer over time. Thus, we sought to examine the impact of a DCIS diagnosis on QOL outcomes, by comparing women with DCIS, women with EIBC, and a comparison group of age-matched women without a history of breast cancer.
The results of QOL studies in women with DCIS have been inconsistent, largely explained by limitations due to small samples of DCIS patients [3, 5] or the lack of comparison groups of either healthy women [3] or women with invasive breast cancer [3, 4, 6, 13]. Many QOL studies of breast cancer patients have combined patients with in situ carcinoma and invasive disease in one group for analysis [14–17], and some studies that included DCIS patients were cross-sectional in design [3–5, 14]. In this longitudinal study, we used examined changes in QOL in a cohort of incident DCIS and EIBC cases and of women without any breast cancer (controls) beginning shortly after definitive surgical treatment (patients) or routine screening mammogram with benign/normal findings (controls). We tested two hypotheses: (1) women with DCIS would report lower levels of QOL compared with controls but would report similar QOL compared with women with EIBC at baseline and (2) DCIS patients’ QOL would improve during 2-year follow-up and approach levels similar to those of controls faster than EIBC patients.
Methods
Participants
We prospectively recruited participants between October 2003 and June 2007 from the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine and from Saint Louis University School of Medicine in Saint Louis, Missouri. Patients diagnosed by surgical pathology with a first primary stage 0-IIA breast cancer (without neoadjuvant chemotherapy) were eligible. Controls were identified two weeks following normal/benign screening mammograms and were frequency-matched by age group (40–49, 50–69, ≥ 70) to patient participants. We included women age 40 and older, since screening mammography is recommended for women in this age group [18] and DCIS is primarily identified using mammography [19]. Additional eligibility criteria included no prior history of in situ or invasive breast cancer, the ability to speak and understand English and no evidence of cognitive impairment on the Orientation-Memory-Concentration (OMC) Test [20], administered to participants 65 years of age or older.
Procedures and measures
Following Institutional Review Board approval at each institution and obtaining participants’ informed consent, computer-assisted telephone interviews were administered at 4–6 weeks (T1), 6 months (T2), 1 year (T3), and 2 years (T4) following definitive surgical treatment (patients) or screening mammogram (controls). We collected participants’ demographic information and administered validated measures of QOL, social support, comorbidity, and history of depression as well as a measure of menopausal symptoms developed for this study. All measures were selected because they were previously found (or hypothesized) to be associated with QOL in breast cancer patients.
QOL was measured using the eight subscales of the RAND 36-Item Health Survey 1.0 [21] – physical functioning, role limitations due to physical problems, role limitations due to emotional health, energy/fatigue, emotional well-being, social functioning, pain, and general health. Standardized scores range from 0–100 with higher scores reflecting better QOL. The reliability and validity of the subscales have been established in studies of both general and patient populations [22–25]. A subscale score change of 3–5 points is considered evidence for a minimally clinically important difference [26, 27].
The 19-item Medical Outcomes Study (MOS) Social Support Survey [28] was used to measure how often social support is available, if needed. Response choices range from “none of the time” (1) to “all the time” (5). Higher mean scores indicate greater availability of social support. We used Katz’ [29] validated adaptation of the Charlson Comorbidity Index [30] to measure history and presence of comorbid conditions. A weighted index taking into account both the number and severity of comorbid diseases was computed; higher scores indicate greater comorbidity. History of depression at study enrollment was determined using two questions: “Has a doctor ever told you that you had depression?” and “Have you ever been treated for depression with medication or psychotherapy?” An affirmative response to either or both questions was coded as having a history of depression. Participants rated the severity of menopausal symptoms (hot flashes, cold sweats, night sweats, and vaginal dryness) “in the last month” using a 5-point scale from “not at all” (1) to “all the time” (5). Higher mean scores on this 4-item scale [31] indicate more severe menopausal symptoms.
In addition, we obtained information about age at diagnosis, race, marital status, employment status, household income, education, height and weight to compute body mass index (BMI), and use of postmenopausal estrogen/hormone replacement therapy (HRT). Patients’ clinical data obtained from the medical record included cancer stage (DCIS, EIBC [stage I or IIA]) [32], surgery type (BCS, mastectomy, bilateral mastectomy), and receipt of adjuvant therapy (radiation therapy, chemotherapy, endocrine therapy) during the study.
Data analysis
We used chi-square tests and analysis of variance (ANOVA) to compare characteristics of participants and non-participants and to compare participants who did and did not complete all four interviews. We used multivariable logistic regression to identify independent predictors of study completion and report adjusted odds ratios (aOR) and 95% confidence intervals (CI) for that analysis.
To test the first hypothesis, we measured the association between diagnostic group (DCIS and EIBC versus controls) and each of the eight QOL domains at T1 in separate general linear regression models for each QOL domain, controlling for selected variables significantly associated with at least one of the QOL domains at T1. To test the second hypothesis, the change in each QOL domain over time across three diagnostic groups was examined using generalized estimating equations (GEE), which account for the correlation among repeated measures within subjects and allow for inclusion of all available data. On the basis of the correlation matrices for eight QOL domains within subjects, an exchangeable correlation structure was specified for the within-subject correlation. In the eight separate GEE models, each QOL domain was the dependent variable and diagnostic group, time since definitive surgery (continuous), and the interaction between diagnostic group and time since definitive surgery were the independent variables of primary interest. The interaction between diagnostic group and time since definitive surgery was tested to determine whether the rates of change over time in each QOL domain (i.e., change in each QOL subscale per six months after definitive surgery) differed by diagnostic group. The procedure GENMOD in SAS (version 9.1, SAS Institute, Cary, NC) was used to fit the GEE models, which were adjusted for the selected covariates. We used the CONTRAST statement in PROC GENMOD to test if the change in each QOL domain over two years differed between DCIS and EIBC patients and between controls and each of DCIS and EIBC patients. Two-sided P values < 0.05 were considered statistically significant.
Results
We enrolled 549 patients (71.1% of 772 invited) and 547 controls (57.8% of 946 invited). A greater proportion of the 1096 participants than of 622 non-participants were white (76.2% vs. 59.0%; P < .001) and married (61.1% vs. 49.9%; P < .001); the two groups did not differ significantly by age or, among patients, by cancer stage or surgery type.
Descriptive statistics are included in Table 1. Telephone interviews were completed a mean 6.7 weeks (T1), 6.2 months (T2), 12.3 months (T3), and 24.4 months (T4) following definitive surgery (patients) or screening mammogram (controls). Retention was high with 1,011 participants completing T4 (92.2% overall; 514 [93.6%] patients, 497 [90.9%] controls). Participants who completed the study reported lower levels of comorbidity at T1 than participants who did not (mean [SD]: 0.5 [0.9] vs. 0.8 [1.3]; P = 0.004). Only race (aOR: 0.577, 95% CI: 0.353–0.943; P = 0.028) and marital status (aOR: 0.566, 95% CI: 0.350–0.915; P = 0.020) independently predicted study completion; a greater proportion of white than non-white (786/837 [93.9%] vs. 225/259 [86.9%]) and of married than unmarried (632/668 [94.6%] vs. 379/428 [88.6%]) participants completed the study. Moreover, non-white participants were less likely to be married (92/259 [35.5%] vs. 576/837 [68.8%]), and they reported higher levels of comorbidity at T1 (0.8 [1.2] vs. 0.4 [0.8]) than white participants (each P < 0.001).
Table 1.
Sample characteristics by diagnostic group at enrollment
| DCIS n = 184 |
EIBC n = 365 |
Controls n = 547 |
P value | |
|---|---|---|---|---|
| Age, mean (SD) | 57.2 (10.3) | 58.9 (10.7) | 57.2 (10.6) | .041 |
| BMI, mean (SD)a | 28.4 (6.6) | 28.5 (6.9) | 28.4 (7.0) | .982 |
| Menopausal Symptoms, mean (SD) | 1.6 (0.8) | 1.7 (0.8) | 1.6 (0.7) | .669 |
| Comorbidity, mean (SD) | 0.5 (0.8) | 0.6 (1.0) | 0.5 (0.9) | .328 |
| Social Support, mean (SD) | 4.5 (0.7) | 4.5 (0.7) | 4.3 (0.8) | < .001 |
| n (%) | n (%) | n (%) | ||
| Race | 0.019 | |||
| White | 146 (79.3%) | 293 (80.3%) | 398 (72.8%) | |
| Non-white | 38 (20.7%) | 72 (19.7%) | 149 (27.2%) | |
| Marital status | 0.306 | |||
| Married | 116 (63.0%) | 215 (58.9%) | 337 (61.6%) | |
| Widowed | 17 (9.2%) | 58 (15.9%) | 57 (10.4%) | |
| Divorced/separated | 36 (19.6%) | 53 (14.5%) | 90 (16.5%) | |
| Never been married | 15 (8.2%) | 39 (10.7%) | 63 (11.5%) | |
| Employment status | 0.070 | |||
| Working at least part time | 104 (56.5%) | 176 (48.2%) | 313 (57.2%) | |
| Retired | 48 (26.1%) | 105 (28.8%) | 139 (25.4%) | |
| Homemaker | 16 (8.7%) | 27 (7.4%) | 36 (6.6%) | |
| Unable to work/unemployed | 16 (8.7%) | 57 (15.6%) | 59 (10.8%) | |
| Annual income | 0.145 | |||
| < $25,000 | 46 (25.0%) | 99 (27.1%) | 117 (21.4%) | |
| $25,000–$75,000 | 68 (37.0%) | 142 (38.9) | 207 (37.8%) | |
| > $75,000 | 58 (31.5%) | 93 (25.5%) | 186 (34.0%) | |
| Refused/don’t know | 12 (6.5%) | 31 (8.5%) | 37 (6.8%) | |
| DCIS n = 184 (%) |
EIBC n = 365 (%) |
Controls n = 547 (%) |
P value | |
| Education | 0.383 | |||
| < High school graduate | 12 (6.5%) | 31 (8.5%) | 30 (5.5%) | |
| At least high school graduate | 172 (93.5%) | 334 (91.5%) | 516 (94.3%) | |
| Refused | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | |
| Postmenopausal hormone therapy | < 0.001 | |||
| Previous use | 86 (46.7%) | 160 (44.0%) | 183 (33.5%) | |
| Current use | 2 (1.1%) | 3 (0.80%) | 98 (17.9%) | |
| Never used | 96 (52.2%) | 201 (55.2%) | 266 (48.6%) | |
| Refused | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | |
| History of depression | 0.084 | |||
| Yes | 75 (40.8%) | 123 (33.7%) | 222 (40.6%) | |
| No | 109 (59.2%) | 242 (66.3%) | 325 (59.4%) | |
| Patients only | ||||
| Surgery type | 0.115 | |||
| Breast-conserving surgery | 111 (60.3%) | 245 (67.1%) | --- | |
| Mastectomy | 73 (39.7%) | 120 (32.9%) | --- | |
| Lymph Node Removal | < 0.001 | |||
| Yes | 80 (43.5%) | 358 (98.1%) | --- | |
| No | 104 (56.5%) | 7 (1.9%) | --- | |
| Radiation therapy during study | 0.012 | |||
| Yesb | 104 (56.5%) | 246 (67.4%) | --- | |
| No | 80 (43.5%) | 119 (32.6%) | --- | |
| Chemotherapy during study | < 0.001 | |||
| Yesc | 0 (0.0%) | 136 (37.3%) | --- | |
| No | 184 (100.0%) | 229 (62.7%) | --- | |
| Endocrine therapy during study | < 0.001 | |||
| Yesd | 79 (42.9%) | 265 (72.6%) | --- | |
| No | 101 (54.9%) | 99 (27.1%) | --- | |
| Unknown | 4 (2.2%) | 1 (0.3%) | ||
SD standard deviation, DCIS ductal carcinoma in situ, EIBC early invasive breast cancer (stages I and IIA)
Tests of significance were one-way analysis of variance for continuous variables and chi-square tests for categorical variables
BMI was not calculated for 5 women lacking height and/or weight data (1 DCIS, 2 EIBC, and 2 Control)
130 patients (55 DCIS, 75 EIBC) self-reported receipt of radiation therapy at T1
59 EIBC patients self-reported taking chemotherapy at T1
100 patients (29 DCIS, 71 EIBC) self-reported taking endocrine therapy at T1
DCIS patients were less likely to have had lymph nodes removed and to have received chemotherapy, radiation and endocrine therapy than EIBC patients (Table 1).
Unadjusted analyses
Table 2 shows the unadjusted mean QOL subscale scores at each interview by diagnostic group (DCIS, EIBC, controls) and the results of post hoc pair-wise comparisons for each subscale at each interview. All eight QOL subscales differed significantly by diagnostic group at T1 (each ANOVA test of main effects P ≤ 0.001).
Table 2.
Unadjusted mean (SD) RAND 36-item Health Survey subscale scores at each interview, by diagnostic group
| DCIS | EIBC | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |
| PF | 78.48a (23.17) | 80.06 (23.95) | 81.53 (23.09) | 80.50 (24.02) | 76.46b (22.56) | 75.66b (25.15) | 77.68b (23.94) | 76.33b (25.34) | 84.07a,b (21.13) | 84.10b (21.66) | 83.48b (21.40) | 83.81b (20.15) |
| RP | 42.26a,c (42.92) | 71.25c (40.29) | 75.00 (38.60) | 74.71 (38.67) | 32.74b,c (39.92) | 55.74b,c (44.60) | 66.86b (42.55) | 70.25b (41.80) | 78.56a,b (35.82) | 77.29b (35.53) | 75.48b (37.39) | 78.17b (36.01) |
| RE | 74.28a (39.61) | 82.78 (32.00) | 82.77 (34.03) | 86.02 (31.69) | 71.42b (39.17) | 80.35b (35.56) | 84.76 (31.76) | 83.48 (34.05) | 87.20a,b (28.90) | 77.29b (35.53) | 86.14 (29.87) | 85.21 (30.94) |
| E/F | 55.51a (23.45) | 61.75c (23.23) | 64.76c (21.62) | 63.39 (22.04) | 51.99b (22.69) | 55.51b,c (25.72) | 58.34b,c (23.97) | 60.57 (24.00) | 63.21a,b (19.97) | 61.72b (20.25) | 62.69b (19.86) | 62.32 (20.22) |
| EWB | 77.61 (17.80) | 80.13 (15.91) | 82.36 (15.75) | 81.56 (15.30) | 76.50b (18.34) | 79.97 (17.40) | 80.99 (17.33) | 82.23b (16.78) | 80.76b (14.98) | 80.41 (15.79) | 81.01 (14.29) | 79.03b (16.91) |
| SF | 78.53a,c (25.86) | 85.97c (23.02) | 87.57 (21.93) | 87.57 (21.06) | 72.36b,c (28.29) | 79.52b,c (26.44) | 83.71 (23.88) | 85.92 (22.80) | 88.16a,b (21.15) | 87.42b (22.42) | 86.82 (21.36) | 86.77 (21.53) |
| Pain | 70.35a (25.41) | 77.08 (22.81) | 77.16 (23.43) | 73.58 (25.11) | 66.66b (25.03) | 71.90b (25.58) | 72.81 (24.38) | 73.25 (24.18) | 76.07a,b (22.15) | 77.14b (24.11) | 76.63 (22.73) | 76.32 (23.91) |
| GH | 70.68 (21.51) | 70.93 (18.76) | 71.52 (19.45) | 69.72 (22.24) | 67.98b (21.48) | 67.17b (22.46) | 68.43b (22.86) | 67.99b (22.55) | 73.20b (20.33) | 73.81b (20.80) | 73.57b (20.58) | 73.42b (21.08) |
SD standard deviation, DCIS ductal carcinoma in situ, EIBC early invasive breast cancer (stages I and IIA), T1 first interview 4–6 weeks after definitive surgical treatment, T2 second interview 6-month follow-up, T3 third interview 1-year follow-up, T4 fourth interview 2-year follow-up, PF physical functioning, RP role limitations due to physical problems, RE role limitations due to emotional problems, E/F energy/fatigue, EWB emotional well being, SF social functioning, GH general health
Scores range from 0 to 100
Analysis of variance post-hoc comparisons between DCIS patients and controls significant at P < 0.05 at the same interview
Analysis of variance post -hoc comparisons between EIBC patients and controls significant at P < 0.05 at the same interview
Analysis of variance post -hoc comparisons between DCIS and EIBC patients significant at P < 0.05 at the same interview
We examined collinearity among potential covariates (Tables 3 and 4). A higher proportion of high school graduates than non-high school graduates were employed at least part time (56.4% vs. 23.3%) and had household income ≥$25,000/year (69.3% vs. 20.5%) (chi-square tests, P < 0.001). Since 7.3% of participants did not report household income and employment status could change over time, we included education as a covariate in addition to race, age, marital status, use of HRT, a history of depression, BMI, social support, comorbidity, and severity of menopausal symptoms.
Table 3.
Unadjusted mean (SD) RAND 36-item Health Survey subscale scores in association with covariates of interest in 1096 study participants at the first interview
| PF |
P value |
RP |
P value |
RE |
P value |
E/F |
P value |
|
|---|---|---|---|---|---|---|---|---|
| Race | ||||||||
| White | 82.86 (20.23) | <.001 | 58.87 (43.73) | .025 | 81.00 (34.02) | .038 | 57.89 (21.78) | .511 |
| Non-white | 73.29 (26.45) | 51.81 (44.83) | 75.80 (38.88) | 58.92 (23.14) | ||||
| Marital status | ||||||||
| Married | 84.54 (18.92) | <.001 | 59.77 (43.60) | .019 | 83.13 (32.15) | <.001 | 59.30 (21.25) | <.001 |
| Widowed | 73.56 (26.65) | 58.27 (41.91) | 83.07 (33.40) | 62.83 (20.52) | ||||
| Divorced/separated | 75.06 (25.42) | 48.37 (45.46) | 70.20 (41.25) | 52.31 (24.95) | ||||
| Never been married | 74.42 (25.27) | 55.34 (45.47) | 72.08 (40.34) | 55.51 (22.02) | ||||
| Employment status | ||||||||
| Working at least part time | 86.07 (17.50) | <.001 | 63.41 (43.22) | <.001 | 80.72 (34.17) | <.001 | 59.28 (21.51) | <.001 |
| Retired | 77.23 (23.21) | 57.02 (42.55) | 86.87 (29.19) | 61.28 (20.97) | ||||
| Homemaker | 82.57 (21.03) | 59.81 (43.38) | 82.28 (34.93) | 60.32 (20.64) | ||||
| Unable to work/unemployed | 62.31 (28.00) | 28.22 (40.32) | 58.33 (43.85) | 44.67 (23.47) | ||||
| Educationa | ||||||||
| < High school graduate | 67.29 (23.21) | <.001 | 39.38 (42.06) | <.001 | 63.24 (41.66) | <.001 | 49.73 (23.09) | .001 |
| At least high school graduate | 81.59 (21.82) | 58.53 (43.94) | 80.94 (34.51) | 58.76 (21.91) | ||||
| Postmenopausal hormone therapy | ||||||||
| Previous use | 80.28 (21.00) | .026 | 56.18 (41.13) | <.001 | 81.47 (33.20) | .018 | 57.54 (22.03) | .027 |
| Current use | 86.22 (17.53) | 75.00 (38.82) | 86.73 (28.90) | 63.72 (19.01) | ||||
| Never used | 79.91 (23.64) | 54.80 (45.03) | 77.18 (37.62) | 57.60 (22.57) | ||||
| History of depression | ||||||||
| Yes | 76.53 (23.89) | <.001 | 49.52 (44.27) | <.001 | 68.17 (41.25) | <.001 | 50.32 (22.47) | <.001 |
| No | 83.12 (20.74) | 61.98 (43.30) | 86.98 (28.76) | 62.98 (20.44) | ||||
| PF |
P value |
RP |
P value |
RE |
P value |
E/F |
P value |
|
| Patients only | ||||||||
| Surgery type | ||||||||
| Breast-conserving surgery | 79.21 (23.23) | .004 | 44.59 (42.43) | <.001 | 71.91 (39.05) | .708 | 55.00 (22.75) | .007 |
| Mastectomy | 73.31 (21.40) | 19.95 (33.32) | 73.23 (39.86) | 49.51 (23.01) | ||||
| Lymph node removal | ||||||||
| Yes | 69.66 (25.36 | .002 | 28.08 (37.95) | .080 | 65.30 (42..10) | .099 | 54.26 (23.16) | .002 |
| No | 78.28 (22.14) | 37.13 (41.53) | 73.46 (38.79) | 45.27 (20.21) | ||||
| Radiation therapy | ||||||||
| Yes | 80.11 (21.77) | <.001 | 42.86 (42.09) | <.001 | 70.76 (39.57) | .203 | 54.27 (22.24) | .103 |
| No | 71.91 (23.57) | 23.74 (36.48) | 75.21 (38.78) | 50.95 (24.12) | ||||
| Chemotherapy | ||||||||
| Yes | 75.08 (23.16) | .368 | 23.16 (35.18) | <.001 | 64.46 (42.20) | .009 | 49.74 (22.64) | .146 |
| No | 77.28 (22.20) | 38.43 (41.52) | 75.55 (36.72) | 53.32 (22.66) | ||||
| Endocrine therapy | ||||||||
| Yes | 78.50 (21.34) | .062 | 38.44 (41.42) | .042 | 72.19 (38.92) | .892 | 53.15 (22.91) | .913 |
| No | 74.74 (24.77) | 31.00 (40.20) | 72.67 (40.07) | 52.93 (23.26) | ||||
| EWB |
P value |
SF |
P value |
Pain |
P value |
GH |
P value |
|
|---|---|---|---|---|---|---|---|---|
| Race | ||||||||
| White | 79.13 (16.20) | .261 | 82.50 (24.39) | .005 | 72.84 (23.36) | .034 | 73.04 (20.31) | <.001 |
| Non-white | 77.79 (18.42) | 77.36 (28.65) | 69.21 (26.06) | 64.55 (22.01) | ||||
| Marital status | ||||||||
| Married | 80.19 (14.92) | <.001 | 83.72 (23.63) | <.001 | 73.52 (22.86) | .003 | 73.84 (19.70) | <.001 |
| Widowed | 84.15 (23.49) | 84.15 (23.49) | 74.17 (22.67) | 71.16 (19.49) | ||||
| Divorced/separated | 73.10 (30.33) | 73.10 (30.33) | 67.16 (27.49) | 64.81 (23.05) | ||||
| Never been married | 77.14 (27.03) | 77.14 (27.03) | 68.40 (25.35) | 64.69 (23.30) | ||||
| Employment status | ||||||||
| Working at least part time | 79.29 (15.12) | <.001 | 83.83 (22.77) | <.001 | 74.70 (21.92) | <.001 | 74.06 (19.35) | <.001 |
| Retired | 83.29 (14.87) | 84.20 (24.53) | 73.49 (23.47) | 71.95 (18.94) | ||||
| Homemaker | 80.96 (14.64) | 87.18 (19.51) | 75.63 (23.32) | 74.27 (19.84) | ||||
| Unable to work/unemployed | 65.53 (21.64) | 59.85 (31.84) | 54.24 (27.50) | 53.50 (24.76) | ||||
| Educationa | ||||||||
| < High school graduate | 72.55 (19.55) | .001 | 72.94 (27.48) | .004 | 67.23 (25.39) | .076 | 60.21 (19.82) | <.001 |
| At least high school graduate | 79.25 (16.46) | 81.92 (25.28) | 72.38 (23.87) | 71.81 (20.92) | ||||
| Postmenopausal hormone therapy | ||||||||
| Previous use | 80.22 (14.99) | .003 | 82.58 (23.97) | <.001 | 72.24 (23.18) | .296 | 70.18 (20.24) | .398 |
| Current use | 81.72 (13.87) | 89.93 (16.61) | 75.10 (20.97) | 73.16 (21.58) | ||||
| Never used | 77.19 (18.29) | 78.69 (27.59) | 71.16 (25.20) | 71.31 (21.53) | ||||
| History of depression | ||||||||
| Yes | 71.90 (19.56) | <.001 | 74.20 (27.77) | <.001 | 66.01 (24.72) | <.001 | 64.88 (22.78) | <.001 |
| No | 83.11 (13.01) | 85.69 (23.00) | 75.69 (22.89) | 74.86 (18.89) | ||||
| EWB |
P value |
SF |
P value |
Pain |
P value |
GH |
P value |
|
| Patients only | ||||||||
| Surgery type | ||||||||
| Breast-conserving surgery | 76.99 (17.96) | .841 | 78.83 (25.17) | <.001 | 71.90 (23.71) | <.001 | 68.54 (21.36) | .616 |
| Mastectomy | 76.66 (18.54) | 66.32 (30.10) | 60.53 (26.23) | 69.51 (21.82) | ||||
| Lymph node removal | ||||||||
| Yes | 71.18 (20.48) | .004 | 66.10 (27.91) | .006 | 58.25 (23.31) | <.001 | 61.51 (23.95) | .002 |
| No | 77.75 (17.63) | 75.71 (27.39) | 69.38 (25.17) | 70.01 (20.91) | ||||
| Radiation therapy | ||||||||
| Yes | 76.26 (17.81) | .296 | 77.98 (25.12) | <.001 | 70.55 (23.90) | .001 | 68.58 (21.09) | .661 |
| No | 77.95 (18.74) | 86.34 (30.12) | 63.24 (26.76) | 69.42 (22.26) | ||||
| Chemotherapy | ||||||||
| Yes | 73.18 (19.75) | .007 | 64.34 (29.44) | <.001 | 62.28 (25.02) | .010 | 66.58 (23.16) | .340 |
| No | 78.48 (17.18) | 77.13 (26.52) | 69.27 (24.73) | 68.80 (20.42) | ||||
| Endocrine therapy | ||||||||
| Yes | 77.26 (17.78) | .531 | 75.76 (27.25) | .160 | 69.18 (24.93) | .110 | 69.76 (21.03) | .191 |
| No | 76.24 (18.20) | 72.31 (28.21) | 65.60 (25.58) | 67.25 (22.42) | ||||
PF physical functioning, RP role limitations due to physical problems, RE role limitations due to emotional problems, E/F energy/fatigue, EWB emotional well being, SF social functioning, GH general health
Scores range from 0 to 100
One person did not report education level and was excluded; n = 1095.
Table 4.
Pearson product-moment correlations among RAND 36-item Health Survey subscale scores and covariates in 1096 study participants at the first interview
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PF | .487a | .282a | .222a | .435a | .545a | .460a | .550a | −.343a | −.105a | −.339a | −.140a | .164a |
| 2. RP | .298a | .332a | .613a | .393a | .531a | .547a | −.120a | −.093b | −.141a | .007 | .052 | |
| 3. EWB | .636a | .539a | .433a | .558a | .387a | −.075c | −.239a | −.071c | .227a | .319a | ||
| 4. RE | .465a | .310a | .409a | .287a | −.074c | −.203a | −.076c | .171a | −.200a | |||
| 5. SF | .394a | .579a | .532a | −.115a | −.169a | −.328a | .016 | .153a | ||||
| 6. GH | .508a | .473a | −.330a | −.194a | −.305a | −.320a | .228a | |||||
| 7. E/F | .517a | −.178a | −.189a | −.156a | .135a | .201a | ||||||
| 8. Pain | −.193a | −.175a | −.138a | .071c | .125a | |||||||
| 9. BMId | .011 | .214a | −.010 | −.095b | ||||||||
| 10. Menopausal symptoms | .090b | −.161a | −.084b | |||||||||
| 11. Comorbidity | .155a | −.088b | ||||||||||
| 12. Age | .030 | |||||||||||
| 13. Social support | 1.000 |
PF physical functioning, RP role limitations due to physical problems, EWB emotional well being, RE role limitations due to emotional problems, SF social functioning, GH general health, E/F energy/fatigue, BMI body-mass index
P < 0.001
P ≤ 0.005
P < 0.02
Five women lacked data to compute BMI, n = 1091.
Multivariable models
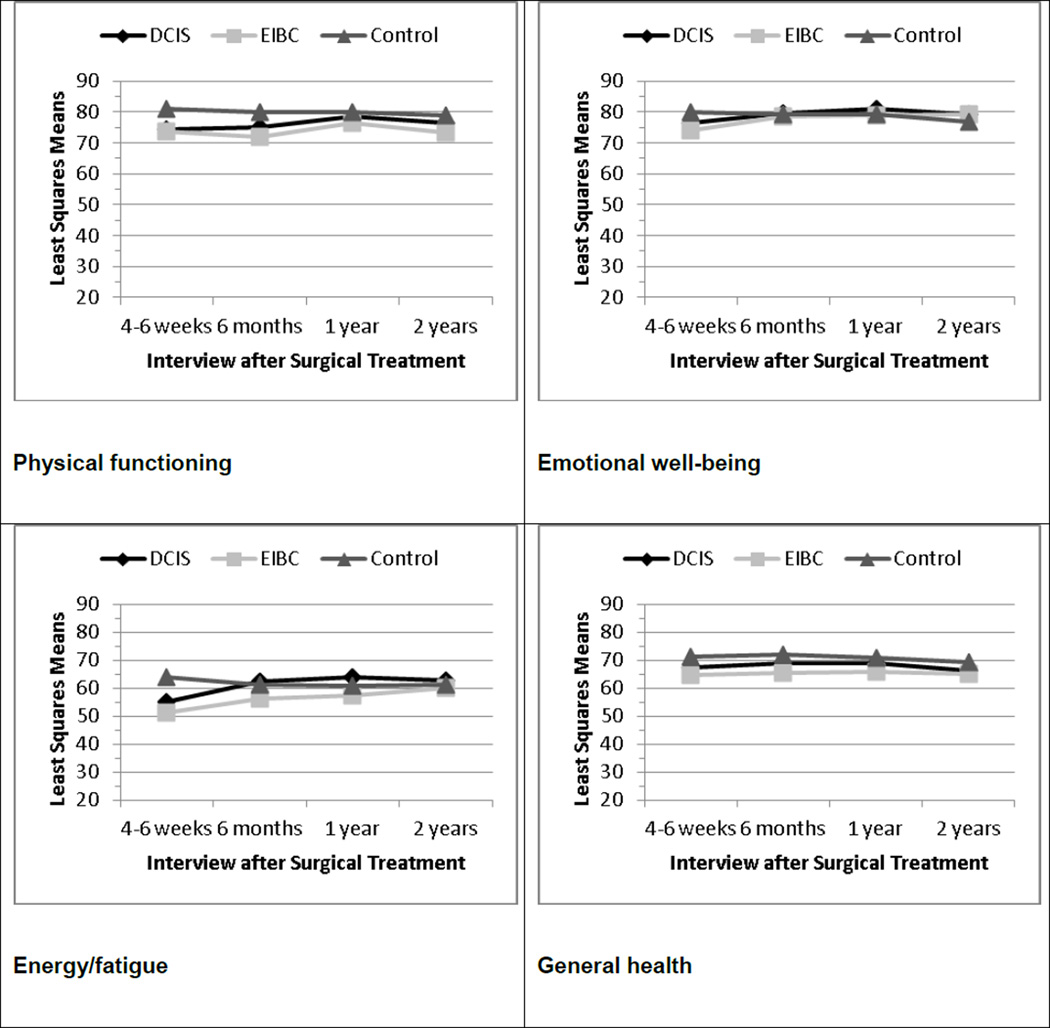
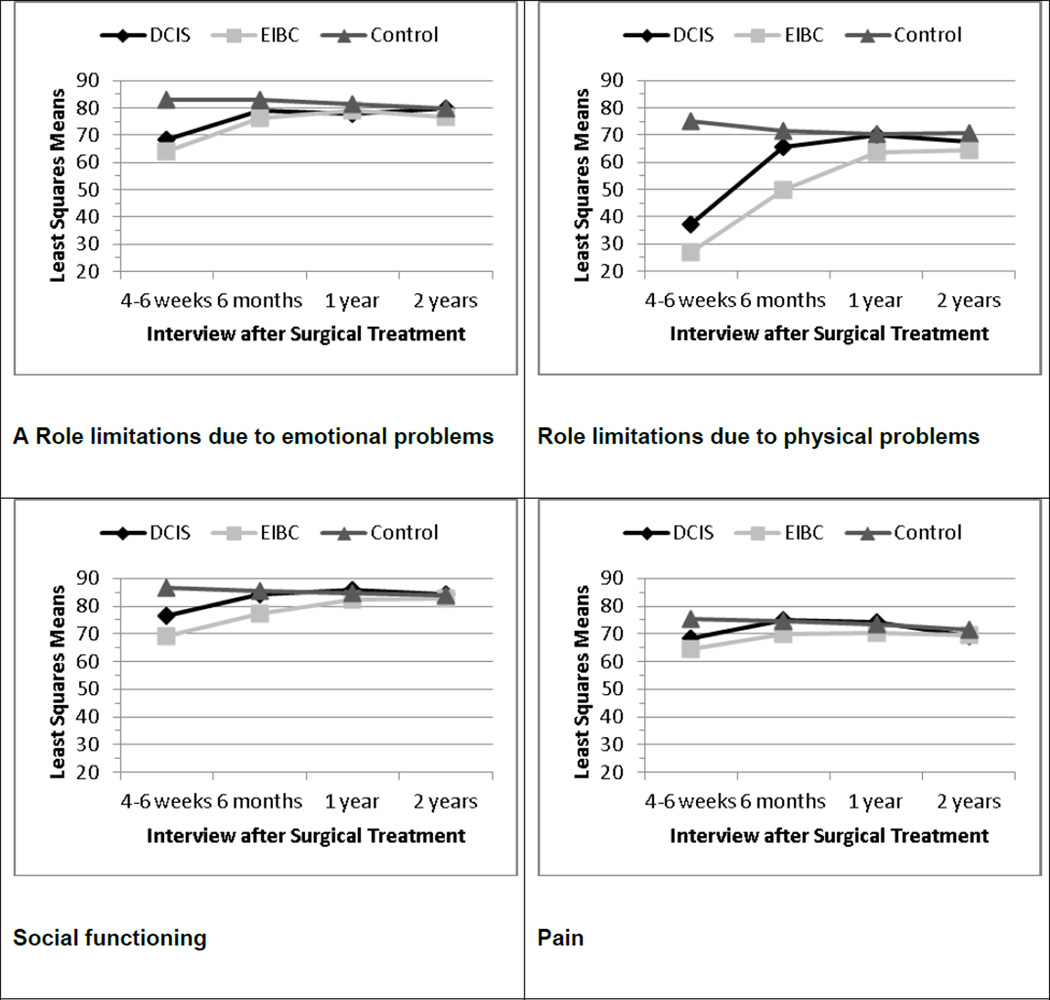
Figure 1 shows the adjusted means for each QOL subscale at each interview. For Hypothesis 1, we observed significant main effects by diagnostic group (DCIS, EIBC, controls) for all eight QOL subscales at T1, controlling for all covariates in the general linear regression models (each P < 0.0001). Controls reported better QOL at T1 on each subscale compared with DCIS patients (each P < 0.02) and with EIBC patients (each P < 0.0001). At T1, DCIS patients reported better QOL than EIBC patients on the role limitations due to physical problems (P = 0.0021), energy/fatigue (P = 0.0231), and social functioning (P = 0.0006) subscales.
Fig. 1.
Least-square means at each interview for RAND 36-item Health Survey subscales adjusting for all covariates in general linear models, by diagnostic group. Higher scores indicate better quality of life. DCIS ductal carcinoma in situ, EIBC early-invasive breast cancer
Both DCIS and EIBC patients showed improvements in QOL over the 2-year follow-up (Fig. 1). However by T2, there were still significant differences by diagnostic group overall in physical functioning (P < 0.0001), role limitations due to physical (P < 0.0001) and emotional (P = 0.0053) problems, energy/fatigue (P = 0.0005), social functioning (P < 0.0001), pain (P = 0.0087), and general health (P < 0.0001). Only emotional well being did not differ significantly by diagnostic group at T2. In support of Hypothesis 2, there were no statistically significant differences in post-hoc contrasts between DCIS patients and controls in any subscale but physical functioning at T2; however, all eight subscales at T2 differed significantly between EIBC patients and controls (each P < 0.005). In addition, DCIS (P = 0.0118) and EIBC (P < 0.0001) patients each reported worse physical functioning compared with controls at T2, and DCIS patients reported significantly better QOL on role limitations due to physical problems (P < 0.0001), energy/fatigue (P = 0.0008), social functioning (P = 0.0004), pain (P = 0.0206), and general health (P = 0.0392) than EIBC patients at T2.
By T3, there were still significant differences by diagnostic group overall in physical functioning (P = 0.0337), role limitations due to physical problems (P = 0.0379), energy/fatigue (P = 0.0010), and general health (P = 0.0011). In post-hoc contrasts, DCIS patients no longer differed significantly from controls on any of the eight QOL subscales, but EIBC patients still reported worse QOL than controls on physical functioning (P = 0.0095), role limitations due to physical problems (P = 0.0155), energy/fatigue (P = 0.0131), and general health (P = 0.0002). Moreover, EIBC patients reported worse energy/fatigue compared with DCIS patients at T3 (P = 0.0004).
By T4, the main effect of diagnostic group was significant only for physical functioning (P = 0.0005) and general health (P = 0.0059). In post-hoc contrasts, EIBC patients still reported worse physical functioning (P = 0.0001) and general health (P = 0.0017) than controls.
In GEE models (Table 5), we tested whether the rates of recovery differed between DCIS and EIBC by including an interaction term between diagnostic group and time since definitive surgical treatment. The rate of recovery differed significantly between DCIS and EIBC patients only for social functioning (P = .03).
Table 5.
Test for differences in change in each RAND 36-item Short Survey quality-of-life domain per 6 months after diagnosis over 2-year follow-up, by diagnostic group.
| Change per 6 Months after Diagnosis (95% CI) |
P value Test for Differences in Change between: |
|||||
|---|---|---|---|---|---|---|
| Controls | DCIS | EIBC | DCIS and EIBC | DCIS and Controls |
EIBC and Controls |
|
| PF | −0.29 (−0.64, 0.06) | 0.75 (−0.09, 1.60) | 0.33 (−0.32, 0.98) | 0.38 | 0.08 | 0.31 |
| RP | −0.33 (−1.14, 0.49) | 7.27 (5.25, 9.30) | 9.22 (7.62, 10.82) | 0.10 | < 0.0001 | < 0.0001 |
| EWB | −0.48 (−0.81, −0.14) | 1.49 (0.84, 2.14) | 1.85 (1.29, 2.40) | 0.32 | < 0.0001 | < 0.0001 |
| RE | −0.73 (−1.44, −0.01) | 3.48 (1.88, 5.08) | 3.67 (2.31, 5.03) | 0.84 | < 0.0001 | < 0.0001 |
| SF | −0.50 (−1.02, 0.02) | 2.52 (1.42, 3.62) | 3.91 (3.02, 4.79) | 0.03 | < 0.0001 | <0.0001 |
| GH | −0.11 (−0.44, 0.22) | −0.01 (−0.69, 0.68) | 0.19 (−0.39, 0.76) | 0.61 | 0.98 | 0.52 |
| E/F | −0.24 (−0.64, 0.17) | 2.22 (1.31, 3.14) | 2.46 (1.72, 3.19) | 0.65 | < 0.0001 | < 0.0001 |
| Pain | −0.12 (−0.61, 0.37) | 0.68 (−0.42, 1.77) | 1.71 (0.82, 2.59) | 0.10 | 0.23 | < 0.001 |
CI Confidence Interval, DCIS ductal carcinoma in situ, EIBC early invasive breast cancer, PF physical functioning, RP role limitations due to physical problems, EWB emotional well being, RE role limitations due to emotional problems, SF social functioning, GH general health, E/F energy/fatigue
Generalized Estimating Equation models were adjusted for age, race, education, marital status, BMI, social support, comorbidity, history of depression, menopausal symptoms, and hormone replacement therapy use
Discussion
Our study contributes to the paucity of knowledge about changes in QOL over the first two years after a diagnosis of DCIS or EIBC and about differences in QOL between these two groups and between each patient group and women without breast cancer. Few studies have directly compared QOL between women with DCIS and EIBC [5, 33], and these studies did not include a comparison group of women without breast cancer. Other studies measuring QOL in breast cancer patients at various times after diagnosis were cross-sectional [3–5, 14, 16, 34–36]. Our findings can help inform treatment decisions and the design of interventions that address early on the QOL needs of DCIS and EIBC survivors, who comprise a growing proportion of all breast cancer survivors in the U.S. due to concomitant increases in early detection by screening mammography and receipt of adjuvant treatment [37]. Overall, the 5-year relative survival for 2001–2007 was 99% for breast cancer survivors with localized disease [38].
Our first hypothesis was that DCIS patients would report similar levels of QOL as EIBC patients 4–6 weeks following definitive surgery, since these patients receive similar surgical treatments. Although DCIS and EIBC patients reported similar levels of QOL on several subscales in the multivariable analysis, DCIS patients reported significantly (and clinically meaningfully [26, 27]) higher scores on energy/fatigue, role limitations due to physical problems, and social functioning than EIBC patients at T1. Several factors might explain differences in these QOL subscales between DCIS and EIBC patients shortly after surgical treatment. Although QOL has been reported to differ by surgery type among breast cancer patients [39–41], these differences by surgery type may be due to lymph node removal to determine extent of invasive disease. Lymph node sampling to diagnose local spread of disease is standard surgical treatment for patients’ with invasive breast cancer [42] but is used less frequently in patients with DCIS [43, 44]. A greater percentage of EIBC patients in our cohort received some type of lymph node sampling procedure than DCIS patients (Table 1). Lymph node removal [45] and the development of lymphedema following axillary lymph node dissection in particular [46–48] have been reported to be associated with poorer QOL outcomes, which may explain QOL differences between EIBC and DCIS patients at T1 in role limitations due to physical problems.
Differences in adjuvant treatment, which are reported to be associated with poorer QOL [33, 49–52], also might contribute to differences between DCIS and EIBC patients’ energy/fatigue, role limitations due to physical problems, and social functioning at T1. Chemotherapy was only received by EIBC patients. In addition at T1, EIBC patients were more likely than DCIS patients to have received radiation therapy and endocrine therapy, which has been found to be associated with diminished QOL [53–55]. Lower social functioning would be expected while patients are receiving treatment. Thus, the lower proportion of DCIS patients than EIBC patients who received adjuvant treatment at T1 could explain poorer energy/fatigue, role functioning due to physical problems, and social functioning scores in EIBC than DCIS patients at T1.
Both DCIS and EIBC patients reported worse QOL than controls at T1. But, in support of Hypothesis 2, by T2, DCIS patients only reported significantly lower physical functioning than controls – a difference no longer apparent at T3. Although EIBC patients also showed improvements in QOL over time, DCIS patients reached QOL levels reported by controls sooner than EIBC patients did. Moreover, EIBC patients’ physical functioning and general health did not reach levels reported by controls during the 2-year study period, which also might be attributable to receipt of adjuvant treatments [33, 52–56].
The rate of change in QOL domains was similar in DCIS and EIBC patients, except for social functioning; EIBC patients, whose social functioning was poorer than that of DCIS patients at T1, showed a greater rate of increase in social functioning after T2 than DCIS patients did. As shown in Table 2 and Figure 1, minimally clinically important changes of at least 3–5 points [26, 27] occurred in both DCIS and EIBC patients on all subscales except physical functioning and general health perceptions in the first six months after surgery, similar to changes reported elsewhere [15]. Like other longitudinal studies [13, 15, 17, 33, 57], we found that DCIS and EIBC patients reported significant improvements in QOL domains over time. Although rates of change for seven subscales were similar for DCIS and EIBC patients, DCIS patients reached QOL levels reported by controls on all eight subscales before EIBC patients did.
Similar to a previous report [33], emotional well being did not differ significantly between DCIS and EIBC patients at T1 despite DCIS patients’ better prognosis and need for less aggressive treatment following definitive surgery. By T2, there was no significant main effect by diagnostic group in this subscale at all (Fig. 1). Similarly, investigators using the Nurses’ Health Study data reported the relative risk of decline in emotional well being (from a pre-diagnosis assessment) was not significantly greater among incident breast cancer cases diagnosed 6–11 months ago (in situ and invasive disease combined) and controls without any breast cancer [17]. Another study using these data observed clinically significant declines in social functioning and mental health in women diagnosed with DCIS < six months before the QOL assessment compared with controls [6], but incident invasive breast cancer cases were not included. It remains to be seen whether and to what extent our cohort of DCIS and EIBC patients and controls might differentially report QOL in these domains after longer-term follow-up.
Strengths of our study include our longitudinal design and high retention rates of a cohort of DCIS and EIBC patients and women without a history of breast cancer. We examined QOL over time in DCIS and EIBC patients separately, comparing each to one another and to a same-aged control group of women without breast cancer, which other studies [13, 15, 33, 57] did not. Although we lacked pre-diagnosis levels of QOL among the patients in our study, our findings of poorer QOL in patients compared with controls at T1 was not unexpected given the greater relative risk of decline in QOL observed among more recently diagnosed breast cancer patients (i.e., QOL assessment < six months after diagnosis) compared with women without a breast cancer diagnosis in the Nurses’ Health Study; this relative risk of decline was attenuated with longer time since diagnosis [17]. However, since we recruited our patients and controls from a National Cancer Institute-designated comprehensive cancer center and another academic medical center in the same city, the generalizability of our findings may be limited. Additionally, our findings may not be generalizable to breast cancer patients who are younger than 40 years of age [47] or who have more advanced disease, since these patients may receive more aggressive treatment regimens. Although the representation of non-white patients in our sample (26%) was comparable to their distribution in the Siteman Cancer Center breast cancer patient population, 95% of non-white participants was African American, limiting generalizability of our findings to other racial/ethnic groups [58–60]. Finally, as reported elsewhere [34], participation rates were somewhat higher for patients than controls and for white than non-white women; a greater proportion of non-participants and of participants not completing the study were non-white and unmarried, thereby introducing potential selection bias.
In closing, our results supported our hypotheses for most QOL domains. DCIS and EIBC patients each reported poorer QOL compared with controls at T1 and showed improvements on all QOL subscales over two-year follow-up. Thus, in the short-term, there are differential QOL outcomes following different breast cancer treatments, and differences between DCIS and EIBC patients might be explained by treatments received. After two years, the significant differences between patients and controls in physical functioning and general health perceptions could be explained by lingering treatment effects among EIBC patients. Further examination of longer-term QOL outcomes in early-stage breast cancer survivors is warranted, since there is a paucity of data published on the impact of breast cancer radiation therapy on either short- or long-term QOL outcomes among DCIS and EIBC survivors [61, 62], and the late effects on QOL of some breast cancer treatments, e.g., brachytherapy and newer endocrine therapies, are largely unknown [55, 63].
Acknowledgments
This study was supported by grants from the National Cancer Institute (NCI) and Breast Cancer Stamp Fund (R01 CA102777; PI: Jeffe, DB) and the NCI Cancer Center Support Grant (P30 CA091842; PI: Eberlein, T) to the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCI or Breast Cancer Stamp Fund.
We thank our participants, the interviewers, and the Siteman Cancer Center’s Health Behavior, Communication, and Outreach Core for data management services (Irene Fisher and Jim Struthers). We also thank the physicians who helped us recruit their patients for this study, including Drs. Barbara Monsees, Jill Dietz, Julie Margenthaler, Virginia Herrmann, Timothy Eberlein, Matthew Ellis, Imran Zoberi, Marie Taylor, Michael Naughton, Antonella Rastelli, Donald Lombardi, Cynthia Ma, Loren Michel, and Rama Suresh at Washington University School of Medicine and Dr. Eddie Hsueh and Pam Hunborg, RN, at Saint Louis University School of Medicine.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Schwartz GF, Solin LJ, Olivotto IA, Ernster VL, Pressman PI Consensus Conference Committee. The Consensus Conference on the treatment of in situ ductal carcinoma of the breast, April 22–25, 1999. Breast J. 2000;6(1):4–13. [Google Scholar]
- 2.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100(22):1643–1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amichetti M, Caffo O, Arcicasa M, Roncadin M, Lora O, Rigon A, Zini G, Armaroli L, Coghetto F, Zorat P, Neri S, Teodorani N. Quality of life in patients with ductal carcinoma in situ of the breast treated with conservative surgery and postoperative irradiation. Breast Cancer Res Treat. 1999;54(2):109–115. doi: 10.1023/a:1006125602353. [DOI] [PubMed] [Google Scholar]
- 4.Claus E, Petruzella S, Carter D, Kasl S. Quality of life for women diagnosed with breast carcinoma in situ. J Clin Oncol. 2006;24(30):4875–4881. doi: 10.1200/JCO.2005.05.2290. [DOI] [PubMed] [Google Scholar]
- 5.van Gestel YR, Voogd AC, Vingerhoets AJ, Mols F, Nieuwenhuijzen GA, van Driel OJ, van Berlo CL, van de Poll-Franse LV. A comparison of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer. 2007;43(3):549–556. doi: 10.1016/j.ejca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Nekhlyudov L, Kroenke CH, Jung I, Holmes MD, Colditz GA. Prospective changes in quality of life after ductal carcinoma-in-situ: results from the Nurses' Health Study. J Clin Oncol. 2006;94(18):2822–2827. doi: 10.1200/JCO.2005.04.6219. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 8.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M UK Coordinating Committee on Cancer Research, Ductal Carcinoma in situ Working Party, DCIS trialists in the UK, Australia, and New Zealand. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M, Strom E, Bassett L, Dershaw DD, Fowble B, Harris JR, O'Malley F, Schnitt SJ, Singletary SE, Winchester DP. Standard for the management of ductal carcinoma in situ of the breast (DCIS) CA Cancer J Clin. 2002;52(5):256–276. doi: 10.3322/canjclin.52.5.256. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein MJ, Barth A, Poller DN, Gierson ED, Colburn WJ, Waisman JR, Gamagami P. Ten year results comparing mastectomy to excision and radiotherapy for ductal carcinoma in situ of the breast. Eur J Cancer. 1995;31A(9):1425–1427. doi: 10.1016/0959-8049(95)00283-o. [DOI] [PubMed] [Google Scholar]
- 11.Bartelink H, Horiot J, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, Fourquet A, Borger J, Jager J, Hoogenraad W, Collette L, Pierart M European Organization for Research and Treatment of Cancer Radiotherapy and Breast Cancer Groups. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 12.Elder E, Kennedy C, Gluch L, Carmalt H, Janu N, Joseph M, Donellan M, Molland J, Gillett D. Patterns of breast cancer relapse. Eur J Surg Oncol. 2006;32:922–927. doi: 10.1016/j.ejso.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, Ligibel J, de Moor JS, Weeks J, Emmons K, Winer E. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100(4):243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 14.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16(2):501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 15.Härtl K, Engel J, Herschbach P, Reinecker H, Sommer H, Friese K. Personality traits and psychosocial stress: quality of life over 2 years following breast cancer diagnosis and psychological impact factors. Psychooncology. 2010;19(2):160–169. doi: 10.1002/pon.1536. [DOI] [PubMed] [Google Scholar]
- 16.Trentham-Dietz A, Sprague B, Klein R, Klein B, Cruickshanks K, Fryback D, Hampton J. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109(2):379–387. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael Y, Kawachi I, Berkman L, Holmes M, Colditz G. The persistent impact of breast carcinoma on functional health status. Cancer. 2000;89(11):2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Leitch AM, Dodd GD, Costanza M, Linver M, Pressman P, McGinnis L, Smith RA. American Cancer Society Guidelines for the early detection of breast cancer: update 1997. CA Cancer J Clin. 1997;47:150–153. doi: 10.3322/canjclin.47.3.150. [DOI] [PubMed] [Google Scholar]
- 19.Carrera C, Payne S. Ductal carcinoma in situ (DCIS) of the breast: the need for psychosocial research. Psychooncology. 1999;8:538–545. doi: 10.1002/(sici)1099-1611(199911/12)8:6<538::aid-pon426>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 21.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 22.Stewart AL, Greenfield S, Hays R, Wells K, Rogers W, Berry S, McGlynn E, Ware J. Functional status and well-being of patients with chronic conditions. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- 23.Stewart AL, Hays R, Ware J. The MOS short-form general health survey: reliability and validity in a patient population. Med Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36) Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 25.Wells KB, Stewart AL, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware JE. The functioning and well-being of depressed patients: results from the medical outcomes study. JAMA. 1989;262:914–919. [PubMed] [Google Scholar]
- 26.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 27.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 28.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 29.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Perez M, Liu Y, Schootman M, Aft R, Schechtman K, Gillanders W, Jeffe D. Changes in sexual problems over time in women with and without early-stage breast cancer. Menopause. 2010;17(5):924–937. doi: 10.1097/gme.0b013e3181d5dd26. PMCID: PMC2939280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. American Joint Committee on cancer staging manual. 6th edition. New York: Springer; 2002. [Google Scholar]
- 33.Lauzier S, Maunsell E, Levesque P, Mondor M, Robert J, Robidoux A, Provencher L. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Res Treat. 2010;120:685–691. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 34.Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, Pozet A, Guizard AV, Henry-Amar M, Velten M. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat. 2011;129:125–134. doi: 10.1007/s10549-011-1408-3. [DOI] [PubMed] [Google Scholar]
- 35.Paskett E, Alfano C, Davidson M, Andersen B, Naughton M, Sherman A, Green P, Hays J. Breast cancer survivors' health-related quality of life. Cancer. 2008;113(11):3222–3230. doi: 10.1002/cncr.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paskett ED, Herndon JE, Day JM, Stark NN, Winer EP, Grubbs SS, Pavy MD, Shapiro CL, List MA, Hensley ML, Naughton MA, Kornblith AB, Habin KE, Fleming GF, Bittoni MA ftCaLGB. Applying a conceptual model for examining health-related quality of life in long-term breast cancer survivors: CALGB study 79804. Psychooncology. 2008;17:1108–1120. doi: 10.1002/pon.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, EJ F. Cancer Intervention and Surveillance Modeling Network (CISNET) collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 38.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. Bethesda, MD: National Cancer Institute; 2011. [Accessed 1 February, 2012]. SEER cancer statistics review, 1975–2008. Updated November10, 2011. http://seer.cancer.gov/csr/1975_2008/. [Google Scholar]
- 39.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of life at the end of primary treatment of breast cancer: first results from the Moving Beyond Cancer Randomized Trial. J Natl Cancer Inst. 2004;96(5):376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 40.Taira N, Shimozuma K, Shiroiwa T, Ohsumi S, Kuroi K, Saji S, Saito M, Iha S, Watanabe T, Katsumata N. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat. 2011;128:735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 41.Arndt V, Stegmaier C, Ziegler H, Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: a population-based study. J Cancer Res Clin Oncol. 2008;134(12):1311–1318. doi: 10.1007/s00432-008-0418-y. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer. [Accessed 1 February, 2012];2011 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 43.Mokbel K. Current management of ductal carcinoma in situ of the breast. Int J Clin Oncol. 2003;8:18–22. doi: 10.1007/s101470300001. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz GF. The current treatment of ductal carcinoma in situ. Breast J. 2001;7:308–331. doi: 10.1046/j.1524-4741.2001.22057.x. [DOI] [PubMed] [Google Scholar]
- 45.Kwan ML, Ergas IJ, Somkin CP, Quesenberry CP, Neugut AI, Hershman DL, Mandelblatt J, Pelayo MP, Timperi AW, Miles SQ, Kushi LH. Quality of life among women recently diagnosed with invasive breast cancer: the Pathways Study. Breast Cancer Res Treat. 2010;123(2):507–524. doi: 10.1007/s10549-010-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed RL, Prizment A, Lazovich D, Schnitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22(10):1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 48.Nesvold IL, Reinertsen KV, Fossa SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv. 2011;5(1):62–72. doi: 10.1007/s11764-010-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mar Fan HG, Houédé-Tchen N, Yi Q, L, Chemerynsky I, Downie FP, Sabate K, Tannock IF. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23(31):8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 50.Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2:25. doi: 10.1186/1477-7525-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Alderman A, Hamilton AS, Graff J, Katz SJ. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganz PA, Land SR, Geyer CE, Cecchini RS, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff JA, Vogel VG, Erban JK, Linvingston RB, Perez EA, Mamounas EP, Womark N, Swain SM. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29(9):1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boehm DU, Lebrecht A, Eckhardt T, Albrich S, Schmidt M, Sigglekow W, Kandelhardt E, Koelbl H. Quality of life and adjuvant tamoxifen treatment in breast cancer patients. Eur J Cancer Care. 2009;18:500–506. doi: 10.1111/j.1365-2354.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 54.Day R. Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. Ann N Y Acad Sci. 2001;949:143–150. [PubMed] [Google Scholar]
- 55.Dixon JM, Renshaw L, Langridge C, Young OE, McHugh M, Williams L, Murray J, Macaskill EJ, McCaig F, Dixon OM, Fallowfield LJ. Anastrozole and letrozole: an investigation and comparison of quality of life and tolerability. Breast Cancer Res Treat. 2011;125(3):741–749. doi: 10.1007/s10549-010-1091-9. [DOI] [PubMed] [Google Scholar]
- 56.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 57.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16(2):487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 58.Ashing-Giwa KT, Lim JW. Examining emotional outcomes among a multiethnic cohort of breast cancer survivors. Oncol Nurs Forum. 2011;38(3):279–288. doi: 10.1188/11.ONF.279-288. [DOI] [PubMed] [Google Scholar]
- 59.Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, Derhagopian RP, Ironson G, Love N. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol. 1999;18(2):159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- 60.Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic Whites. Psychooncology. 2002;11(6):495–504. doi: 10.1002/pon.615. [DOI] [PubMed] [Google Scholar]
- 61.Goodwin A, Parker S, Ghersi D, Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 2009;(Issue 4) doi: 10.1002/14651858.CD000563.pub4. Art. No.: CD000563. [DOI] [PubMed] [Google Scholar]
- 62.James ML, Lehman M, Hider PN, Jeffery M, Hickey BE, Francis DP. Fraction size in radiation treatment for breast conservation in early breast cancer. Cochrane Database Syst Rev. 2010;(Issue 11) doi: 10.1002/14651858.CD003860.pub3. Art. No.: CD003860. [DOI] [PubMed] [Google Scholar]
- 63.Hattangadi JA, Taback N, Neville BA, Harris JR, Punglia RS. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104:29–41. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]