Thiolate ligation to iron plays a vital role in the enzymatic deactivation of reactive oxygen species. Superoxide reductase (SOR) found in microaerophilies catalyzes the reduction of superoxide (O2−•), yielding H2O2[1] and has an active site that consists of an iron center with four equatorial His ligands and an axial Cys ligand. The thiolate is proposed to stabilize the O–O bond of RS-FeIII(η1-OO(−/H)) intermediates and to weaken the Fe–(OO) bond to facilitate formation of H2O2.[1,2] Evidence for an intermediate in the reaction between O2−• and SOR has been obtained, which exhibits a visible absorption feature at ~600 nm.[3] Pulse radiolysis studies showed diffusion-controlled pH-independent formation of the intermediate, while its decay was found to be pH-dependent. These results led to the postulation of the intermediate as either an RS-FeII(OO•) adduct or the isoelectronic RS-FeIII(OO−); however its short lifetime has made it difficult to establish the iron oxidation state experimentally. We have thus endeavored to obtain a synthetic analog of this intermediate.
A number of synthetic non-heme RS-FeIII(η1-OOX) (X = H, alkyl) complexes have been reported recently,[4] providing insights into the role thiolate ligation plays in modulating the reactivity of peroxide complexes, and the nature of the RS-FeIII(η1-OOH) intermediate observed in SOR (Table 1). However, there are no reports detailing the isolation of an RS-FeIII(η1-OO−) complex. Herein, we describe the spectroscopic characterization of a synthetic complex that serves to model the putative RS-FeIII(η1-OO−) intermediate found in SOR.
Table 1.
Properties of SOR and related RS-FeIII(OOX) complexes.
| λmax (nm) (ε, M−1cm−1) | r (Fe–S) (Å) | r (Fe–OO) (Å) | |
|---|---|---|---|
| wt-SORox [RS-FeIII(Glu)] [3c,9a] | 644 (1900) | 2.42–2.46 | |
| wt-SORred + superoxide [3] | 600 (2800) | ||
| E114A-SORox + H2O2 [RS-FeIII(OOH)] [3b,9b] | 560 | 2.5 | 2.0 |
| chloroperoxidase Cpd 0 [10] | 2.4 | 1.9 | |
| 2 [FeIII(TMCS)(η1-OO−)] | 460 (6100), 610 (1200) | 2.41 | 1.89 |
| [FeIII(cyclam-PrS)-(OOH)]+ [4c] | 535 (1350) | ||
| [FeIII(SMe2N4tren)-(OOH)]+ [4a,4h] | 452 (2780) | 2.33 | 1.86 |
| [FeIII([15]aneN4)(SAr)-(OOR)]+ [4b,4d] | ~ 525 | 2.29–2.32 | 1.82–1.85 |
| [FeIII(Me4[15]aneN4)-(SPh)(OOR)]+ [4f,4g] | 340 (3500), 405 (2300), 650 (2300) |
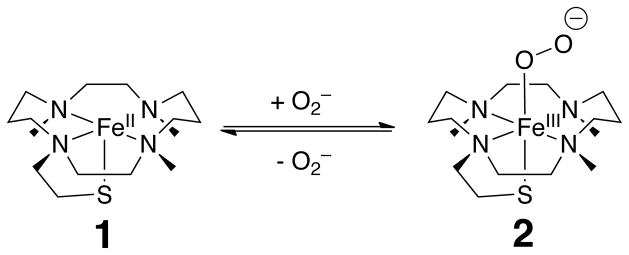
[FeII(TMCS)]PF6 (1) acts as an ideal mimic of the SOR active site because it contains four equatorial N ligands and an axial S bound to the iron center (Scheme 1).[5] 1 reacted with KO2 (dissolved in pure DMF in the presence of 2 equiv. of 18-crown-6) at −90 °C in a 4:1 THF/DMF solvent mixture. The characteristic UV feature of 1 (λmax = 320 nm, Figure 1) disappeared over the course of 10 s and was replaced by two new intense features (λmax = 460, 610 nm), assigned to transient species 2. The visible features achieved maximum intensity when an excess (>50-fold) of KO2 was used. Intermediate 2 was reasonably stable at −90 °C, decaying over the course of ~ 3 h to produce 1 nearly quantitatively (Figure S1). 2 could then be regenerated by replenishing the KO2. These observations suggest that superoxide binding to 1 is reversible, with the decay of 2 presumably reflecting the loss of KO2 due to its gradual disproportionation in the reaction medium and a shift of the equilibrium back to 1 (Scheme 1).[6] Similar chemistry has been reported for a crown-ether functionalized porphyrin ligand that facilitates the reversible formation of a heme-FeIII(OO) complex from the heme-FeII precursor and KO2.[7]
Scheme 1.
Equilibrium between 1 and 2.
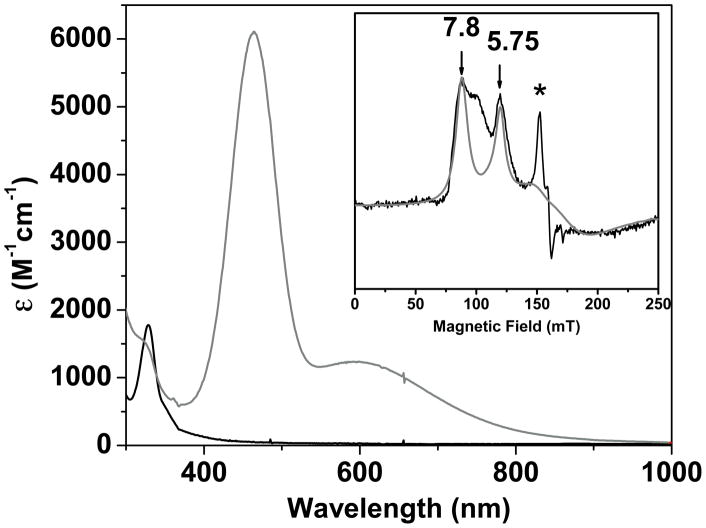
Figure 1.
UV-vis spectrum of 1 (black trace, 0.2 mM in 4:1 THF/DMF (v/v) at −90 °C) that reacted with KO2 to yield 2 (gray trace). Molar absorptivity values were calculated using a combination of UV-vis and Mössbauer experiments. Inset: X-band EPR spectrum of 2 in 4:1 THF/DMF. T = 20 K; microwave power, 2 mW, microwave frequency, 9.645 GHz, modulation amplitude, 10 G, modulation frequency, 100 Hz. Gray line is a theoretical spectrum for an S = 5/2 species with E/D = 0.08, and E/D distribution σ(E/D) = 0.03 (see text). The sharp feature marked with * arises from minor (≈0.5 % of Fe) contaminant. The high-field region is not shown as it is dominated by the intense absorption of free KO2.
The highly chromophoric nature of 2 suggests that the iron center has been oxidized to FeIII, concomitant with the reduction of the superoxide ligand to the peroxide level. However the electronic absorption spectrum of 2 is quite distinct from those reported for related non-heme RS-FeIII(OOX) (X = H or alkyl) complexes (Table 1). While all complexes exhibit broad absorption features in the 500–800 nm region typically associated with peroxo-to-FeIII charge transfer transitions, 2 has an additional feature at 460 nm (ε = 6100 M−1cm−1) that is sharper and more intense (Figure 1). For comparison, treatment of [FeII(TMC)(OTf)]+ with KO2 afforded a product with a broad band at 850 nm (Figure S2), nearly identical to that observed for [FeIII(TMC)(η 2-OO)]+ in CH3CN at −40 °C.[8] It would thus appear that the more basic axial ligand of 2 is required to elicit its novel absorption features. We therefore postulate that 2 represents a thus far unprecedented example of an RS-FeIII(η1-OO−) complex.
EPR spectra of 2 revealed broad features belonging to an S = 5/2 species (Figure 1, inset). The spectra indicate that the rhombicity parameter, E/D, is distributed around a mean value of E/D ≈ 0.08. The gray curve is an illustrative simulation for the major contributing species: the feature at g = 7.9 belongs to the MS = ±1/2 ground doublet and the resonance g = 5.75 results from the MS = ±3/2 excited state. The feature marked with the star is a minor rhombic contaminant (≈ 0.5% of total Fe) (see Figure S5 for more details).[8b] The S = 5/2 species could arise from a ferromagnetically coupled [SFe(II) = 2/Ssuperoxide = 1/2] center or from a high-spin FeIII-peroxide complex. The 57Fe magnetic hyperfine interactions as well as the isomer shift of 2, obtained from Mössbauer spectra (Figure 2) establish that the iron center is in the high-spin ferric state.
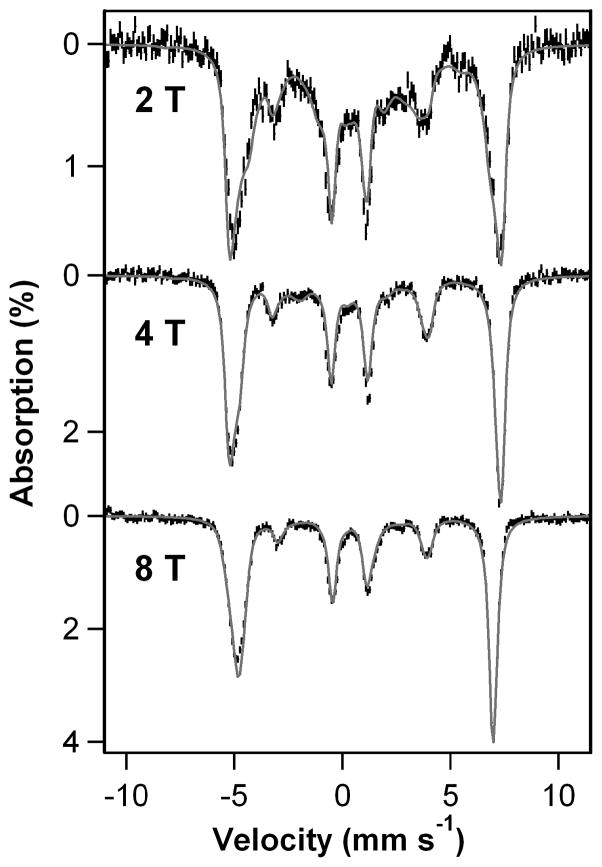
Figure 2.
4.2 K Mössbauer spectra of 2 recorded in parallel applied magnetic fields indicated in the figure. The gray curves are spectral simulations using the S = 5/2 spin-Hamiltonian of eq. 1 with D = 2.5 cm−1, E/D = 0.08, gx,y,z = 2.0, δ = 0.71(3) mm/s, ΔEQ = −1.9(1) mm/s, η = −0.20, Ax,/gnβn = Ay,/gnβn = −17.5(4) T, Az/gnβn = −21.0(5) T.
We have simulated the 4.2 K Mössbauer spectra of 2 using the S = 5/2 spin Hamiltonian (parameters listed in the caption of Figure 2)
| (1) |
where D and E are the axial and rhombic zero-field splitting parameters, A is the 57Fe magnetic hyperfine tensor and HQ describes the nuclear quadrupole interactions. ΔEQ = (eQVzz/12)(1 + η2/3)1/2 is the quadrupole splitting, while η = (Vxx − Vyy)/Vzz is the asymmetry parameter of the electric field gradient tensor. The shape of the Mössbauer spectrum recorded in an applied field, B = 50 mT (Figure S6), just like the EPR spectrum, indicates that E/D is distributed; we were not able describe the E/D distribution by a symmetric function such as a Gaussian, and therefore we have focused the Mössbauer analysis on the high field spectra (B ≥ 2 T), which are essentially independent of E/D. The 8.0 T spectrum reveals that 2 represents at least 95% of total Fe. Importantly, 2 has a negative quadrupole splitting, ΔEQ = −1.9 mm/s, the largest value yet observed for a high-spin [FeIII(TMC)] complex.[8b, 11] The 57Fe A tensor of 2 is characteristic of high-spin FeIII. Its isotropic component, Aiso/gnβn = −18.7 T (Aiso = (Ax +Ay +Az)/3), is comparable to the −20.0 T value reported for the high-spin FeIII(TMC)(η1-OOH) complex,[8b] and the smaller value observed for 2 can be attributed to the presence of the more covalent thiolate ligand. Interestingly, the D and (dominant) E/D values of 2 are quite similar to those observed for [FeIII(TMC)(η1-OOH)]2+ (D = +2.5 cm−1, E/D = 0.097).[8b] However the isomer shift of 2, δ = 0.71(3) mm/s, is distinctly larger than the δ = 0.51 mm/s observed for [FeIII(TMC)(η1-OOH)]2+.[8b]
The Mössbauer parameters of 2 reveal a high-spin ferric center with unique properties. It has the largest isomer shift for any FeIII-peroxide complex reported thus far,[8b,12] a large and negative ΔEQ (with the largest component of the field gradient along z), and an 57Fe A-tensor that is rather anisotropic for a high-spin FeIII center.[13] These three properties have a common origin, as may be inferred from the following considerations. For a high-spin FeIII center, the five d-orbitals are occupied by α electrons. In order to explain the large and negative ΔEQ, we postulate transfer of β electron density from the two filled peroxo π* orbitals to the empty βdxz(Fe) and βdyz(Fe) orbitals. If only one orbital were involved (dxz or dyz), a positive ΔEQ would result, with the largest component of the quadrupole tensor in the xy plane, in contrast to what is observed. However, if βdxz(Fe) and βdyz(Fe) were equally populated, a negative ΔEQ with the major component along z would result, as observed.[14] Since the spin-dipolar contribution to 57Fe A-tensor is proportional to the valence part of the quadrupole tensor, we expect the donation to increase the magnitude of Az and to decrease the magnitude of Ax,y. Indeed, we observe that |Ax,y| < |Az|. Finally, electron donation by the peroxo ligand into β dxz and β dyz orbitals enhances the d-electron density at the iron, thereby imbuing the iron center with some ferrous character and resulting in an increase in its isomer shift as observed.
X-ray absorption spectroscopy (XAS) provided insight into the structural and electronic properties of 2. The XAS edge energy of 7123.1 eV observed for 2 falls at the low energy end of the range reported for FeIII(OO) complexes (~7123 to 7125 eV), consistent with an FeIII center ligated by highly basic thiolate and peroxide donors (Figure S8). There is a small pre-edge peak arising from a 1s → 3d transition at 7113.6 eV with a peak area of 11.0 units (Table S1), suggesting the presence of a fairly distorted 6-coordinate FeIII center.[15]
Analysis of EXAFS data for 2 yielded a principal shell of four N/O scatterers at 2.17 Å, which are attributed to the equatorial nitrogen donors of the TMCS ligand. This distance resembles that for [FeIII(TMC)(η1-OOH)]2+ (2.15–2.16 Å) more closely than for its conjugate base [FeIII(TMC)(η2-OO)]+ (2.20–2.23 Å).[8b,8c] The inclusion of an S scatterer at 2.41 Å significantly improved the quality of the fit, indicating coordination of the thiolate moiety to the iron center. However the Fe–S distance found for 2 is rather long, relative to those observed for other synthetic RS-FeIII(OOX) model complexes (~2.30 Å), but comparable to values associated with biological RS-FeIII(OOX) intermediates (Table 1). For the peroxo ligand, we considered two possible binding modes, η2-side-on and η1-end-on. Attempts to model 2 using an η2-side-on peroxo moiety yielded unreasonably large σ2 values (>15, Table S2). In contrast, the inclusion of a single O/N scatterer at 1.89 Å significantly improved the quality of the fit (Figure S9 and Table S2). Taken together, the EXAFS fits favor a six-coordinate structure, with a pentadentate TMCS and an η1-end-on peroxo moiety trans to the thiolate ligand.[16]
As the RS-FeIII(η1-OO−) complex would be expected to be prone to protonation, the reactivity of 2 towards weak acids was probed. The addition of 100 equiv. 2,2,2-trifluoroethanol (TFE, pKa = 12.5) to a solution of 2 resulted in the rapid (10 s) disappearance of the features assigned to 2 and the formation of new features (λmax = 550, 720 nm) assigned to a new species 3 (Figure S3). Under the same conditions, addition of 100 equiv. of stronger acids such as ammonium acetate (pKa = 9.2) or pyridinium triflate (pKa = 5.2) also yielded intermediate 3 on the same time scale. In contrast, the reaction between 2 and 100 equiv. MeOH (pKa = 15.2) did not yield 3 but instead afforded 1, presumably due to accelerated decay of KO2 bydisproportionation (Figure S7).
The behavior of 1 with KO2 is notably different from the RS-FeII complexes studied by Kovacs, for which no reaction with KO2 was observed until the addition of MeOH, leading to the generation of RS-FeIII(η1-OOH) intermediates.[4a, 4c] This was true even for the complex of the N-(3-mercaptopropyl)cyclam ligand,[4c] which is closely related to TMCS. In contrast, superoxide reduction readily occurred in the reaction between 1 and KO2 in an aprotic solvent, to generate 2, which could be easily protonated to yield a postulated RS-FeIII(η1-OOH) intermediate (3) (Scheme S1).
Reactivity studies provided further insights into the properties of the peroxide ligand in 2. Complex 2 did not react at −90 °C with substrates that contain weak C-H bonds, such as dihydroanthracene, thus demonstrating that the bound peroxo moiety did not possess any electrophilic character. Its nucleophilic nature, however, was demonstrated in its reactivity towards electrophilic substrates. Menadione (2-methyl-1,4-naphthoquinone) is a substrate often used for this purpose, affording menadione epoxide (2,3-epoxy-2-methyl-1,4-naphthoquinone) in high yield (Scheme S2).[17] For example, Valentine showed that [FeIII(F20TPP)(η2-OO)]− (F20TPP = meso-tetrakis(pentafluorophenyl)porphinato) did not react with menadione in CH3CN at 25 °C but afforded a 70% yield of the epoxide product by change of solvent to dimethyl sulfoxide (DMSO).[17a, 17c] The latter reactivity was proposed to result from the binding of DMSO to the FeIII center, converting the η2-side-on peroxo moiety to its η1-end-on isomer. When 2 was treated with menadione at −90 °C in 4:1 THF/DMF, menadione epoxide was obtained in 100 (±20) % yield within 60 s, supporting the assignment of 2 as an η1-end-on peroxide complex.
Aldehyde substrates are also useful probes of metal-peroxide nucleophilicity.[18] At −90 °C 2 reacted with 20 equiv. of 2-phenylpropionaldehyde (PPA) within 5 s, yielding acetophenone and formate according to GC-MS. Accurate kinetic analysis, and thus determination of second order rate constants (k2), at such rapid rates was not possible; however 2 was clearly a very active reactant for nucleophilic oxidation reactions. UV-vis analysis of the reaction between 2 and PPA (Figure S4) showed isosbestic behaviour and a clean conversion to a new FeIII product that displayed features typical of an RS-FeIII complex (λmax = 480 nm).[19] In stark contrast, we found that [FeIII(TMC)(η2-OO)]+ was not reactive towards PPA at −90 °C in 4:1 THF/DMF, in agreement with Nam’s earlier report that side-on [FeIII(TMC)(η2-OO)]+ was not reactive towards PPA at −40 °C.[8c] On the other hand, the end-on hydroperoxide analogue [FeIII(TMC)(η1-OOH)]2+ reacted with PPA over the course of 800 s at −40 °C.[8c]. Based on the accumulated observations on the reactivity of all the above FeIII(L)(OO) complexes, it is clear that 2 contains a very reactive nucleophilic peroxide ligand, which reacts rapidly, even at −90 °C. These results further support our assignment of 2 as an RS-FeIII(η1-OO−) complex.
In summary, the reaction between an RS-FeII complex 1 and KO2 at −90 °C in aprotic solvent generates a reversible adduct 2 that is best described as an RS-FeIII(η1-OO−) complex on the basis of its spectroscopic properties. We postulate that 2 represents the first synthetic model of the initial SOR-superoxide adduct prior to its protonation in the SOR catalytic cycle. Two recent DFT studies favor an RS-FeII(OO•) description for this adduct using simplified active sites that did not include second sphere residues.[3d,20] However our results provide experimental evidence that the isomeric RS-FeIII(η1-OO−) complex can be generated and stabilized in an aprotic medium and in fact exhibits an absorption band at 610 nm like that found for the SOR intermediate.[3] For 2, the anionic peroxo ligand may be stabilized in part by the potassium ion introduced through the use of KO2. Related porphyrin-FeIII(OO−) complexes have been reported, but they do not have an axial thiolate ligand.[7,21] 2, therefore, represents a unique example of a thiolato-iron(III)-peroxy anion model compound that mimics the structure and function of the postulated RS-FeIII(η1-OO−) intermediates in SOR and the P450 family. We have also shown that the peroxide ligand on 2 is highly nucleophilic in nature and is readily protonated by weak acids, consistent with a possible role for this species in the SOR cycle. Furthermore 2 reacts rapidly with carbonyl compounds, even at −90 °C, making it relevant to the nucleophilic chemistry postulated for corresponding RS-FeIII(η1-OO−) intermediates in the catalytic cycles of hemethiolate enzymes such as aromatase and NO synthase.[18, 22]
Supplementary Material
Acknowledgments
This work was supported by the US National Science Foundation (grant CHE1058248 to L.Q.) and the US National Institutes of Health (grant EB001475 to E.M. and postdoctoral fellowships to A.R.McD. (GM087895) and K.M.V.H. (GM093479). XAS data were collected at beamline X3B of the National Synchrotron Light Source at the Brookhaven National Laboratory, which is supported by the U.S. NIH and DOE.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Dr. Aidan R. McDonald, Department of Chemistry, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455, USA
Dr. Katherine M. Van Heuvelen, Department of Chemistry, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455, USA
Dr. Yisong Guo, Department of Chemistry, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, PA 15213, USA
Dr. Feifei Li, Department of Chemistry, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455, USA
Prof. Dr. Emile L. Bominaar, Department of Chemistry, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, PA 15213, USA
Prof. Dr. Eckard Münck, Email: emunck@cmu.edu, Department of Chemistry, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, PA 15213, USA
Prof. Dr. Lawrence Que, Jr., Email: larryque@umn.edu, Department of Chemistry, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455, USA
References
- 1.a) Nivière V, Bonnot F, Bourgeois D. In: Handbook of Metalloproteins. Messerschmidt A, editor. 4 & 5. John Wiley & Sons, Ltd; Chichester, UK: 2011. pp. 246–258. [Google Scholar]; b) Pereira AS, Tavares P, Folgosa F, Almeida RM, Moura I, Moura JJG. Eur J Inorg Chem. 2007:2569–2581. [Google Scholar]; c) Kurtz DM., Jr Acc Chem Res. 2004;37:902–908. doi: 10.1021/ar0200091. [DOI] [PubMed] [Google Scholar]
- 2.a) Mathé C, Weill CO, Mattioli TA, Berthomieu C, Houée-Levin C, Tremey E, Nivière V. J Biol Chem. 2007;282:22207–22216. doi: 10.1074/jbc.M700279200. [DOI] [PubMed] [Google Scholar]; b) Kovacs JA, Brines LM. Acc Chem Res. 2007;40:501–509. doi: 10.1021/ar600059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Lombard M, Houée-Levin C, Touati D, Fontecave M, Nivière V. Biochemistry. 2001;40:5032–5040. doi: 10.1021/bi0023908. [DOI] [PubMed] [Google Scholar]; b) Mathé C, Mattioli TA, Horner O, Lombard M, Latour JM, Fontecave M, Nivière V. J Am Chem Soc. 2002;124:4966–4967. doi: 10.1021/ja025707v. [DOI] [PubMed] [Google Scholar]; c) Nivière V, Asso M, Weill CO, Lombard M, Guigliarelli B, Favaudon V, Houée-Levin C. Biochemistry. 2004;43:808–818. doi: 10.1021/bi035698i. [DOI] [PubMed] [Google Scholar]; d) Bonnot F, Molle T, Ménage S, Moreau Y, Duval S, Favaudon V, Houée-Levin C, Nivière V. J Am Chem Soc. 2012;134:5120–5130. doi: 10.1021/ja209297n. [DOI] [PubMed] [Google Scholar]; e) Coulter ED, Emerson JP, Kurtz DM, Jr, Cabelli DE. J Am Chem Soc. 2000;122:11555–11556. [Google Scholar]; f) Emerson JP, Coulter ED, Cabelli DE, Phillips RS, Kurtz DM., Jr Biochemistry. 2002;41:4348–4357. doi: 10.1021/bi0119159. [DOI] [PubMed] [Google Scholar]
- 4.a) Shearer J, Scarrow RC, Kovacs JA. J Am Chem Soc. 2002;124:11709–11717. doi: 10.1021/ja012722b. [DOI] [PubMed] [Google Scholar]; b) Krishnamurthy D, Kasper GD, Namuswe F, Kerber WD, Sarjeant AAN, Moenne-Loccoz P, Goldberg DP. J Am Chem Soc. 2006;128:14222–14223. doi: 10.1021/ja064525o. [DOI] [PubMed] [Google Scholar]; c) Kitagawa T, Dey A, Lugo-Mas P, Benedict JB, Kaminsky W, Solomon E, Kovacs JA. J Am Chem Soc. 2006;128:14448–14449. doi: 10.1021/ja064870d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Namuswe F, Kasper GD, Sarjeant AAN, Hayashi T, Krest CM, Green MT, Moenne-Loccoz P, Goldberg DP. J Am Chem Soc. 2008;130:14189–14200. doi: 10.1021/ja8031828. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Jiang Y, Telser J, Goldberg DP. Chem Commun. 2009;44:6828–6830. doi: 10.1039/b913945a. [DOI] [PubMed] [Google Scholar]; f) Namuswe F, Hayashi T, Jiang Y, Kasper GD, Sarjeant AAN, Moenne-Loccoz P, Goldberg DP. J Am Chem Soc. 2009;132:157–167. doi: 10.1021/ja904818z. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Stasser J, Namuswe F, Kasper GD, Jiang Y, Krest CM, Green MT, Penner-Hahn J, Goldberg DP. Inorg Chem. 2010;49:9178–9190. doi: 10.1021/ic100670k. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Nam E, Alokolaro PE, Swartz RD, Gleaves MC, Pikul J, Kovacs JA. Inorg Chem. 2011;50:1592–1602. doi: 10.1021/ic101776m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiedler AT, Halfen HL, Halfen JA, Brunold TC. J Am Chem Soc. 2005;127:1675–1689. doi: 10.1021/ja046939s. [DOI] [PubMed] [Google Scholar]
- 6.As peroxide is a byproduct of superoxide disproportionation, control experiments between 1 and H2O2 or Na2O2 were carried out in THF/DMF at −90 °C. In neither case was a reaction observed over the course of 1 h.
- 7.a) Duerr K, Macpherson BP, Warratz R, Hampel F, Tuczek F, Helmreich M, Jux N, Ivanovic-Burmazovic I. J Am Chem Soc. 2007;129:4217–4228. [Google Scholar]; b) Duerr K, Jux N, Zahl A, van Eldik R, Ivanovic-Burmazovic I. Inorg Chem. 2010;49:11254–11260. doi: 10.1021/ic102092h. [DOI] [PubMed] [Google Scholar]; c) Duerr K, Olah J, Davydov R, Kleimann M, Li J, Lang N, Puchta R, Hubner E, Drewello T, Harvey JN, Jux N, Ivanovic-Burmazovic I. Dalton Trans. 2010;39:2049–2056. doi: 10.1039/b920237d. [DOI] [PubMed] [Google Scholar]
- 8.a) Annaraj J, Suh Y, Seo MS, Kim SO, Nam W. Chem Commun. 2005:4529–4531. doi: 10.1039/b505562h. [DOI] [PubMed] [Google Scholar]; b) Li F, Meier KK, Cranswick MA, Chakrabarti M, Van Heuvelen KM, Münck E, Que L., Jr J Am Chem Soc. 2011;133:7256–7259. doi: 10.1021/ja111742z. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cho J, Jeon S, Wilson SA, Liu LV, Kang EA, Braymer JJ, Lim MH, Hedman B, Hodgson KO, Valentine JS, Solomon EI, Nam W. Nature. 2011;478:502–505. doi: 10.1038/nature10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Yeh AP, Hu Y, Jenney FE, Adams MWW, Rees DC. Biochemistry. 2000;39:2499–2508. doi: 10.1021/bi992428k. [DOI] [PubMed] [Google Scholar]; b) Katona G, Carpentier P, Nivière V, Amara P, Adam V, Ohana J, Tsanov N, Bourgeois D. Science. 2007;316:449–453. doi: 10.1126/science.1138885. [DOI] [PubMed] [Google Scholar]
- 10.Kühnel K, Derat E, Terner J, Shaik S, Schlichting I. Proc Nat Acad Sci USA. 2007;104:99–104. doi: 10.1073/pnas.0606285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry JF, Bill E, Bothe E, Neese F, Wieghardt K. J Am Chem Soc. 2006;128:13515–13528. doi: 10.1021/ja063590v. [DOI] [PubMed] [Google Scholar]
- 12.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 13.Lang G. Quart Rev Biophys. 1970;3:1–60. doi: 10.1017/s0033583500004406. [DOI] [PubMed] [Google Scholar]
- 14.The valence contribution to the principal components of the quadrupole tensor (Q′xx,yy,zz, in atomic units) is proportional to (−2/7, +4/7, −2/7) and (+4/7, −2/7, −2/7) for an electron in dxz and dyz, respectively. Adding these contributions yields (2/7, 2/7, −4/7). (See Gütlich P, Link R, Trautwein A. Mössbauer Spectroscopy and Transition Metal Chemistry. Springer-Verlag; Berlin-Heidelberg-New York: 1978.
- 15.DeBeer George S, Petrenko T, Neese F. J Phys Chem A. 2008;112:12936–12943. doi: 10.1021/jp803174m. [DOI] [PubMed] [Google Scholar]
- 16.Because of the importance of vibrational information, we made a number of attempts to obtain such data to probe the peroxide binding mode in 2, but these experiments were all unsuccessful. These experiments are described in the Supporting Information.
- 17.a) Selke M, Sisemore MF, Valentine JS. J Am Chem Soc. 1996;118:2008–2012. [Google Scholar]; b) Sisemore MF, Burstyn JN, Valentine JS. Angew Chem Int Ed. 1996;35:206–208. [Google Scholar]; c) Selke M, Valentine JS. J Am Chem Soc. 1998;120:2652–2653. [Google Scholar]
- 18.Wertz DL, Valentine JS. Struct Bonding. 2000;97:38–60. [Google Scholar]
- 19.a) Kennepohl P, Neese F, Schweitzer D, Jackson HL, Kovacs JA, Solomon EI. Inorg Chem. 2005;44:1826–1836. doi: 10.1021/ic0487068. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bukowski MR, Koehntop KD, Stubna A, Bominaar EL, Halfen JA, Münck E, Nam W, Que L., Jr Science. 2005;310:1000–1002. doi: 10.1126/science.1119092. [DOI] [PubMed] [Google Scholar]
- 20.Dey A, Jenney FE, Adams MWW, Johnson MK, Hodgson KO, Hedman B, Solomon EI. J Am Chem Soc. 2007;129:12418–12431. doi: 10.1021/ja064167p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JG, Shimizu Y, Ohta T, Naruta Y. J Am Chem Soc. 2010;132:3672–3673. doi: 10.1021/ja1001955. [DOI] [PubMed] [Google Scholar]
- 22.a) Akhtar MRCM, Corina DL, Wright JN. Biochem J. 1982;201:569–580. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Korzekwa KR, Trager WF, Smith SJ, Osawa Y, Gillette JR. Biochemistry. 1991;30:6155–6162. doi: 10.1021/bi00239a011. [DOI] [PubMed] [Google Scholar]; c) Pufahl RA, Wishnok JS, Marletta MA. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]; d) Woodward JJ, Chang MM, Martin NI, Marletta MA. J Am Chem Soc. 2008;131:297–305. doi: 10.1021/ja807299t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.