Abstract
In both chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), abnormally high collagen remodeling occurs within the lung tissue. Matrix metalloproteinase (MMP)-degraded type I, III, IV, V and VI collagen and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-degraded type III collagen were assessed in serum of patients diagnosed with mild COPD (n = 10) or IPF (n = 30), and healthy controls (n = 15). The collagen degradation markers C1M, C3M, C5M and C6M were significantly elevated in serum of both mild COPD and IPF patients, versus controls. C3A and C4M were only elevated in patients with mild COPD, compared with controls. The most reliable indicators of mild COPD versus controls were: C1M (area under the receiver-operating characteristics (AUROC = 0.94, P < 0.0001), C3M (AUROC = 0.95, P < 0.0001), and C5M (AUROC = 0.95, P < 0.0001). The most reliable markers for the diagnosis of IPF were achieved by C1M (AUROC = 0.90, P < 0.0001) and C3M (AUROC = 0.93, P < 0.0001). Collagen degradation was highly up-regulated in patients with IPF and mild COPD, indicating that degradation fragments of collagens are potential markers of pulmonary diseases. Interestingly, C4M and C3A were only elevated in patients with mild COPD, indicating that these markers could be used to distinguish between the two pathologies.
Keywords: collagen, extracellular matrix remodeling, biochemical marker, neoepitope, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, matrix metalloproteinases
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by narrowing of small conducting airways and chronic changes in lung parenchyma which develop over many years.1 Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease characterized by fibroblast proliferation and extracellular remodeling.2,3 Common to both diseases is the highly altered interaction between fibrogenesis and fibrolysis leading to functional impairment of the lungs. Fibrosis of the lungs is seen as increased deposition and abnormal distribution of extracellular matrix (ECM) components such as collagens, elastin and proteoglycans. The turnover rate of type I and III collagen in particular is changed significantly4–6 in fibrotic lungs, leading to excessive remodeling and accumulation of structural proteins.
The fibril-forming type I collagen is the most abundant in the lung7 and is mainly found together with type III collagen, the second most abundant collagen type. Together they provide the structural framework of the alveolar wall, pulmonary blood vessels, visceral pleura and the connective tissue sheaths that surround the tracheobronchial tree.8,9 Type III collagen is correlated to extensibility of tissues and may contribute to elasticity, a property that is uniquely connected to this type of collagen.10 The most abundant non- fibrillar collagen of the lung is type IV collagen which is present in the basement membrane (BM) of tissue.11 It provides the blood-air barrier with tensile strength and prevents stress failure of the pulmonary capillaries under normal conditions.12 Other types of collagens, such as type V and VI, are present to a smaller extent, and are important in processes such as collagen fibril assembly and adhesion.13–15 All of these collagens are aggressively remodeled during pulmonary fibrosis,16 and the peptide fragments released systemically during their degradation may serve as potential markers of lung tissue turnover.
Lack of sensitive parameters of lung injury and lung tissue destruction makes short-term evaluation of lung diseases difficult. To assess impaired lung function, computed tomography analysis and biochemical measurements of ECM degradation have been described as tools.17 The pathogenesis of lung diseases such as COPD and IPF involves an inflammatory response,1–3 and the activation of macrophages partly mediates tissue turnover by the secretion of signature proteases, including matrix metalloproteinase (MMP)-9 and -12,2,3,18,19 as well as other MMPs and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs).20 To date, the lack of sensitive parameters of lung injury and lung tissue destruction makes shortterm evaluation of lung diseases difficult. Reported tools for the assessment of impaired lung function are computed tomography analysis and biochemical measurements of ECM degradation.17 The most promising serological markers of fibrotic pulmonary diseases are desmosine and isodesmosine, two molecules involved in elastin cross-linking;17 Krebs von den Lungen 6 antigen (KL-6), a high molecular weight glycoprotein expressed on the surface of alveolar epithelial cells and released as a response to injury, proliferation or stimulation;21 CC-chemokine ligand 18 (CCL18), highly expressed in the lungs and a marker of the alternative macrophage activation seen in fibrotic lungs;22 and finally serum surfactant proteins A (SP-A) and D (SP-D), C-type lectins which are only expressed in the lungs and are produced by alveolar epithelial cells, the number of which increase with the exacerbation of fibrosis.23 All of these markers, however, are in need of better validation, and there is still a lack of non-invasive markers of lung fibrosis.24,25
MMPs and ADAMTSs have been associated with collagen degradation and respiratory diseases.18–20,26 Collagen degradation fragments may be released into the circulation and potentially assessed systemically as markers of collagen degradation. Such protein fragments, referred to as neoepitopes or protein fingerprints,27,28 have proven to be more accurate than their unmodified intact protein of origin in detecting and quantifying certain pathophysiological processes assessed by standard technologies.29 As an example, fragments of types III, IV and VI collagen generated by MMPs have been shown to be markers of generalized and liver fibrosis.30–33 while fragments of type II collagen degradation by MMP-9 have been demonstrated to be markers of osteoarthritis and rheumatoid arthritis.34 An assay for the assessment of type I collagen fragments generated by cathepsin K has already been approved by the US Food and Drug Administration for monitoring bone resorption.29
The current hypothesis was that MMP-mediated fragments of types I, III, IV, V and VI collagen and fragments of ADAMTS-mediated degradation of type III collagen had diagnostic power when assessed in serum of patients with the respiratory diseases COPD or IPF.
Materials and Methods
Patient samples
Serum was collected from patients diagnosed with COPD (n = 10) or IPF (n = 30), and healthy controls (n = 15). The COPD and IPF serum samples were obtained as a part of the “lung tissue research consortium” (www.ltrcpublic.com) and were de-identified. Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) readings were obtained from patients and controls and also de-identified. IPF patients were divided into 3 groups according to their FVC: mild (FVC > 80%), moderate (FVC = 50%–80%) or severe (FVC < 50%). All COPD patients had a FEV1 > 80% defined as mild COPD.
ELISA procedure
Fasting serum samples were collected from patients and healthy controls and stored at −80 °C until assayed. Levels of the MMP-degraded types I, III, IV, V and VI collagen marker (C1M,35 C3M,31 C4M,32 C5M36 and C6M,33 respectively) and ADAMTS-4-degraded type III collagen (C3A, unpublished) marker were assessed in the collected serum samples. Briefly, each marker was run on a 96-well streptavidin plate coated with the appropriate biotinylated synthetic peptide dissolved in an optimized assay buffer and incubated for 30 minutes at 20 °C. 20 μL of peptide calibrator or sample was added to appropriate wells, followed by 100 μL of a conjugated monoclonal antibody raised against the specific sequence of interest. This was incubated for 1 hour or overnight at 4 °C or 20 °C, depending on the individual assay. Finally, 100 μL tetramethylbenzinidine (TMB) (Kem-En-Tec cat.438OH) was added and the plate was incubated for 15 minutes at 20 °C in the dark. All the above incubation steps included shaking at 300 rpm. After each incubation step the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). The TMB reaction was stopped by adding 100 μL of stopping solution (1% HCl) and measured at 450 nm with 650 nm as the reference. A calibration curve was plotted using a 4-parametric mathematical fit model.
Statistical analysis
The serum levels of the individual biochemical markers in healthy controls and each patient group were compared by non-parametric Mann-Whitney t-test for two-tailed observations, and are presented as box plots indicating the 5th and 95th percentiles. Area under the receiver operating characteristic (AUROC) was calculated for each biochemical marker. All statistical analyses were performed using Graph Pad Prism software v.5 (Graph Pad S oftware, San Diego, CA). P values less than 0.05 were considered significant.
Results
Connective tissue degradation
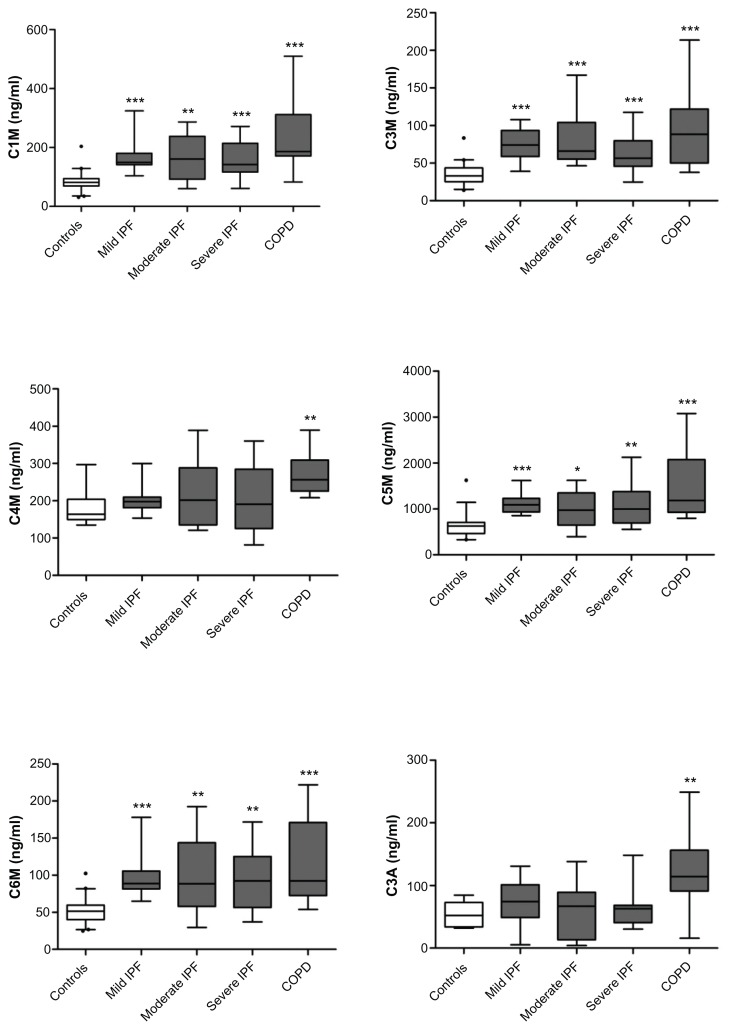
Fragments of types I, III, V and VI collagen degradation by MMPs were significantly elevated in the serum of both COPD and IPF patients versus healthy controls, as presented in Figure 1. C1M, C3M, C3A, C4M, C5M and C6M were all significantly elevated in mild COPD compared with controls, (P < 0.05–0.01 and up to >189% for C1M). C1M, C3M, C5M and C6M were all highly elevated in all severity groups of IPF patients compared with controls, (P < 0.05–0.0001, and up to ≥133% for C3M). Interestingly, the C3A and C4M markers were not elevated in any of the IPF patient groups.
Figure 1.
Biochemical markers of collagen degradation as measured by six different ELISAs. Levels of the markers reflecting types I (C1M), III (C3M), IV (C4M), V (C5M) and VI (C6M) collagen degradation by MMPs, as well as fragments of type III collagen degraded by aggrecanase (C3A), were measured in serum of patients with mild (n = 10), moderate (n = 10) and severe (n = 10) IPF, mild COPD (n = 10), and healthy controls (n = 15).
Notes: Patient groups were compared with healthy controls using the non-parametric Mann-Whitney test, and results are presented as box plots. The boundaries of each box indicate the 25th and 75th percentiles, the line within the box marks the median, and the whiskers indicate the 5th and 95th percentiles. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001.
Diagnostic power
The power of the individual markers to discriminate between disease and healthy controls was calculated by the AUROC and presented in Table 1. The three groups of IPF patients were combined for these calculations, since the markers were not able to differentiate severity in IPF patients. The diagnostic powers of C1M, C3M, C5M and C6M were highly significant with an AUROC > 85% (P < 0.0001). C4M and C3A presented an AUROC > 89% for COPD patients versus controls (P < 0.01), but was unable to distinguish between IPF patients and controls. The best discriminators between mild COPD and controls were C1M (AUROC = 0.94, P < 0.0001), C3M (AUROC = 0,95, P < 0.0001), and C5M (AUROC = 0.95, P < 0.0001). The most reliable differentiation between IPF and controls was observed using C1M (AUROC = 0.90, P < 0.0001) and C3M (AUROC = 0.93, P < 0.0001).
Table 1.
Area under the receiver operating characteristic (AUROC) for biochemical marker levels in healthy controls vs. IPF and COPD.
| Comparison | AUROC | Std. error | P value |
|---|---|---|---|
| C1M | |||
| IPF | 0.90 | 0.05 | <0.0001*** |
| COPD | 0.94 | 0.05 | <0.0001*** |
| C3M | |||
| IPF | 0.93 | 0.03 | <0.0001*** |
| COPD | 0.95 | 0.04 | <0.0001*** |
| C4M | |||
| IPF | 0.59 | 0.09 | 0.36 |
| COPD | 0.89 | 0.07 | 0.0018** |
| C5M | |||
| IPF | 0.85 | 0.05 | <0.0001*** |
| COPD | 0.95 | 0.03 | <0.0001*** |
| C6M | |||
| IPF | 0.86 | 0.05 | <0.0001*** |
| COPD | 0.90 | 0.06 | <0.0001*** |
| C3A | |||
| IPF | 0.52 | 0.08 | 0.78 |
| COPD | 0.82 | 0.11 | 0.0049** |
Notes: AUROC > 80% are highlighted in italics. Significant separation of controls and patients is indicated by asterisks. Significance levels:
P < 0.05,
P < 0.01,
P < 0.001.
Discussion
To our knowledge, this is the first study investigating the serological profile of collagen turnover in fibrotic lung diseases. We tested a range of novel ECM degradation serum markers in patients with COPD or IPF and in healthy controls. Interestingly, significant differences in marker levels were seen between healthy and disease-affected individuals, and also between the two lung diseases. The markers of ECM degradation provided diagnostic information and suggest that tissue degradation is highly elevated in patients with mild COPD and mild to severe IPF. C1M, C3M, C5M and C6M showed highly statistically significant power to discriminate between IPF and mild COPD patients versus healthy individuals, while C4M and C3A were only able to diagnose COPD patients. The fact that four out of six collagen degradation markers were able to detect even a mild form of IPF is interesting, since it is this patient group that will benefit the most from a diagnosis. These preliminary findings need to be validated in larger clinical settings. This study, although the first and small, has identified collagen turnover markers with the potential to separate healthy individuals from COPD and IPF patients. In future these markers may assist in the identification of patients that are fast progressers and may respond to a given intervention.
Endopeptidases play a major role in the degradation of ECM proteins such as collagens and proteoglycans.26,37,38 MMP-2 and -9, in particular, have been shown to be highly up-regulated in connective tissue diseases leading to fibrosis.39–41 A wide range of ADAMs/ADAMTSs are expressed in the lung42 and many have been associated with different respiratory diseases such as COPD, IPF and asthma.20 COPD has been coupled with changes in ADAM-33, ADAM-17, and ADAMTS-4 expression,43–45 while only a weak association has been observed for a few ADAMs/ADAMTSs in IPF.46 The C3A marker showed elevated levels of ADAMTS-4-mediated degradation of type III collagen in COPD but not IPF patients. This finding is in agreement with previous experiments showing that ADAMTS-4 is up-regulated in COPD while there is no evidence of a relationship in IPF.45,46 This highlights that the differences in the pathological processes and the proteases involved in these two diseases result in distinct tissue turnover profiles.
Fibrotic lungs have an overall increased ECM turnover rate, but the normal balance between formation and degradation is changed, leading to increased deposition and increased degradation. The change in remodeling balance is not the same for different types of collagen; a key feature is the increased deposition and degradation of type I collagen in pulmonary fibrosis.4,5,47 This is in line with our C1M results showing significantly elevated levels in both patients with mild to severe IPF and mild COPD and thus indicating an increased degradation of type I collagen. Contradictory results have been published in relation to type III collagen deposition in pulmonary fibrosis, demonstrating that both up—and down-regulation of type III collagen occurs.4–6 The serum C3M data presented here suggest an increased type III collagen degradation level in both IPF and mild COPD, indicating a high level of formation of type III collagen as well. Indications of a decreased content of type V collagen in fibrotic rat lungs have been presented.48 However, Parra et al demonstrated that type V collagen levels in biopsies from IPF patients increased with disease severity,49 supporting an increased degradation of deposited type V collagen indicated by our C5M data. Type IV and type VI collagen expression, as well as protein levels, have been reported to be elevated in lung tissue of patients with COPD50 and IPF,51 further supporting the findings of our biochemical marker study.
Desmosine, isodesmosine, KL-6, CCL18, SP-A and SP-D have all been extensively discussed as serological markers of pulmonary diseases.17,21–23 However, none of these markers have demonstrated optimal sensitivity and diagnostic value. Thus, more sensitive and accurate biochemical markers are needed for fibrotic lung diseases. Stratification of patients with pulmonary fibrosis is most likely not feasible using a single marker since would require a marker specific for a protein only expressed during lung fibrosis. A panel of markers reflecting different pathophysiological processes involved in pulmonary fibrosis during the two different diseases will almost certainly be required for diagnosis, prognosis and assessment of the efficacy of interventions.
The systemic level of a biochemical marker is the sum of all tissue sites generating this one fragment, and also depends on the extent of disease- affected tissue, the aggressiveness of the disease and the protein specificity of the fragment. This was elegantly investigated by Meulenbelt et al52 who demonstrated that the level of a MMP-generated fragment of the signature protein of cartilage, type II collagen, was correlated to the number of affected joints in osteoarthritis. Collagen expression is not restricted to the lung tissue, but is found ubiquitously throughout the body. Thus, several co-morbidities may influence the systemic level of fragments produced by MMP degradation of collagen molecules. Further investigations are needed to determine the individual contribution of different tissues to the total pool of collagen neoepitopes.
There are several limitations with the current study. The sample size was very small, and thus the findings are preliminary. The lack of information available for patients does not allow for further analysis and correlation with clinical parameters. Furthermore, this is a cross-sectional study and the prognostic value of the biomarkers could not be validated.
In conclusion, by using protein fingerprint technology, we have developed assays measuring novel biochemical markers which enable us to assess peptides generated during degradation of collagens in pulmonary fibrosis. These markers were able to distinguish between healthy controls and patients with mild COPD and/or IPF in a small clinical population. The collagen degradation markers demonstrated promising discriminative diagnostic power and may provide an improved tool for identification of those patients most in need of treatment, as well as for monitoring potential efficacy of interventions. These data need to be validated in larger clinical settings.
Footnotes
Author Contributions
Conceived and designed the experiments: MK, DJL, MH. Data analysis: DJL, JSA, MJU. Scientific writers: JSA, DJL, MK, FGE. Agree with manuscript results and conclusions: ALL. Made critical revisions and approved final version: DJL, MK. Clinical study samples retrieval: LK, FJM, CMH, MH.
Competing Interests
DL, MK and FG are full-time employees of Nordic Bioscience. MK is stockholder of Nordic Bioscience. FM is a board member for COPD and/or IPF for Actelion, Almirall/Forest, Nycomed/Takeda, Bayer, GSK, Ikaria, MedImmune, Merck, Pearl, Pfizer, Jannsen, Elan, Gilead, Centocor, Novartis, has consulted for Nycomed/Takeda, American Institute for Research, AstraZeneca, Boom Comm, Elan, HCRC, IntraMed, JK Associates, Merion, Schering, Sudler and Hennessey, Cardiomems, Janssens, spoken for Forest, Nycomed/Takeda, AstraZeneca, GSK, Sanofi, Boeheringer Ingelheim, developed educational presentations for UpToDate, American College of Chest Physicians, Almirall/Forest, Bayer. DL’s institution has received a grant from Den Danske Forsknings Fond. MH is a consultant to Boehringer Ingelheim, Pfizer, GSK, Medimmune, Novartis, Grifols Therapeutics, United Biosource Corporation, has grants/grants pending from NHLBI K23 award, Chicago Community Trust and has developed educational presentations for WebMD, National Association for potential competing interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but Consulting Education. Other authors disclose no not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Funding
We acknowledge the funding from the Danish Research Foundation (Den Danske Forskningsfond). The authors would like to acknowledge The Lung Tissue Research Consortium (LTRC) and The National Heart, Lung, and Blood Institute (NHLBI) for kindly providing the COPD and IPF lung samples.
References
- 1.Hogg JC, McDonough JE, Gosselink JV, Hayashi S. What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2009;6:668–72. doi: 10.1513/pats.200907-079DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 3.Desai B, Mattson J, Paintal H, et al. Differential expression of monocyte/macrophage- selective markers in human idiopathic pulmonary fibrosis. Exp Lung Res. 2011;37:227–38. doi: 10.3109/01902148.2010.538132. [DOI] [PubMed] [Google Scholar]
- 4.Last JA, Siefkin AD, Reiser KM. Type I collagen content is increased in lungs of patients with adult respiratory distress syndrome. Thorax. 1983;38:364–8. doi: 10.1136/thx.38.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyer JM, Hutcheson ET, Kang AH. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976;57:1498–507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk JM, Bateman ED, Haslam PL, Laurent GJ, Turner-Warwick M. Serum type III procollagen peptide concentration in cryptogenic fibrosing alveolitis and its clinical relevance. Thorax. 1984;39:726–32. doi: 10.1136/thx.39.10.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Kuppevelt TH, Veerkamp JH, Timmermans JA. Immunoquantification of type I, III, IV and V collagen in small samples of human lung parenchyma. Int J Biochem Cell Biol. 1995;27:775–82. doi: 10.1016/1357-2725(95)00047-s. [DOI] [PubMed] [Google Scholar]
- 8.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 2005;98:1892–9. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 9.Dunsmore SE. Treatment of COPD: a matrix perspective. Int J Chron Obstruct Pulmon Dis. 2008;3:113–22. doi: 10.2147/copd.s1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konomi H, Hayashi T, Nakayasu K, Arima M. Localization of type V collagen and type IV collagen in human cornea, lung, and skin. Immunohistochemical evidence by anti-collagen antibodies characterized by immunoelectroblotting. Am J Pathol. 1984;116:417–26. [PMC free article] [PubMed] [Google Scholar]
- 12.West JB, Mathieu-Costello O. Structure, strength, failure, and remodeling of the pulmonary blood-gas barrier. Annu Rev Physiol. 1999;61:543–72. doi: 10.1146/annurev.physiol.61.1.543. [DOI] [PubMed] [Google Scholar]
- 13.Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–7. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 14.Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272:26522–9. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- 15.Engvall E, Hessle H, Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol. 1986;102:703–10. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luisetti M, Ma S, Iadarola P, et al. Desmosine as a biomarker of elastin degradation in COPD: current status and future directions. Eur Respir J. 2008;32:1146–57. doi: 10.1183/09031936.00174807. [DOI] [PubMed] [Google Scholar]
- 18.Joos L, He JQ, Shepherdson MB, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet. 2002;11:569–76. doi: 10.1093/hmg/11.5.569. [DOI] [PubMed] [Google Scholar]
- 19.Marciniak SJ, Lomas DA. What can naturally occurring mutations tell us about the pathogenesis of COPD? Thorax. 2009;64:359–64. doi: 10.1136/thx.2008.099408. [DOI] [PubMed] [Google Scholar]
- 20.Paulissen G, Rocks N, Gueders MM, et al. Role of ADAM and ADAMTS metalloproteinases in airway diseases. Respir Res. 2009;10:127. doi: 10.1186/1465-9921-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoyama A, Kohno N, Hamada H, et al. Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1680–4. doi: 10.1164/ajrccm.158.5.9803115. [DOI] [PubMed] [Google Scholar]
- 22.Prasse A, Pechkovsky DV, Toews GB, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56:1685–93. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- 23.Greene KE, King TE, Jr, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439–46. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 24.Thomeer M, Grutters JC, Wuyts WA, Willems S, Demedts MG. Clinical use of biomarkers of survival in pulmonary fibrosis. Respir Res. 2010;11:89. doi: 10.1186/1465-9921-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140:221–9. doi: 10.1378/chest.10-2572. [DOI] [PubMed] [Google Scholar]
- 26.Oikonomidi S, Kostikas K, Tsilioni I, Tanou K, Gourgoulianis KI, Kiropoulos TS. Matrix metalloproteinases in respiratory diseases: from pathogenesis to potential clinical implications. Curr Med Chem. 2009;16:1214–28. doi: 10.2174/092986709787846587. [DOI] [PubMed] [Google Scholar]
- 27.Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay-Jensen AC. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers—are they the cause or the consequence of the disease? Clin Biochem. 2010;43:793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Karsdal MA, Delvin E, Christiansen C. Protein fingerprints—relying on and understanding the information of serological protein measurements. Clin Biochem. 2011;44:1278–9. doi: 10.1016/j.clinbiochem.2011.08.1135. [DOI] [PubMed] [Google Scholar]
- 29.Karsdal MA, Henriksen K, Leeming DJ, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 30.Veidal SS, Vassiliadis E, Barascuk N, et al. Matrix metalloproteinase-9- mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int. 2010;30:1293–304. doi: 10.1111/j.1478-3231.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 31.Barascuk N, Veidal SS, Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Veidal SS, Karsdal MA, Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: A fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:22. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veidal SS, Karsdal MA, Vassiliadis E, et al. MMP Mediated Degradation of Type VI Collagen Is Highly Associated with Liver Fibrosis— Identification and Validation of a Novel Biochemical Marker Assay. PLoS ONE. 2011;6:e24753. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bay-Jensen AC, Liu Q, Byrjalsen I, et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM—increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin Biochem. 2011;44:423–9. doi: 10.1016/j.clinbiochem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Leeming DJ, He Y, Veidal S, et al. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011;16:616–28. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 36.Veidal SS, Larsen DV, Chen X, et al. MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin Biochem. 2012 doi: 10.1016/j.clinbiochem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Bay-Jensen AC, Hoegh-Madsen S, Dam E, et al. Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatol Int. 2010;30:435–42. doi: 10.1007/s00296-009-1183-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhen EY, Brittain IJ, Laska DA, et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58:2420–31. doi: 10.1002/art.23654. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- 40.Boeker KH, Haberkorn CI, Michels D, Flemming P, Manns MP, Lichtinghagen R. Diagnostic potential of circulating TIMP-1 and MMP-2 as markers of liver fibrosis in patients with chronic hepatitis C. Clin Chim Acta. 2002;316:71–81. doi: 10.1016/s0009-8981(01)00730-6. [DOI] [PubMed] [Google Scholar]
- 41.Kirimlioglu H, Kirimlioglu V, Yilmaz S. Expression of matrix metalloproteinases 2 and 9 in donor liver, cirrhotic liver, and acute rejection after human liver transplantation. Transplant Proc. 40:3574–7. doi: 10.1016/j.transproceed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Rocks N, Paulissen G, Quesada CF, et al. Expression of a disintegrin and metalloprotease (ADAM and ADAMTS) enzymes in human non-small-cell lung carcinomas (NSCLC) Br J Cancer. 2006;94:724–30. doi: 10.1038/sj.bjc.6602990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Diemen CC, Postma DS, Vonk JM, Bruinenberg M, Schouten JP, Boezen HM. A disintegrin and metalloprotease 33 polymorphisms and lung function decline in the general population. Am J Respir Crit Care Med. 2005;172:329–33. doi: 10.1164/rccm.200411-1486OC. [DOI] [PubMed] [Google Scholar]
- 44.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5 AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L420–7. doi: 10.1152/ajplung.00019.2004. [DOI] [PubMed] [Google Scholar]
- 45.Ezzie ME, Crawford M, Cho JH, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67:122–31. doi: 10.1136/thoraxjnl-2011-200089. [DOI] [PubMed] [Google Scholar]
- 46.Pardo A, Selman M, Kaminski N. Approaching the degradome in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:1141–55. doi: 10.1016/j.biocel.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Bateman ED, Turner-Warwick M, mann-Grill BC. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax. 1981;36:645–53. doi: 10.1136/thx.36.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiser KM, Last JA. Type V collagen. Quantitation in normal lungs and in lungs of rats with bleomycin-induced pulmonary fibrosis. J Biol Chem. 1983;258:269–75. [PubMed] [Google Scholar]
- 49.Parra ER, Teodoro WR, Velosa AP, de Oliveira CC, Yoshinari NH, Capelozzi VL. Interstitial and vascular type V collagen morphologic disorganization in usual interstitial pneumonia. J Histochem Cytochem. 2006;54:1315–25. doi: 10.1369/jhc.6A6969.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kranenburg AR, Willems-Widyastuti A, Moori WJ, et al. Enhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;126:725–35. doi: 10.1309/jc477fael1ykv54w. [DOI] [PubMed] [Google Scholar]
- 51.Specks U, Nerlich A, Colby TV, Wiest I, Timpl R. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med. 1995;151:1956–64. doi: 10.1164/ajrccm.151.6.7767545. [DOI] [PubMed] [Google Scholar]
- 52.Meulenbelt I, Kloppenburg M, Kroon HM, et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage. 2007;15:379–85. doi: 10.1016/j.joca.2006.09.007. [DOI] [PubMed] [Google Scholar]