Abstract
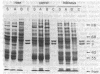
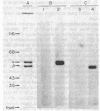
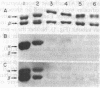
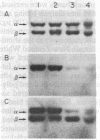
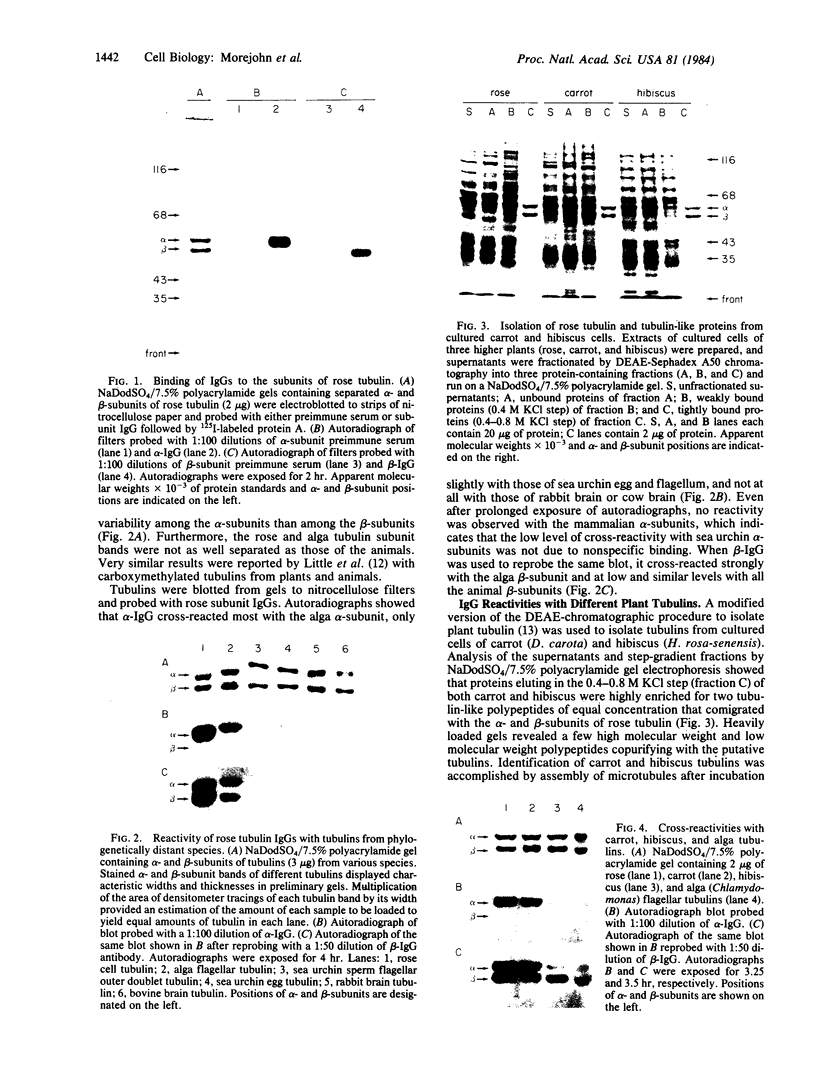
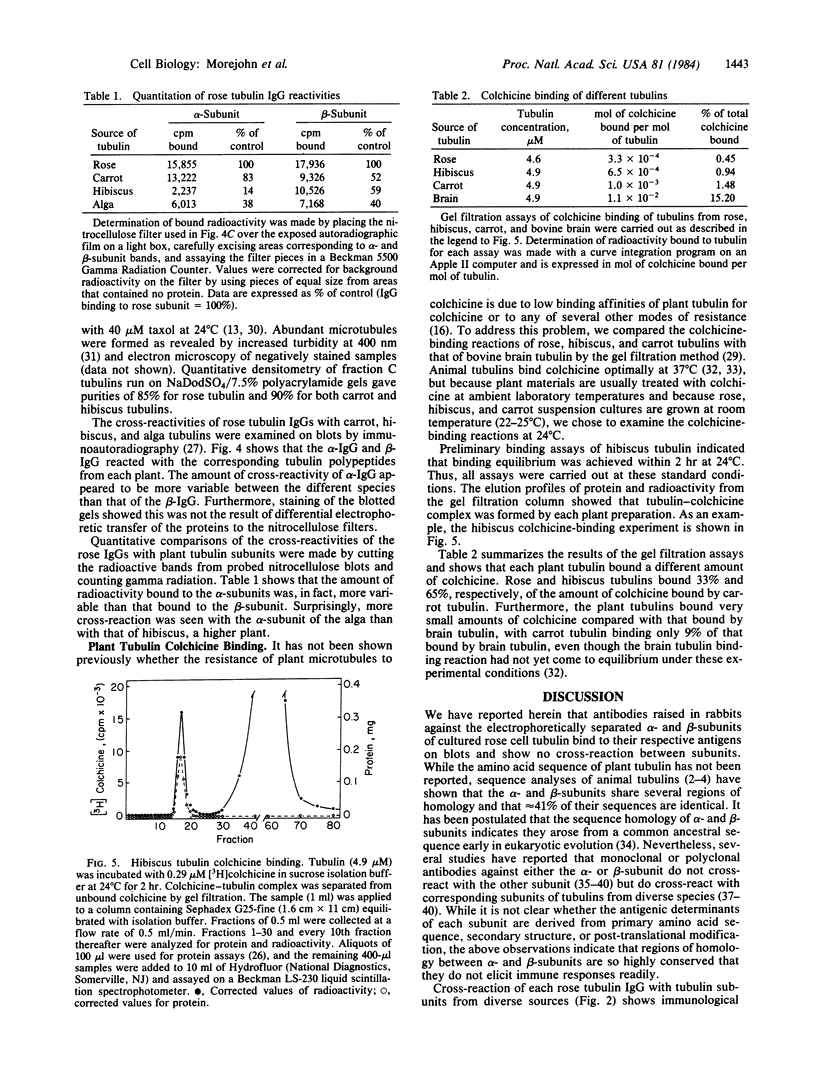
We have initiated immunological and drug-binding studies on the tubulins from different higher plant species. Antibodies were raised against electrophoretically separated rose (Rosa sp.) tubulin α- and β-subunits and characterized by immunoblot autoradiographic assays. Each IgG preparation bound to its antigen and cross-reacted differentially with the respective tubulin subunits from an alga, sea urchin, rabbit, and cow. Antigenic determinants were shared more among the β-subunits than among the α-subunits from these organisms. Tubulins were isolated from cultured cells of carrot (Daucus carota) and hibiscus (Hibiscus rosa-senensis). Immunoautoradiography and quantitation of cross-reactivity on blots showed nonidentity among homologous subunits from rose, carrot, hibiscus, and alga tubulins, with more antigenic differences among α-subunits than among β-subunits. Comparative colchicine-binding assays showed that rose and hibiscus tubulins bound 33% and 65%, respectively, of the colchicine bound by carrot tubulin and that higher plant tubulins bound much less colchicine than bovine brain tubulin under identical conditions.
Keywords: microtubules, cultured plant cells, tubulin antibodies, immunoautoradiography, antimicrotubule drugs
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai D. J., Brokaw C. J., Thompson W. C., Wilson L. Two different monoclonal antibodies to alpha-tubulin inhibit the bending of reactivated sea urchin spermatozoa. Cell Motil. 1982;2(6):599–614. doi: 10.1002/cm.970020608. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- De Mey J., Lambert A. M., Bajer A. S., Moeremans M., De Brabander M. Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1898–1902. doi: 10.1073/pnas.79.6.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Williams R. C., Jr, Wilson L. Effect of colchicine binding on the reversible dissociation of the tubulin dimer. Biochemistry. 1982 May 11;21(10):2392–2400. doi: 10.1021/bi00539a018. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Gozes I., Barnstable C. J. Monoclonal antibodies that recognize discrete forms of tubulin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2579–2583. doi: 10.1073/pnas.79.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn J. A., Olsen R. W. Radioimmune assay of tubulin applied to oligomers, synaptic membranes, and plants. Biochem Biophys Res Commun. 1978 Jan 30;80(2):391–397. doi: 10.1016/0006-291x(78)90689-7. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Purification and properties of flagellar outer doublet tubulin from Naegleria gruberi and a radioimmune assay for tubulin. J Biol Chem. 1974 Jun 10;249(11):3638–3646. [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir A., Engelborghs Y. A fluorescence stopped flow study of colchicine binding to tubulin. J Biol Chem. 1981 Apr 10;256(7):3279–3282. [PubMed] [Google Scholar]
- Lee J. C., Frigon R. P., Timasheff S. N. The chemical characterization of calf brain microtubule protein subunits. J Biol Chem. 1973 Oct 25;248(20):7253–7262. [PubMed] [Google Scholar]
- Lee J. C., Tweedy N., Timasheff S. N. In vitro reconstitution of calf brain microtubules: effects of macromolecules. Biochemistry. 1978 Jul 11;17(14):2783–2790. doi: 10.1021/bi00607a013. [DOI] [PubMed] [Google Scholar]
- Little M., Ludueña R. F., Keenan R., Asnes C. F. Tubulin evolution: two major types of alpha-tubulin. J Mol Evol. 1982;19(1):80–86. doi: 10.1007/BF02100226. [DOI] [PubMed] [Google Scholar]
- Little M., Ludueña R. F., Langford G. M., Asnes C. F., Farrell K. Comparison of proteolytic cleavage patterns of alpha-tubulins and beta-tubulins from taxonomically distant species. J Mol Biol. 1981 Jun 15;149(1):95–107. doi: 10.1016/0022-2836(81)90262-x. [DOI] [PubMed] [Google Scholar]
- Luduena R. F., Woodward D. O. Isolation and partial characterization of alpha and beta-tubulin from outer doublets of sea-urchin sperm and microtubules of chick-embryo brain. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3594–3598. doi: 10.1073/pnas.70.12.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Higher plant tubulin identified by self-assembly into microtubules in vitro. Nature. 1982 Jun 3;297(5865):426–428. doi: 10.1038/297426a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. L., Holladay C. R., Spooner B. S. Species-dependent immunological differences between vertebrate brain tubulins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1414–1417. doi: 10.1073/pnas.75.3.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer T. A., Asnes C. F., Wilson L. Properties of tubulin in unfertilized sea urchin eggs. Quantitation and characterization by the colchicine-binding reaction. J Cell Biol. 1976 Jun;69(3):599–607. doi: 10.1083/jcb.69.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Microtubular proteins of Chlamydomonas reinhardtii. An immunochemical study based on the use of an antibody specific for the beta-tubulin subunit. J Biol Chem. 1977 Jan 10;252(1):383–391. [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Timasheff S. N. Aggregation of microtubule subunit protein. Effects of divalent cations, colchicine and vinblastine. Biochemistry. 1970 Oct 13;9(21):4110–4116. doi: 10.1021/bi00823a012. [DOI] [PubMed] [Google Scholar]
- Wick S. M., Seagull R. W., Osborn M., Weber K., Gunning B. E. Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol. 1981 Jun;89(3):685–690. doi: 10.1083/jcb.89.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]