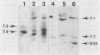
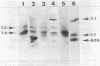
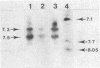
Abstract
This work was aimed at elucidating the environmental conditions that account for the production of embryonic erythrocytes in the mouse yolk sac (YS), while the adult-type hemoglobin and erythrocytes are generated in the fetal liver. Differentiation of YS hemopoietic stem cells (YS-HSC) of 9.5-day mouse embryos (prior to the colonization of the liver rudiment by HSC) was investigated in vitro. The influence of well-characterized erythroid growth factors, burst-promoting activity (BPA) and erythropoietin (EPO), which trigger the differentiation of early erythroid burst-forming units (BFU-E) and late erythroid colony-forming units (CFU-E), respectively, was tested on the YS-HSC in two different systems of culture: (i) organ culture and (ii) clonal culture in methylcellulose. When studied in organ culture, where the YS microenvironment was maintained, YS-HSC required only additional EPO to attain complete maturation into adult erythrocytes within 7 days. In contrast, YS hemopoietic single cells grown in methylcellulose, and thus released from the influence of the YS, required the presence of both BPA and EPO to generate BFU-E-derived colonies synthesizing high concentrations of hemoglobin. It is concluded that 9.5-day YS from mouse embryos is by itself able to promote the first differentiation steps of the adult lineage YS-HSC due to an intrinsic production of a BPA-like activity. In contrast, these experiments demonstrate that EPO or an EPO-like activity is not produced by YS tissues. As demonstrated earlier, if embryonic tissue is added to YS organ culture, although separated from it by a filter preventing cell contact, YS-HSC differentiate into adult erythrocytes producing adult-type hemoglobins. This shows that, in contrast to YS tissues, the early embryo produces EPO or a factor that can substitute for EPO.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. E. Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev Biol. 1968 Jul;18(1):14–29. doi: 10.1016/0012-1606(68)90020-1. [DOI] [PubMed] [Google Scholar]
- CRAIG M. L., RUSSELL E. S. A DEVELOPMENTAL CHANGE IN HEMOGLOBINS CORRELATED WITH AN EMBRYONIC RED CELL POPULATION IN THE MOUSE. Dev Biol. 1964 Oct;10:191–201. doi: 10.1016/0012-1606(64)90040-5. [DOI] [PubMed] [Google Scholar]
- Cudennec C. A., Thiery J. P., Le Douarin N. M. In vitro induction of adult erythropoiesis in early mouse yolk sac. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2412–2416. doi: 10.1073/pnas.78.4.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg B. Is erythropoietin the only factor which regulates late erythroid differentiation? Nature. 1981 Jan 15;289(5794):184–186. doi: 10.1038/289184a0. [DOI] [PubMed] [Google Scholar]
- Fantoni A., Bank A., Marks P. A. Globin composition and synthesis of hemoglobins in developing fetal mice erythroid cells. Science. 1967 Sep 15;157(3794):1327–1329. doi: 10.1126/science.157.3794.1327. [DOI] [PubMed] [Google Scholar]
- Gilman J. G., Smithies O. Fetal hemoglobin variants in mice. Science. 1968 May 24;160(3830):885–886. doi: 10.1126/science.160.3830.885. [DOI] [PubMed] [Google Scholar]
- Gruber D. F., Zucali J. R., Mirand E. A. Identification of erythropoietin producing cells in fetal mouse liver cultures. Exp Hematol. 1977 Sep;5(5):392–398. [PubMed] [Google Scholar]
- Houssaint E. Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell Differ. 1981 Nov;10(5):243–252. doi: 10.1016/0045-6039(81)90007-5. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Roitsch C. A., Williams N., Guilbert L. J. Molecules stimulating early red cell, granulocyte, macrophage, and megakaryocyte precursors in culture: similarity in size, hydrophobicity, and charge. J Cell Physiol Suppl. 1982;1:65–78. doi: 10.1002/jcp.1041130412. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Nature of cells forming erythroid colonies in agar after stimulation by spleen conditioned medium. J Cell Physiol. 1978 Mar;94(3):243–252. doi: 10.1002/jcp.1040940302. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Moore M. A. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975 Dec 25;258(5537):726–728. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Perah G., Feldman M. In vitro activation of the in vivo colony-forming units of the mouse yolk sac. J Cell Physiol. 1977 May;91(2):193–199. doi: 10.1002/jcp.1040910205. [DOI] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche M. A., Cudennec C. A. Adult hemoglobins are synthesized in yolk sac microenvironment obtained from murine cultured blastocysts. Cell Differ. 1983 Oct;13(2):125–131. doi: 10.1016/0045-6039(83)90104-5. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Clarke B. J., Carr D. H., Chui D. H. Adult hemoglobins are synthesized in erythroid colonies in vitro derived from murine circulating hemopoietic progenitor cells during embryonic development. Proc Natl Acad Sci U S A. 1982 May;79(9):2952–2956. doi: 10.1073/pnas.79.9.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucali J. R., Ulatowski J. A., Mirand E. A. Erythroid progenitor cells and cells capable of producing erythropoietic activity from fetal mouse liver. Exp Hematol. 1980 Sep;8(8):971–979. [PubMed] [Google Scholar]