Abstract
Buprenorphine, a synthetic opioid that acts at both μ and κ opioid receptors, can decrease cocaine use in individuals with opioid addiction. However, the potent agonist action of buprenorphine at μ opioid receptors raises its potential for creating opioid dependence in non–opioid-dependent cocaine abusers. Here, we tested the hypothesis that a combination of buprenorphine and naltrexone (a potent μ opioid antagonist with weaker δ and κ antagonist properties) could block compulsive cocaine self-administration without producing opioid dependence. The effects of buprenorphine and various doses of naltrexone on cocaine self-administration were assessed in rats that self-administered cocaine under conditions of either short access (noncompulsive cocaine seeking) or extended access (compulsive cocaine seeking). Buprenorphine alone reproducibly decreased cocaine self-administration. Although this buprenorphine-alone effect was blocked in a dose-dependent manner by naltrexone in both the short-access and the extended-access groups, the combination of the lowest dose of naltrexone with buprenorphine blocked cocaine self-administration in the extended-access group but not in the short-access group. Rats given this low dose of naltrexone with buprenorphine did not exhibit the physical opioid withdrawal syndrome seen in rats treated with buprenorphine alone, and naltrexone at this dose did not block κ agonist–induced analgesia. The results suggest that the combination of buprenorphine and naltrexone at an appropriate dosage decreases compulsive cocaine self-administration with minimal liability to produce opioid dependence and may be useful as a treatment for cocaine addiction.
INTRODUCTION
Although effective pharmacological treatments for opioid, tobacco, and alcohol dependence have been successfully developed (1–3), no effective medication yet exists for cocaine dependence. Buprenorphine, a potent mixed opioid receptor agonist and antagonist, has been used to treat opioid dependence in humans (4–6) and may have some promise in the treatment of cocaine addiction. In laboratory animals, buprenorphine reduces cocaine self-administration in rhesus monkeys (7) and rats (8, 9) and attenuates cocaine seeking in rats (10, 11). Previous reports also indicated that buprenorphine decreases cocaine craving and use in opioid-dependent humans who were concurrently abusing cocaine (12–14).
Buprenorphine has potent μ opioid receptor partial agonist actions (15) and κ opioid receptor antagonist actions (16). As a result, we hypothesized that the potent κ opioid receptor antagonist action of buprenorphine may decrease compulsive cocaine seeking. However, one major concern with the use of buprenorphine is that its μ agonist action during prolonged treatment may result in opioid dependence in previously non–opioid-dependent cocaine abusers. One way to minimize this effect would be to attenuate the μ action of buprenorphine by coadministering a μ receptor antagonist such as naltrexone. Naltrexone is a highly potent μ opioid antagonist with weaker δ and κ antagonist properties (17).
Buprenorphine is currently used to treat opioid dependence as a maintenance medication (18), so if it is used to treat cocaine abuse and the μ agonist activity is not blocked, it could likely produce opioid dependence in these individuals. Buprenorphine is prescribed with or without the potent opioid antagonist naloxone under the trade names of Suboxone or Subutex, respectively. When Suboxone, which contains naloxone, is taken sublingually (as prescribed), the naloxone is rendered inactive because it is not locally absorbed and is destroyed in the gastrointestinal system. However, if Suboxone is taken via other means (intravenously, smoked, or inhaled), the naloxone remains active and causes opioid withdrawal, typically making the abuse of Suboxone undesirable. Thus, the addition of naloxone to the buprenorphine preparation significantly reduces the likelihood of abuse of Suboxone (19). Naltrexone, in contrast, is orally active.
The rationale for the proposed efficacy of a buprenorphine-naltrexone combination with spared κ antagonistic activity is based on the observations that the dynorphin–κ opioid system is activated during the development of cocaine dependence and that κ antagonists can reverse compulsive cocaine self-administration. Increased levels of κ opioid receptors and the endogenous κ opioid receptor ligand dynorphin are found in the basal ganglia of chronic cocaine abusers (20, 21) and rodents after repeated cocaine administration (22–24). κ Antagonists decrease compulsive cocaine seeking (25). In a rodent model of cocaine self-administration, κ opioid receptor inhibition effectively blocks the increased motivation for cocaine in rats with extended access to the drug without altering cocaine intake in rats with short access (25). κ Receptor antagonists also inhibit the stress-induced reinstatement of cocaine-seeking behavior, and a κ receptor agonist reinstates cocaine-seeking behavior in mice (26).
A conceptual framework that links the activation of the dynorphin–κ opioid system to addiction is that negative emotional states during withdrawal via the recruitment of brain stress–aversive systems drive drug addiction in humans (27–30). Indeed, activation of the κ opioid system produces dysphoric-like, depressive-like, and aversive effects in animals and humans (31–33). Together, these results suggest a role for enhanced κ opioid activity in the negative emotional state that drives compulsive cocaine seeking in cocaine addiction (34, 35) via a negative reinforcement mechanism (that is, increased cocaine use reduces the aversive effects of dynorphin/κ activation) (36).
The present study was designed to determine whether a buprenorphine-naltrexone combination could be found that does not produce opioid dependence yet blocks cocaine self-administration. The studies were undertaken in a rodent model of cocaine self-administration that uses an extended-access procedure known to reflect the compulsivity associated with cocaine addiction (37, 38). We selected the μ opioid receptor antagonist naltrexone because it was designed to be used orally (ReVia, Depade) and is also available in a long-acting, slow-release intramuscular preparation (Vivitrol). A previous study compared the effects of a buprenorphine-naltrexone combination and naltrexone alone on morphine and cocaine use in opioid-dependent humans and found that a significantly lower percentage of subjects had positive urine for morphine and cocaine in the drug combination–treated group than in the naltrexone-alone group at 12 weeks of abstinence (4.5% versus 25% for morphine-positive urine; 9.1% versus 33.3% for cocaine-positive urine) (39). The present data extend and suggest the possibility of using buprenorphine and naltrexone combination for cocaine dependence in non–opioid-dependent humans.
RESULTS
Buprenorphine + naltrexone decreases cocaine self-administration
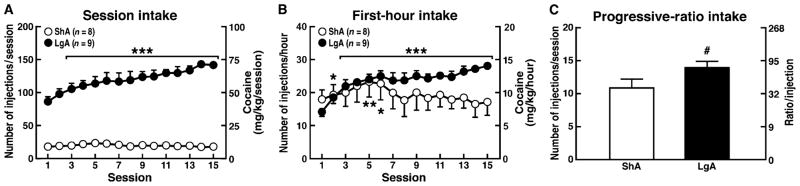
Consistent with previous findings (38, 40), 6-hour extended access to cocaine produced an escalation of cocaine intake in rats during the entire session [defined henceforth as the long-access group (LgA)] and during the first hour of each session (Fig. 1, A and B). Notice a significant escalation of intake from day 3 on. Additionally, LgA rats worked harder for cocaine on the progressive-ratio (PR) schedule (in which each successive injection required more responses so the ratio of responses to rewards increased with each subsequent reward) than did rats with limited 1-hour access [defined henceforth as the short-access group (ShA)] under a PR schedule (Fig. 1C).
Fig. 1.
Cocaine self-administration in rats with short or long access to drug. The rats were allowed to self-administer cocaine (0.5 mg/kg per injection) with either 1-hour (ShA) or 6-hour (LgA) access under an FR schedule. (A) Increase in cocaine intake over the entire 1-hour (ShA) and 6-hour (LgA) sessions. (B) Increase in cocaine over the 1-hour session in ShA rats and during the first hour of the 6-hour session in LgA rats. Cocaine intake significantly increased in the extended-access groups by day 3. (C) Cocaine self-administration under a PR schedule. After 15 sessions under an FR schedule, the number of injections per session was determined under a PR schedule for both groups. A two-way ANOVA revealed the following for the session intake: access × session interaction (F14,210 = 10.5, P < 0.001), main effect of session (F14,210 = 8.6, P < 0.001), and main effect of access (F1,210 = 128, P < 0.001). A two-way ANOVA revealed the following for the first-hour intake: access × session interaction (F14,210 = 5.5, P < 0.001), main effect of session (F14,210 = 4.8, P < 0.001), and main effect of access (F1,210 = 1.3, P > 0.05). Student’s t test revealed the following for the PR intake: t = 2.0, P < 0.05. *P < 0.05, ***P < 0.001, compared with session 1 [(A) and (B)]; #P < 0.05, compared with ShA group (C).
Under a fixed-ratio (FR) schedule, in which one response resulted in one injection, buprenorphine (3 mg/kg) significantly decreased cocaine self-administration in both the ShA and the LgA groups (Fig. 2, A and B). However, the effect of buprenorphine on cocaine self-administration was greater in ShA rats than in LgA rats. The addition of naltrexone dose-dependently reversed the effect of buprenorphine (3 mg/kg) on cocaine self-administration under an FR schedule in both groups. However, in LgA rats but not ShA rats, the lowest dose of naltrexone, 0.3 mg/kg, did not effectively antagonize the effect of buprenorphine in reducing cocaine self-administration. On a PR schedule for cocaine self-administration, buprenorphine (3 mg/kg) again decreased cocaine self-administration in both groups. Significant only in LgA rats, the low dose of naltrexone (0.3 mg/kg) added to buprenorphine again blocked cocaine self-administration produced by buprenorphine under a PR schedule (Fig. 2, C and D). However, the addition of naltrexone (0.3 mg/kg) completely reversed the attenuating effect of buprenorphine on cocaine self-administration in ShA rats. These results suggest that in a measure of compulsive responding for cocaine (extended-access self-administration), buprenorphine retained its ability to block cocaine self-administration in LgA rats despite the addition of naltrexone but not in ShA rats, supporting the hypothesis that the κ antagonist actions of buprenorphine become effective in LgA rats.
Fig. 2.
Effects of buprenorphine + naltrexone on cocaine self-administration in rats under FR and PR schedules. After the determination of baseline cocaine self-administration under a PR schedule, the rats received an injection of buprenorphine (3 mg/kg) plus naltrexone (0, 0.3, 1, 3, and 10 mg/kg) for 2 consecutive days. Cocaine self-administration was assessed under an FR schedule on the first treatment day and under a PR schedule on the second treatment day. The testing sequence of the drug mixtures was counterbalanced across rats. (A and B) Cocaine self-administration in response to buprenorphine + naltrexone, assessed on an FR schedule for the ShA group (1-hour session) (A) and LgA group (first hour) (B). (C and D) Cocaine self-administration in response to buprenorphine + naltrexone on PR responding in both ShA and LgA groups. The data are expressed as a percentage of baseline responding in the baseline FR and PR sessions that immediately preceded the FR and PR test sessions to account for the differences in baseline responding observed in Fig. 1. A two-way ANOVA revealed the following for the FR data (A and B): access × naltrexone interaction (F5,75 = 2.5, P < 0.05), main effect of access (F1,75 = 0.01, P > 0.05), and main effect of naltrexone (F5,75 = 11.4, P < 0.001). A two-way ANOVA revealed the following for the PR data (C and D): access × naltrexone interaction (F5,75 = 3.1, P < 0.05), main effect of access (F1,75 = 1.5, P > 0.05), and main effect of naltrexone (F5,75 = 6.7, P < 0.001). For the effects of buprenorphine-naltrexone on cocaine self-administration compared with vehicle with a given access group, the Bonferroni post hoc test yielded *P < 0.05, **P < 0.01, and ***P < 0.001 compared with vehicle treatment. For the effects of buprenorphine on cocaine self-administration between the ShA and the LgA groups, the Bonferroni post hoc test yielded P < 0.05.
Repeated administration of buprenorphine + naltrexone does not produce dependence
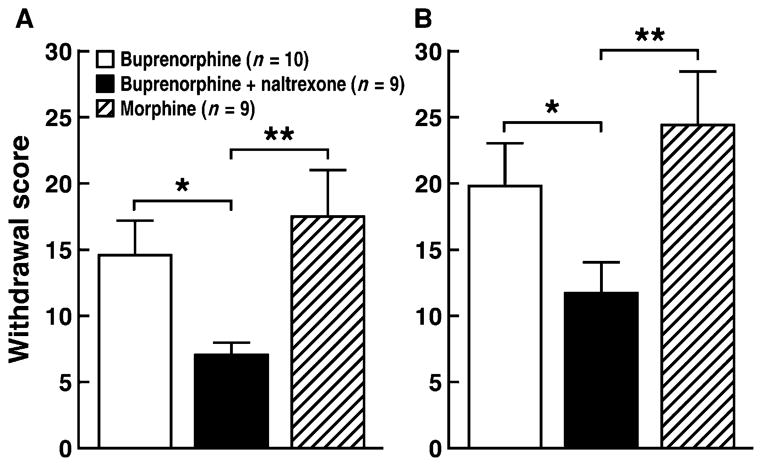
An important question is whether the combination of naltrexone and buprenorphine at a dose that effectively reduces cocaine self-administration [naltrexone (0.3 mg/kg); Fig. 2, B and D] can produce dependence, given that buprenorphine treatment produces dependence in humans and animal models (41–44). Daily treatment with buprenorphine (3 and 6 mg/kg) (3 mg/kg twice per day) for 5 days produced dependence, as measured by naloxone-precipitated withdrawal symptoms, to a similar degree to morphine (10 and 20 mg/kg) (10 mg/kg twice per day; Fig. 3). However, naloxone elicited markedly fewer withdrawal signs in the group that received the buprenorphine-naltrexone combination [buprenorphine (3 mg/kg) plus naltrexone (0.3 mg/kg)] than in the group that received buprenorphine alone or morphine.
Fig. 3.
Naloxone-precipitated withdrawal in rats. (A) Animals were treated once daily with buprenorphine, buprenorphine + naltrexone, or morphine. (B) Animals were treated twice daily with buprenorphine, buprenorphine + naltrexone, or morphine. The rats were given a naloxone challenge injection (1 mg/kg, sc) 4 hours after the last injection of buprenorphine (3 mg/kg), buprenorphine (3 mg/kg) + naltrexone (0.3 mg/kg), or morphine (10 mg/kg), and the somatic signs of withdrawal were evaluated for 10 min. The withdrawal score was based on several signs of somatic withdrawal (see Materials and Methods). A two-way ANOVA revealed the following: treatment × buprenorphine dose interaction (F2,25 = 0.2, P > 0.05), main effect of treatment (F2,25 = 4.5, P < 0.05), and main effect of buprenorphine dose (F1,25 = 15.6, P < 0.001). *P ≤ 0.05, **P ≤ 0.001, significantly different from buprenorphine + naltrexone. n = 9 to 10.
Naltrexone does not block κ opioid analgesia at doses that blocked cocaine self-administration
Treatment with naltrexone (0.3 and 3.0 mg/kg) blocked heroin-induced analgesia measured by the tail flick response and partially blocked buprenorphine analgesia but failed to block analgesia produced by the κ opioid agonist U50,488 (Table 1).
Table 1.
Antinociceptive effect of naltrexone.
| Treatment | Naltrexone pretreatment (% maximal possible inhibition)
|
||
|---|---|---|---|
| 0 mg/kg | 0.3 mg/kg | 3.0 mg/kg | |
| Heroin (1 mg/kg)* | 100 ± 0 | 4 ± 3† | 8 ± 4† |
| Buprenorphine (3 mg/kg)* | 82 ± 15 | 20 ± 9† | 22 ± 6† |
| U50,488 (50 mg/kg)‡ | 54 ± 11 | 51 ± 4 | 49 ± 7 |
Maximal effect at 20 min.
P < 0.001, compared with controls (0 mg/kg).
Maximal effect at 40 min.
DISCUSSION
Our overall hypothesis is that compulsive cocaine intake is driven in part by negative emotional states during withdrawal, mediated by increased activity of the κ opioid system, and that attenuation of κ opioid activity by buprenorphine can limit compulsive cocaine seeking and use in cocaine addiction. However, considering the potent μ opioid agonist action of buprenorphine, we specifically hypothesized that an appropriate dose of naltrex-one added to buprenorphine (that is, a naltrexone dose that selectively inhibits the μ agonistic action of buprenorphine) would leave the κ antagonist action intact, thus allowing limitation of cocaine intake while simultaneously minimizing the liability of buprenorphine to produce opioid dependence.
Our results supported the hypothesis that a combination of buprenorphine and low-dose naltrexone is sufficient to prevent the dependence potential of buprenorphine without interfering with the ability of buprenorphine to decrease compulsive cocaine self-administration. Consistent with the literature (25, 38), in our study, rats with extended access to the drug exhibited increased cocaine intake and increased motivation to take cocaine, as measured by their responses on a PR schedule compared to rats with short-term access to cocaine. Previous studies showed that this increase in cocaine intake in rats with extended access is accompanied by an increased dysphoric-like state (that is, increased thresholds for brain stimulation reward) and an anxiety-like state (45, 46). Such negative emotional states have been hypothesized to increase cocaine intake and to supply the motivation for cocaine in rats with extended access via negative reinforcement mechanisms in which the animal escalates drug intake to reduce the negative emotional state of withdrawal (47).
Buprenorphine significantly decreased cocaine self-administration under FR and PR schedules in both ShA and extended-access rats, consistent with the results of previous studies (8, 9, 48). Buprenorphine reduces cocaine self-administration in rats, nonhuman primates, and human drug abusers (7–9, 12–14, 49). Nonetheless, the effect of buprenorphine alone on cocaine self-administration was greater in the ShA group than in the extended-access group with both FR and PR schedules, suggesting a more important role for the μ opioid actions of buprenorphine in the ShA group. Indeed, it has been suggested that the buprenorphine-induced decrease in cocaine intake in rhesus monkeys occurs via its μ opioid agonistic action because naltrexone attenuated the buprenorphine-induced decrease in cocaine intake (50). Here, the buprenorphine-induced decrease in cocaine self-administration was blocked by naltrexone in a dose-dependent fashion in both the ShA and the extended-access groups, but the dose-effect functions were shifted to the right in the extended-access group. This suggests that there is less of a role for naltrexone-sensitive μ opioid activation in buprenorphine’s suppression of extended-access cocaine self-administration and more of a role for κ antagonist action that is not blocked by naltrexone. Indeed, at a low dose (0.3 mg/kg), naltrexone only blocked the effects of buprenorphine on cocaine self-administration in ShA rats and not in extended-access rats. The low dose of naltrexone (0.3 mg/kg) that did not alter the effects of buprenorphine on cocaine intake in extended-access rats prevented the buprenorphine-associated opioid withdrawal syndrome, demonstrating that this dose of naltrexone effectively blocked any dependence liability of buprenorphine and, by extrapolation, effectively blocked μ opioid receptors. Moreover, this low dose of naltrexone (0.3 mg/kg) did not alter κ agonist–induced analgesia, indicating that naltrexone itself was not conveying κ agonist or antagonist activity and supporting our hypothesis that the κ antagonist action of buprenorphine was spared by the combination of buprenorphine and low-dose naltrexone.
We tested the effects of the buprenorphine-naltrexone combination on cocaine intake under both an FR schedule (one response resulted in one injection) and a PR schedule (each successive injection required more responses so the ratio of responses to reward increased with each subsequent reward). Although the FR schedule allowed the measurement of the effects of the drug combination on general cocaine intake, the PR schedule measured a more motivational aspect of cocaine use or the compulsive nature of cocaine seeking by imposing a greater amount of work or adversity for each subsequent cocaine reward. That is, the PR schedule measures the amount of work that the animal is willing to do for cocaine. The data suggested that the combination of buprenorphine with low-dose naltrexone attenuated cocaine intake and the motivation for cocaine in extended-access rats (which serves as a rodent model of compulsive cocaine seeking) without the development of opioid dependence.
Buprenorphine has similar affinities for μ and κ opioid receptors, whereas naltrexone is a μ receptor–preferring antagonist (Table 2). Therefore, the observation that the effect of buprenorphine on cocaine self-administration was more sensitive to naltrexone in ShA rats than extended-access rats suggests that the μ agonist actions of buprenorphine may play a greater role in suppressing the acute positive reinforcing effects of cocaine than in blocking the negative reinforcing effects of cocaine (that is, compulsive cocaine intake). Thus, the hypothesis is that the μ agonist action of buprenorphine mediates the buprenorphine-induced decrease in cocaine intake in ShA rats and other animal models with limited access to drug, in which there is no escalation in intake, as opposed to extended-access cocaine self-administration, in which there is an escalation of intake (50). Consistent with this hypothesis, other μ receptor agonists decrease cocaine self-administration in monkeys (51).
Table 2.
Comparison of opioid receptor binding affinities of buprenorphine and naltrexone. Ki, inhibition constant.
Previous results regarding the ability of naltrexone alone and κ agonists to reduce cocaine self-administration have been mixed or hypothesized to reflect nonspecific effects. Naltrexone reduced the acquisition of cocaine self-administration and low-dose cocaine self-administration in rats (52, 53), but it failed to alter established cocaine self-administration in other studies (54, 55). Similarly, intravenously administered naltrexone decreased cocaine self-administration in non-human primates in one study (56), but it had no effect in another study (50). Additionally, κ receptor agonists can acutely decrease cocaine self-administration and reward (57–59), although, as noted above, activation of the κ opioid system produces dysphoric-like, depressive-like, and aversive effects (31–33). Thus, these effects of κ agonists in nondependent animals have been hypothesized to result from a suppression of reward in general (60).
A major concern with the use of buprenorphine for the treatment of cocaine addiction in humans is the dependence liability of buprenorphine in cocaine-dependent subjects who are not opioid-dependent. Indeed, daily buprenorphine treatment produces dependence, as measured by precipitated withdrawal symptoms, in humans (41, 42), monkeys (43), and rats (44). Here, animals repeatedly injected with buprenorphine alone showed substantial signs of physical opioid dependence when withdrawal was precipitated by naloxone. The dependence was as robust as that produced by moderate to high doses of morphine. However, repeated injections of the buprenorphine-naltrexone combination that successfully reduced the motivation for cocaine in extended-access rats produced very few signs of opioid withdrawal. Furthermore, the same lack of dependence was observed when the treatment doses were increased to two injections per day. A previous study described the inhibition of μ-mediated, buprenorphine-induced antinociception by concomitant naltrexone treatment in mice (61), corroborating our findings. Therefore, these results suggest that the buprenorphine-naltrexone combination may be a viable pharmacological approach for the treatment of cocaine addiction. Indeed, sublingual buprenorphine administration in humans at a daily dose of 16 mg was well tolerated and effectively reduced concomitant opioid and cocaine use, demonstrating that buprenorphine has efficacy in reducing cocaine use (14).
In summary, a combination of the drugs buprenorphine and naltrexone at an appropriate dosage selectively decreased compulsive cocaine seeking and intake and had minimal potential for producing opioid dependence.
MATERIALS AND METHODS
All of the procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Animals
Male Wistar rats (n = 45; Charles River), weighing 225 to 250 g at the beginning of the study, were used in all of the experiments. They were housed in groups of three in plastic cages with a 12-hour/12-hour light/dark cycle with lights off at 8:00 a.m. Food and water were available ad libitum throughout the study, except during behavioral testing. Experimental sessions were conducted once per day during the dark (active) cycle.
Effects of a buprenorphine-naltrexone combination on cocaine self-administration
Apparatus
A standard operant chamber for intravenous self-administration was placed in a light- and sound-attenuating cubicle (28 cm × 26 cm × 20 cm; Med Associates). The chamber had two retractable response levers mounted on one side of the opaque walls. A stimulus light was mounted above each lever. A cocaine injection (0.5 mg/kg per injection) was delivered by a syringe pump (Razel Scientific Instruments) located on top of the cubicle. The experimental sessions were controlled and recorded by a computer with a custom interface and software in the experimental room. The start of a session was signaled by the extension of two response levers into the chamber. Responding on the right lever resulted in the delivery of 0.1 ml of cocaine solution over 4 s. During an injection, a stimulus light above the active lever was illuminated and lasted throughout a 20-s timeout period that followed each injection when additional lever presses did not result in a drug infusion. Pressing the left lever was counted but had no other programmed consequences. No priming injection was used in this paradigm.
Procedure
The rats (n = 17) were prepared with indwelling catheters (0.3-mm inner diameter, 0.64-mm outer diameter; Dow Corning) into the right external jugular vein and trained daily to self-administer cocaine for 1 hour under an FR schedule. The self-administration sessions ran 7 days per week. When the rats maintained stable responding (≤10% variation of the running mean of the number of injections) for at least three consecutive sessions, they were divided into two groups balanced by the number of injections per session during the last three sessions. For 15 sessions under an FR schedule, the rats in the ShA group (n = 8) were allowed to self-administer cocaine in 1-hour sessions, whereas rats in the LgA group (n = 9) were allowed to self-administer cocaine in 6-hour sessions (escalation period).
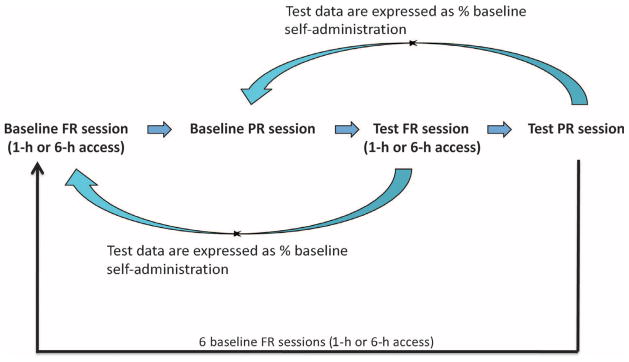
After cocaine self-administration increased and reached a maximum in LgA rats under an FR schedule, cocaine self-administration under a PR schedule was determined in both groups in two sessions per week that were alternated by one FR cocaine session with 1- or 6-hour access. The rationale for conducting both the FR and the PR sessions was to include PR responding, another measure of the motivation for cocaine during compulsive drug taking (25). Previous work has shown that this procedure produces stable and reliable responding with repeated testing in control groups (25). After baseline cocaine intake under a PR schedule was determined, daily treatment with a buprenorphine-naltrexone combination or vehicle began in both the ShA and the LgA groups, and the effect of the treatment on cocaine self-administration was assessed. Five combinations of doses of buprenorphine and naltrexone (Table 3) were chosen on the basis of the literature and the potency of the drugs at μ receptors (62). Buprenorphine, at a dose of 3 mg/kg, decreases cocaine intake under FR and PR schedules in rats (8). Therefore, using buprenorphine at a dose of 3 mg/kg, we identified a dose of naltrexone that did not alter the effects of buprenorphine on cocaine intake. Each combination of drugs was tested in two consecutive sessions (one FR session and one PR session) and seven baseline sessions (1 or 6 hours) under an FR schedule without any treatment, followed by two test sessions. Before each test session, one PR session without any previous treatment served as baseline for the following PR test session (Fig. 4). The testing sequence of the drug combinations was counterbalanced across rats according to a Latin square design (Table 3). The effect of buprenorphine on cocaine self-administration was apparent during the first hour of the 6-hour FR test session and wore off within 6 hours of the FR test session; therefore, the efficacy of the dose of buprenorphine (3 mg/kg) did not last 6 hours in rats. Additionally, we did not observe any carryover effects of buprenorphine in the baseline FR session (1- or 6-hour sessions) that immediately followed the test PR sessions.
Table 3.
Dose combinations of buprenorphine and naltrexone.
| Buprenorphine (mg/kg) | Naltrexone (mg/kg) | |
|---|---|---|
| Mixture 1 | 0 | 0 |
| Mixture 2 | 3 | 0 |
| Mixture 3 | 3 | 0.3 |
| Mixture 4 | 3 | 1 |
| Mixture 5 | 3 | 3 |
Fig. 4.
Schematic presentation of a test sequence to measure the effect of the buprenorphine + naltrexone combination on cocaine self-administration. After cocaine self-administration increased and reached a maximum in LgA rats under an FR schedule, daily treatment with a buprenorphine + naltrexone combination or vehicle began in both the ShA and the LgA groups, and the effects of the treatment on cocaine self-administration were determined. Each combination of the drugs was tested in two consecutive sessions (one FR session and one PR session), with seven regular sessions (1 or 6 hours) under an FR schedule without any treatment followed by two test sessions for each combination. Before each test session, one PR session without any treatment served as the baseline for the following PR test session.
Data analysis
The cocaine self-administration data are expressed as the mean number of injections and milligrams per kilogram for each group of rats. For the tests of the effects of the buprenorphine-naltrexone combination, the numbers of injections in the FR and PR test sessions were normalized as percentages of the number of injections in the last baseline FR and PR sessions without any treatment that immediately preceded the test sessions. Such a transformation is necessary to make direct comparisons of the ShA and LgA interaction given that their baseline responding was markedly different after escalation, as we have previously observed. The data were compared with two-way repeated-measures analysis of variance (ANOVA) followed by the appropriate Bonferroni post hoc test for individual means comparisons, where there was an access × treatment interaction (Prism 5.0, GraphPad).
Measurement of naloxone-precipitated withdrawal
Procedure
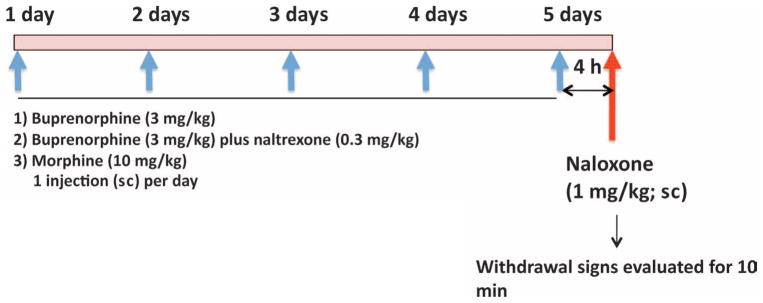
To evaluate whether buprenorphine (3 mg/kg) in conjunction with naltrexone (0.3 mg/kg) once per day for 5 days produces signs of opioid dependence, we measured naloxone-precipitated withdrawal scores with a modified Gellert and Holtzman (63) scale of somatic opiate withdrawal (64). Three separate groups of Wistar rats (n = 28) were given daily subcutaneous injections of the following drugs for 5 consecutive days: (i) buprenorphine alone (3 mg/kg), (ii) buprenorphine (3 mg/kg) + naltrexone (0.3 mg/kg), or (iii) morphine (10 mg/kg). On the test day, the rats were given a challenge dose of naloxone [1 mg/kg, subcutaneously (sc)] 4 hours after the last injection of buprenorphine, buprenorphine + naltrexone, or morphine and immediately placed in a 1-ft3 Plexiglas box for the observation of somatic opiate withdrawal signs (Fig. 5). Two classes of withdrawal signs were measured for 10 min: graded signs (body weight loss in 60 min, escape attempts, wet dog shakes, and abdominal constrictions) and checked signs (defecation/diarrhea, teeth chattering, swallowing movements, salivation, ptosis, penile erection/ejaculation/grooming, hyperirritability upon touch, and abnormal posture). Each withdrawal sign was assigned a multiplier on the basis of a weighted measure of somatic opioid withdrawal (Table 4). After this first experiment, the animals were left undisturbed for 1 week and then treated twice per day (instead of once per day) with the same dosages as described above. The effects of the treatment on naloxone-precipitated withdrawal were determined on the fifth day, 4 hours after the first of the two treatment injections [that is, the rats received a total of nine treatment injections plus a challenge injection with naloxone (1 mg/kg) on the last day; see Fig. 5].
Fig. 5.
Schematic presentation of the measurement of naloxone-precipitated withdrawal in rats treated with the buprenorphine + naltrexone combination. Three separate groups of Wistar rats were given daily subcutaneous injections of the following drugs for 5 consecutive days: buprenorphine alone (3 mg/kg), buprenorphine + naltrexone (3 mg/kg + 0.3 mg/kg), or morphine (10 mg/kg). On the test day, the rats were given a challenge injection of naloxone (1 mg/kg, sc) 4 hours after the last drug treatment and immediately placed in a box for the observation of somatic opiate withdrawal.
Table 4.
Summary of somatic withdrawal rating scale.
| Withdrawal sign | Points assigned |
|---|---|
| Body weight loss in 60 min (per each 1%) | 1 |
| Wet dog shakes | |
| 1–2 | 2 |
| 3+ | 4 |
| Jump attempts | |
| 2–4 | 1 |
| 5–9 | 2 |
| 10+ | 3 |
| Abdominal spasms (per observation) | 1 |
| Penile erection/ejaculation/grooming | 3 |
| Teeth chattering | 2 |
| Irritability/vocalization | 3 |
| Swallowing movements | 2 |
| Ptosis (drooping eyes) | 2 |
| Abnormal posture | 3 |
| Defecation/diarrhea | 2 |
| Profuse salivation | 7 |
Data analysis
The data are expressed as means and SEM. Opioid withdrawal scores were compared with repeated-measures ANOVA with treatment (buprenorphine × buprenorphine + naltrexone × morphine) as the between-subjects factor and dose (one injection per day × two injections per day) as the within-subjects factor. Fisher’s least significant difference post hoc test was used for comparisons of the means. The accepted level of significance for all of the tests was P ≤ 0.05.
Tail immersion antinociception test
The rats were acclimated to the test room 30 min before testing. Baseline measures were then taken for their latency to withdrawal of the terminal 4 cm of their tail from a 55 ± 0.5°C heated water bath. The rats were wrapped in towels and had their tails first dipped in room temperature water to prevent premature responses and then in heated water, and immersion was timed with a stopwatch by a separate, treatment-blinded observer. A baseline of 2 to 4 s was considered normal, and a cutoff of 10 s was used to prevent tissue damage. Immediately after the baseline measures, the rats received an injection of naltrexone, followed by a test opioid drug [heroin (1 mg/kg), buprenorphine (3 mg/kg), or U50,488 (50 mg/kg)] 5 min later. Tail withdrawal latencies were measured every 20 min until maximal drug effects were reached and subsided, typically by 60 min. The rats were allowed at least a 3-day washout period between drug tests, and no animal received the same dose of naltrexone more than once.
Data analysis
The percent maximal possible effect (% MPE) was calculated as follows: 100 × [(test − baseline)/(10 − baseline)]. The data were analyzed with repeated-measures ANOVA followed by Dunnett’s post hoc comparisons.
Acknowledgments
We thank N. D. Volkow for valuable comments on the design of the study, F. E. Bloom for cogent comments on the manuscript, Y. Grant for her excellent technical assistance, and M. Arends for editorial assistance. We also thank A. Azizoddin and C. Muhlfeld, undergraduate student interns from the University of California, San Diego, and San Diego State University, respectively, for assistance with testing the animals in cocaine self-administration. This is publication number 21020 from The Scripps Research Institute.
Funding: This study was supported by National Institute on Drug Abuse grants DA004398 (G.F.K.) and DA025785 (S.W.) and the Pearson Center for Alcoholism and Addiction Research.
Footnotes
Author contributions: All of the authors contributed to the experimental design and data analysis. Additionally, S.W. conducted the cocaine self-administration experiment and wrote the manuscript. L.F.V. and K.K.M. performed the naloxone-precipitated withdrawal test and helped with the preparation of the manuscript. J.E.S. performed the tail immersion antinociception test and helped with the preparation of the manuscript. G.F.K. helped design the study and prepare the manuscript.
Competing interests: G.F.K. is a consultant for Alkermes and Alkeo. The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Kakko J, Grönbladh L, Svanborg KD, von Wachenfeldt J, Rück C, Rawlings B, Nilsson LH, Heilig M. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: A randomized controlled trial. Am J Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- 2.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Burke MV, Ebbert JO, Hays JT. Treatment of tobacco dependence. Mayo Clin Proc. 2008;83:479–483. doi: 10.4065/83.4.479. [DOI] [PubMed] [Google Scholar]
- 4.Kleber HD. Pharmacologic treatments for heroin and cocaine dependence. Am J Addict. 2003;12(Suppl 2):S5–S18. [PubMed] [Google Scholar]
- 5.Kosten TR. Current pharmacotherapies for opioid dependence. Psychopharmacol Bull. 1990;26:69–74. [PubMed] [Google Scholar]
- 6.Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- 7.Mello NK, Lukas SE, Kamien JB, Mendelson JH, Drieze J, Cone EJ. The effects of chronic buprenorphine treatment on cocaine and food self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1992;260:1185–1193. [PubMed] [Google Scholar]
- 8.Sorge RE, Stewart J. The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology. 2006;188:28–41. doi: 10.1007/s00213-006-0485-1. [DOI] [PubMed] [Google Scholar]
- 9.Carroll ME, Lac ST. Effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology. 1992;106:439–446. doi: 10.1007/BF02244812. [DOI] [PubMed] [Google Scholar]
- 10.Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- 11.Comer SD, Lac ST, Curtis LK, Carroll ME. Effects of buprenorphine and naltrexone on reinstatement of cocaine-reinforced responding in rats. J Pharmacol Exp Ther. 1993;267:1470–1477. [PubMed] [Google Scholar]
- 12.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 13.Foltin RW, Fischman MW. Effects of methadone or buprenorphine maintenance on the subjective and reinforcing effects of intravenous cocaine in humans. J Pharmacol Exp Ther. 1996;278:1153–1164. [PubMed] [Google Scholar]
- 14.Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero DV, Partilla JS, Zheng QX, Heyliger SO, Ni Q, Rice KC, Lai J, Rothman RB. Opioid peptide receptor studies. 12. Buprenorphine is a potent and selective μ/κ antagonist in the [35S]-GTP-γ-S functional binding assay. Synapse. 1999;34:83–94. doi: 10.1002/(SICI)1098-2396(199911)34:2<83::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- 18.Kraus ML, Alford DP, Kotz MM, Levounis P, Mandell TW, Meyer M, Salsitz EA, Wetterau N, Wyatt SA. Statement of the American Society of Addiction Medicine consensus panel on the use of buprenorphine in office-based treatment of opioid addiction. J Addict Med. 2011;5:254–263. doi: 10.1097/ADM.0b013e3182312983. [DOI] [PubMed] [Google Scholar]
- 19.Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, Saccone P, Kleber HD. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin users. Addiction. 2010;105:709–718. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 22.Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 24.Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extra-pyramidal and limbic dynorphin systems. J Pharmacol Exp Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- 25.Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 29.Goeders NE. Potential involvement of anxiety in the neurobiology of cocaine. Ann N Y Acad Sci. 1992;654:357–367. doi: 10.1111/j.1749-6632.1992.tb25981.x. [DOI] [PubMed] [Google Scholar]
- 30.Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- 31.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 33.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The κ-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 35.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 36.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 37.Wee S, Mandyam CD, Lekic DM, Koob GF. α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 39.Gerra G, Fantoma A, Zaimovic A. Naltrexone and buprenorphine combination in the treatment of opioid dependence. J Psychopharmacol. 2006;20:806–814. doi: 10.1177/0269881106060835. [DOI] [PubMed] [Google Scholar]
- 40.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- 41.Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine’s physical dependence potential: Antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther. 1996;276:449–459. [PubMed] [Google Scholar]
- 42.Kosten TR, Krystal JH, Charney DS, Price LH, Morgan CH, Kleber HD. Opioid antagonist challenges in buprenorphine maintained patients. Drug Alcohol Depend. 1990;25:73–78. doi: 10.1016/0376-8716(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 43.Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. J Pharmacol Exp Ther. 2011;336:488–495. doi: 10.1124/jpet.110.173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glover EM, Davis M. Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: Tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology. 2008;198:167–180. doi: 10.1007/s00213-008-1112-0. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 46.Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- 47.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 48.Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on i.v. cocaine self-administration in rats maintained under FR schedules. Psychopharmacology. 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- 49.Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine suppresses cocaine self-administration by rhesus monkeys. Science. 1989;245:859–862. doi: 10.1126/science.2772637. [DOI] [PubMed] [Google Scholar]
- 50.Mello NK, Lukas SE, Mendelson JH, Drieze J. Naltrexone-buprenorphine interactions: Effects on cocaine self-administration. Neuropsychopharmacology. 1993;9:211–224. doi: 10.1038/npp.1993.57. [DOI] [PubMed] [Google Scholar]
- 51.Negus SS, Mello NK. Effects of μ-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: Role of μ-agonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- 52.Ramsey NF, van Ree JM. Intracerebroventricular naltrexone treatment attenuates acquisition of intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1991;40:807–810. doi: 10.1016/0091-3057(91)90090-o. [DOI] [PubMed] [Google Scholar]
- 53.Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology. 1991;104:167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- 54.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: Mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- 55.Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- 56.Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1990;254:926–939. [PubMed] [Google Scholar]
- 57.Glick SD, Visker KE, Maisonneuve IM. Effects of cyclazocine on cocaine self-administration in rats. Eur J Pharmacol. 1998;357:9–14. doi: 10.1016/s0014-2999(98)00548-2. [DOI] [PubMed] [Google Scholar]
- 58.Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of mixed-action κ/μ opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- 59.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of κ-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 60.Wee S, Koob GF. The role of the dynorphin–κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kögel B, Christoph T, Strassburger W, Friderichs E. Interaction of μ-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in mice. Eur J Pain. 2005;9:599–611. doi: 10.1016/j.ejpain.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- 63.Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- 64.Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–242. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Villiger JW, Taylor KM. Buprenorphine: Characteristics of binding sites in the rat central nervous system. Life Sci. 1981;29:2699–2708. doi: 10.1016/0024-3205(81)90529-4. [DOI] [PubMed] [Google Scholar]