Abstract
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) is usually an incidental diagnosis in patients with early–intermediate stage disease. However, most patients with a diagnosis of CLL will subsequently have significant morbidity and die from their disease and its complications. For these patients, CLL is not the ‘good leukemia’ with a predictably ‘benign’ outcome. Indeed, we can now identify a cohort of patients with high-risk CLL at diagnosis who will have rapid disease progression, poor response to treatment, and poor survival based on prognostic methods developed from an improved understanding of the biology of CLL. The concomitant development of improved treatments has led to risk-adjusted management approaches that could improve outcomes. We discuss the clinical and laboratory components of comprehensive risk evaluation of patients with CLL and our approach to the management of patients with a high to very high risk of disease progression and poor outcome. In addition, we review the challenges and prospects for improving prognostic precision and the development of new drugs to improve the treatment of patients with CLL with a high risk of adverse outcome.
Keywords: Chronic lymphocytic leukemia, CLL, SLL, high risk, therapy, risk stratified management
Introduction
Although the variability of the course of chronic lymphocytic leukemia (CLL) has been recognized for decades, high-risk CLL is a more recent clinical concept. The concept of high risk of adverse outcome in patients with CLL is based on: (a) the recognition that patients with CLL with treatment refractory progressive disease or a short duration of response to treatment have a poor prognosis, (b) the ability to predict poor prognosis at diagnosis, and (c) the recognition that comorbidity, poor organ function, and poor performance status can limit management options and worsen outcome in patients with CLL. Patients with high-risk disease require more careful active monitoring than patients with low- to intermediate-risk CLL, and can benefit from risk stratified management (Table I).
Table I.
Key points in the management of high-risk CLL.
| 1. | Accurate diagnosis of CLL |
| 2. | Determine disease burden and etiology of cytopenias |
| 3. | Evaluate TP53 pathway integrity |
| 4. | Active monitoring for early detection of disease progression and complications |
| 5. | Reevaluate CLL risk before any treatment |
| 6. | Use risk stratified treatment of progressive disease |
| 7. | Early consideration of RIC allotransplant in patients with TP53 defective or purine analog refractory CLL |
CLL, chronic lymphocytic leukemia; RIC allotransplant, allogeneic hematopoietic stem cell transplant from matched donor using a reduced-intensity conditioning regimen.
CLL is the most prevalent hematological malignancy in the USA, and the *25% of patients with high-risk disease are an important part of the practice of hematology. The estimated annual incidence of CLL (including small lymphocytic lymphoma [SLL]) is 5.5:100 000 resulting in over 15 000 new diagnoses per year, with a median age at diagnosis of 72 years [1] and a median age at treatment in the late 70s. There are multiple parameters other than individual prognostic factors (discussed below) that can influence the course of all patients with CLL. Advanced age and poor biological fitness, defined as impaired physical fitness and organ function, considerably increase the risk for adverse consequences of progressive disease and contribute to the decreased survival for patients with CLL compared to the age-matched population [2]. Although the extent of age-related risk is still not fully defined, it is important to consider that the median age of patients with CLL enrolled in most clinical trials is about 60 years, and results are thus often difficult to generalize to the average patient with CLL. Gender is an additional consideration in risk evaluation in the patient with CLL. CLL is a male-predominant disease (1.3–1.5:1) [2,3], although the relative rate in females does increase with age [2]. Females tend to have a better prognosis than males, although the reason for this difference is not known [2]. Thus, age and gender need to be considered in risk evaluation of patients with CLL.
CLL is associated with an increased risk of serious complications including autoimmune cytopenia, infections, and second malignancies. Cytopenias secondary to autoimmune disease do not have the same serious prognostic implications as cytopenia secondary to bone marrow failure, but the independent implications of autoimmune complications on CLL risk are still not fully defined [4–7]. Patients with CLL can have profound immunodeficiency early in the course of their disease prior to any treatment. The first detectable defect is usually hypogammaglobulinemia, which increases the risk of infection by encapsulated organisms [8]. Progressive disease and treatment are associated with additional defects in cellular immunity that result in an increased risk of opportunistic infections [8]. The risk of second malignancies is markedly increased in patients with CLL. In addition to the well-described risk of transformation of CLL to clonally related diffuse large B-cell lymphoma, CLL is also associated with a significant increase in the risk of development of clonally unrelated hematopoietic, skin, and solid tumors, which can have an adverse effect on outcome for these patients [9,10]. Melanoma and non-melanoma skin cancers are a particular concern because they occur at a considerably higher frequency. Non-melanoma skin cancers tend to be more locally aggressive and have a higher risk of metastases even in patients with earlier stage and untreated CLL [11]. Patients with CLL should thus all be considered at increased risk for these complications and require monitoring during all phases of the disease with appropriate preventive measures (e.g. sun protection and vaccinations) and surveillance (annual skin and primary health examinations).
Evaluation of individual risk in patients with chronic lymphocytic leukemia
Patients with CLL can be evaluated for biological predictors of risk of disease progression, poor response to treatment, complications, and death using clinical and laboratory testing at diagnosis and then throughout the course of their disease. Ongoing reevaluation is essential, because changes in CLL biology and the health of the patient can profoundly influence these risks. Risk evaluation is increasingly valuable in planning overall management strategy and treatment interventions, and can help patients and their families adapt to the disease and its consequences. However, despite considerable improvements over the past two decades, the accuracy of predictive methods can still be limited for a given individual, and thus definitions of risk are both non-standard and in constant flux due to continuous improvements in methods and knowledge. For the purposes of this discussion, high risk in an individual patient with CLL is defined as disease that has a high probability of early progression, poor response to treatment, or considerable morbidity and mortality from CLL and its complications.
Risk stratification
CLL risk stratification began with the staging systems initially described by Rai et al. [12] and validated by Binet et al. [13]. The characterization of molecular markers of risk of disease progression and poor prognosis in CLL cells subsequently provided important new tools for predicting the natural history of early–intermediate stage CLL at diagnosis [14–16]. The current challenge is to implement this knowledge, which is constantly being refined and expanded, into the daily care of patients with CLL throughout the course of their disease.
Prognosis at diagnosis
All patients with newly diagnosed CLL are likely to benefit from a comprehensive risk evaluation. This includes confirmation of the diagnosis of CLL, clinical staging, evaluation of biological fitness and comorbidities, and measurement of appropriate molecular prognostic markers. Advanced clinical stage is defined as cytopenia caused by progressive CLL, and this should be confirmed by clinical evaluation and a bone marrow study to exclude other causes of cytopenia, including autoimmune complications of CLL [6]. The small percentage (<10%) of patients with CLL with bone marrow failure at diagnosis have a significantly poorer survival than other patients with CLL (median 6.2 years vs. 9.7 years [6], median 3.7 years vs. 9 years [7]). Of note, patients with cytopenia caused by autoimmune complications (5–10% of patients with CLL in the course of their disease) have considerably better survival and should not be considered to have high-risk CLL based on cytopenia alone [4–7]. Clinical staging also provides a measure of tumor burden based on the degree of adenopathy and visceromegaly detectable on clinical examination. Patients with early stage disease (Rai 0, Binet A) have a better prognosis than patients with intermediate stage disease (Rai I–II, Binet B) [12,13]. However, because the rate of disease progression cannot usually be determined at diagnosis, the determination of tumor burden is not fully informative of the biology of the disease and, hence, risk of progression especially in patients with early–intermediate clinical stage. In these patients, molecular markers can be especially helpful in determining disease risk.
Patients with newly diagnosed CLL are likely to benefit from the most comprehensive molecular biological evaluation of their disease that can be performed. The following tests are very helpful for assessment and counseling when you first see a patient with CLL.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) for known chromosome abnormalities is the single most useful clinical test for evaluation of clinical risk in patients with CLL [16]. Approximately 5–10% of newly diagnosed patients with CLL have 17p13 deletion (17p—) resulting in the loss of one allele of TP53 (tumor protein p53) [16,17]. This is usually associated with a dysfunctional mutation in the remaining TP53 allele, a short time to disease progression, poor response to conventional chemoimmunotherapy (CIT), and poor overall survival [18,19]. An 11q22 deletion (11q—) resulting in the loss of one allele of ATM (ataxia telangiectasia mutated) was initially reported as more common in younger males with bulky CLL and a short time to treatment [20], and is associated with an increased risk of clonal evolution [21] and poorer survival [16]. In contrast, patients in whom deletion of 13q14 (13q—) is the sole genetic abnormality on FISH analysis have a lower risk of CLL progression [16,17].
IGHV
Somatic hypermutation of the B cell receptor (BCR) variable region is a physiological event during antigen driven maturation of B cells in secondary lymphoid tissue. The extent of somatic hypermutation can be measured by comparing the clonal immungolobulin heavy variable gene (IGHV) sequence to known germline sequences in the clinical laboratory. CLL clones that utilize an unmutated IGHV (by convention a sequence <2% different from rearranged germline) or the VH3-21 family gene segment (irrespective of mutation status) have decreased time from diagnosis to first treatment and poorer survival [14,15,22].
ZAP-70
ZAP-70 is an intracellular molecule associated with BCR signaling in a small subset of normal B cells [23]. In patients with CLL, higher levels of ZAP-70 expression are associated with shorter time to treatment and poorer survival [24]. However, the routine clinical measurement of ZAP-70 expression is difficult and non-standardized, and the clinical utility of this assay is thus limited to centers that do well-validated assays on fresh specimens. Although there is a statistical correlation between increased expression of ZAP-70 and unmutated IGHV status [24], this relationship is not sufficiently precise to allow mutation status to be reliably predicted by the level of ZAP-70 expression [25].
CD38
CD38 is a surface protein that is expressed at variable levels in CLL and can be reliably measured by flow cytometry. CD38 expression levels have a well-validated statistical correlation with time to treatment and prognosis, but use as a single parameter has limited value in defining high-risk CLL in individual patients [26].
Other prognostic factors
There are a large number of other prognostic factors reported in the literature. A comprehensive review of their role in determining CLL risk is outside of the scope of this review.
Relapsed/refractory disease
Optimal management of patients with relapsed/refractory CLL requiring treatment for progressive disease requires reevaluation and revision of risk stratification. Patients with abnormal TP53 function, purine analog refractory disease, transformation, and poor biological fitness are at the highest risk of poor treatment response and outcome.
TP53 function
The risk of defective TP53 function increases with disease duration and treatment and especially with the use of purine analog containing CIT. Patients with CLL are at significant risk of clonal evolution, with over 25% having an additional defect detected by FISH at 5 years after diagnosis [27]. The majority of these additional defects increase disease risk, and about one-third involve the TP53 pathway (17p— or 11q—) [21,27]. At present, most clinicians can only infer TP53 dysfunction by detecting 17p— by FISH, but new developments in the ability to routinely test for TP53 mutations and function should improve both the sensitivity and precision of detection of TP53 dysfunction, which is essential for planning treatment in patients with progressive CLL.
Purine analog refractory
Patients with CLL who do not respond to a purine analog containing regimen or who have a short duration of response (time to progression of less than 1 year) are considered to be purine analog refractory and have a poor prognosis. These patients require similar treatment approaches to patients with defective TP53 function.
Biological fitness
Poor biological fitness in patients with CLL can markedly increase the risk of adverse effects of disease progression, complications, and treatment. However, evaluation of biological fitness is complex, not yet standardized, and can be difficult in some patients. Although age is an independent poor prognostic factor in CLL [28], the reasons why older patients with CLL are at increased risk are not well defined, and risk can vary widely between different patients of the same age [29]. The loss of organ reserve caused by comorbidities and aging are major determinants of an individual’s tolerance of the complications and treatment of CLL. Thus, the assessment of organ function is usually a more important determinant of CLL risk than chronological age alone.
Irreversible decreases in functional status are an important cause of increased risk for poor outcome in patients with CLL. However, objective measurement of the overall functional status can be difficult in patients with CLL. Although functional status can be evaluated using the Eastern Cooperative Oncology Group (ECOG) performance score and the activities of daily living (ADL) and Instrumental Activities of Daily Living (IADL) scales, the results of these assessments have only a moderate correlation in individual patients [30] and are thus of limited clinical value in patient care. In addition, the results of these evaluations can be misleading in patients with CLL with potentially treatment responsive anemia or profound fatigue. The future development of better tools for assessing the biological fitness of patients with CLL would be very useful for more accurate identification of those patients at higher risk of poor outcome.
What we do
Initial evaluation
We do a comprehensive clinical evaluation of all patients with CLL at their first visit. The accuracy of the diagnosis of CLL is reviewed using published immunophenotypic criteria, and mantle cell lymphoma in leukemic phase is excluded by either FISH with an IGH probe or a cyclin D1 (CCND1, BCL-1) immunostain to detect t(11;14) [31–33]. Patients whose monoclonal B cells have an immunophenotype that is atypical for CLL require a diagnostic lymphoid tissue biopsy prior to initiation of treatment [34,35]. Physical examination is used to detect bulky disease. Computed tomography (CT) scanning and a bone marrow study are not routinely required at diagnosis and are performed only if clinically indicated. However, a bone marrow study should be done in all patients before treatment.
Prognosis at diagnosis
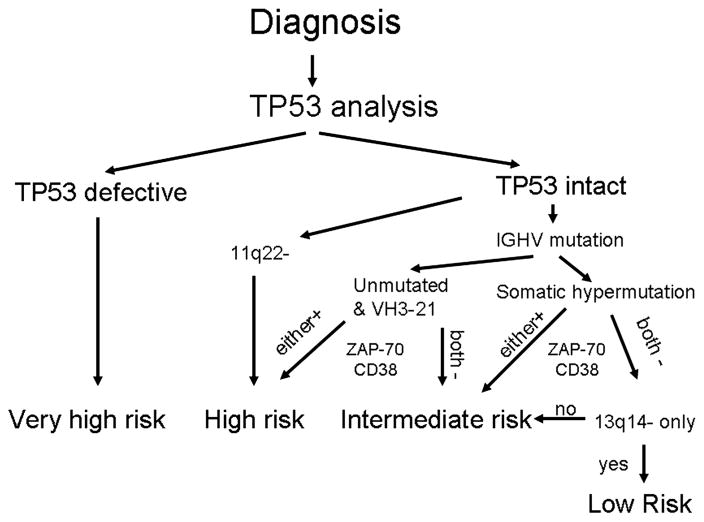
Our approach is to do FISH analysis, IGHV mutation analysis, and ZAP-70, CD38, and CD49d [36] expression analysis by flow cytometry on all patients with newly diagnosed CLL who are potential candidates for treatment, when indicated. Patients are then risk stratified to very high, high, intermediate, and low risk of disease progression (Figure 1) and managed accordingly. The major limitation to our current approach is the inability to detect patients with loss of TP53 function not caused by 17p—. These patients, who constitute approximately 5% of those newly diagnosed with CLL, have a very poor prognosis at disease progression, equivalent to patients with 17p—, and are thus classified as having very-high-risk CLL [19]. We are currently only able to test for TP53 mutations in selected patients as part of ongoing clinical trials. However, we believe that this testing is very important, and plan to start using routine TP53 mutation analysis in 2011 in both patients with earlier stage disease and those who require treatment for progressive disease. However, we do appreciate that the currently available assays are still not capable of identifying all patients with defects in the TP53 pathway and that further improvements of clinical tests for this critical pathway are required.
Figure 1.
Risk stratification for patients with non-progressive early–intermediate stage CLL.
Another important component of the TP53 pathway is the ATM protein located at 11q22. The 11q— defect is usually monoallelic, and the consequences of the loss of one allele of ATM on TP53 pathway function depend on the integrity of the remaining allele. Analysis of the residual allele in patients with CLL with 11q— has shown that it is dysfunctional in approximately 30% of patients, resulting in poorer responses to therapy and inferior clinical outcome [37]. Unfortunately, mutation analysis of the very large ATM gene is not routinely available. Several additional approaches are being tested for utility in measuring the integrity of the TP53 pathway in CLL, including measurement of miR34a expression [38]. However, none of these approaches are currently available for clinical use.
CLL is clearly a genetically complex disease in which additional genetic defects are frequently acquired during the course of the disease. Although FISH analysis is a clinically accessible and very useful test of genomic integrity, it provides a limited insight into the genetic defects that could be used to determine risk in patients with CLL. Whole genome approaches such as comparative genomic hybridization (CGH) and single nucleotide polymorphism (SNP) analysis are currently being used in research projects to measure genomic complexity and discover novel defects in CLL, and could potentially be clinically useful for prognostication in the future [39].
Relapsed/refractory disease
All patients with CLL who have progressive relapsed/refractory disease requiring treatment according to standard criteria [40] should be carefully reevaluated for changes in their risk parameters. These patients require a repeat bone marrow study to determine the extent of involvement by CLL and their myeloid reserve. Repeat FISH analysis is also required to examine for clonal evolution and especially the very-high-risk 17p—. Imaging with CT scans can be useful, but is not required to determine tumor burden. Positron emission tomography (PET) scanning is of limited value in the evaluation of patients with CLL because the disease usually has low fluorodeoxyglucose (FDG) avidity, and should be reserved for those patients with features causing concern for transformation to diffuse large B-cell lymphoma. Although the negative predictive value of PET scanning for detection of transformation to diffuse large B-cell lymphoma is high in patients with CLL, the positive predictive value is low [41]. Our unpublished experience is that patients with 17p— can have FDG-avid CLL, and an excision biopsy of FDG-avid tissue is always required to distinguish between progressive CLL, diffuse large B-cell lymphoma, other malignancies, fungal infections (e.g. histoplasmosis), and other etiologies.
Biological fitness
Assessment of biological fitness requires a comprehensive clinical evaluation to determine whether patients are fit for CIT, can tolerate only less toxic therapy, or are candidates for palliative care [42]. Our basic assessment includes performance status, effort tolerance, and organ function (renal, hepatic, and hematopoietic). Additional organ function testing is done as clinically indicated.
Management of the patient with high-risk chronic lymphocytic leukemia
Initial treatment
Early–intermediate stage with high–very-high-risk CLL
Randomized trials have shown that early intervention with chemotherapy is not beneficial for patients with CLL [43]. Patients with CLL who have early–intermediate clinical stage and do not meet standard criteria for disease progression [40] should thus not be treated outside of a clinical trial. Even among patients with 17p— and non-progressive CLL there is a small cohort who have an unexplained long time to treatment [44,45]. However, the improving ability to predict prognosis and identify patients with high risk of progression of their CLL could mean that some patients with CLL would benefit from early treatment with more targeted non-chemotherapy treatment modalities. A phase II clinical trial of early treatment of high-risk CLL (at least one of following risk factors: 17p—, 11q—, IGHV unmutated, and CD38+ or ZAP-70+) with a short course of alemtuzumab and rituximab showed that this regimen is effective and has acceptable toxicity [46]. However, assessment of the clinical value of these early interventions will require a randomized controlled trial comparing early treatment versus conventional management.
Patients diagnosed with advanced stage/progressive CLL
Only 5–10% of patients have advanced stage or progressive disease at diagnosis that requires immediate treatment [6]. In these patients, the single most important parameter for risk stratification and planning treatment is currently TP53 function. Patients with TP53 deletions/mutations have suboptimal responses to standard CIT and require alternative therapies [19].
Patients with no known defect in TP53
The standard of care for fit patients is CIT using a purine analog, alkylating agent, and rituximab containing treatment regimen [47–49]. The results of the CLL8 study of the German CLL Study Group (GCLLSG) showed a survival advantage for patients with progressive CLL without 17p— who were treated with fludarabine, cyclophosphamide, and rituximab (FCR) compared to those receiving fludarabine and cyclophosphamide (FC) [49]. This study also showed that patients with 11q— treated with FCR did not have inferior responses to treatment [49]. There are also data from the CLL4 study of the GCLLSG published in abstract form that suggest that the addition of cyclophosphamide to fludarabine improves treatment responses in patients with 11q— [50], and data from our phase II trial using pentostatin and rituximab support the importance of the use of cyclophosphamide in CIT [51]. There have not been any rigorous randomized phase III trials comparing the use of FCR and pentostatin, cyclophosphamide, and rituximab (PCR) for initial treatment of CLL. However, our clinical experience suggests that PCR is better tolerated than FCR in older and frailer patients.
Patients with defective TP53
There is no standard of care for this group of patients, and CIT rarely results in complete (CR) or durable responses [19]. Therapies with TP53-independent mechanisms of action such as high-dose corticosteroids and alemtuzumab can be effective, but rarely result in durable responses [52,53]. Combination therapies using alemtuzumab and rituximab [54] and high-dose methylprednisolone in combination with either rituximab [55,56] or alemtuzumab [57] have utility and can achieve response rates of over 50%, but rarely achieve a CR. A major limiting factor in the use of these combination therapies is severe immunosuppression and infections. There is thus a clear need to develop more effective regimens for these patients.
Allogeneic peripheral blood stem cell transplant using a reduced-intensity conditioning regimen (RIC allotransplant) can achieve long-term remission in patients with high-risk CLL including those with 17p— [58,59]. This should be an early consideration in the management of all patients with high-risk relapsed/refractory CLL who have progressive disease requiring treatment. In biologically fit patients with a suitable donor, the initial step in treatment is to achieve a substantial reduction of tumor burden with non-transplant therapy. Thus, effective RIC allotransplant requires strategic long-term planning and coordination of therapy with early involvement of the transplant team. Patients who are not eligible for RIC allotransplant should be considered for early use of experimental therapy on clinical trials.
Later disease progression
Patients with high-risk CLL who do not require treatment for progressive CLL at diagnosis should be actively monitored for CLL progression and complications with clinic visits at least every 3 months. When these patients have progressive disease, they require reevaluation of their risk status prior to deciding on treatment. These patients require both FISH testing to detect clonal evolution and clinical reevaluation to detect any changes in biological fitness that could affect treatment choices. Based on this evaluation, patients can be managed according to the same principles as described above for patients requiring treatment at diagnosis.
Relapsed/refractory CLL
Patients with previously treated CLL who have progressive disease requiring treatment require risk reevaluation. Patients with purine analog sensitive CLL who do not have a detectable TP53 defect are likely to respond to repeat treatment with CIT. In contrast, patients with CLL whose disease is purine analog refractory or who have defective TP53 have very-high-risk disease with poor response to conventional treatment and short survival. In these patients, the initial approach should focus on the possibility of RIC allotransplant. Patients with TP53 dysfunction who are not candidates for RIC allotransplant have the same limited treatment options as those for initial treatment of patients with TP53 dysfunction, as described above. Patients with purine analog refractory disease without known TP53 defects who are not candidates for RIC allotransplant can also be considered for additional therapeutic options including lenalidomide [60], ofatumumab [61], and other experimental options via clinical trials.
Biologically frail patients
Therapy of biologically frail patients needs to be individualized based on the CLL risk, fitness, and comorbidities. Important considerations are anemia and fatigue, which can cause a considerable but reversible decrease in performance status. Patients who are less biologically fit usually do not tolerate standard CIT and should be considered for less toxic therapy with regimens such as pentostatin and rituximab (PR) [51], dose-reduced FCR (FCRlite) [62], and PCR [48]. In addition, experimental options that do not include chemotherapy such as alemtuzumab and rituximab (Intergroup-ECOG1908, NCT01013961) could be appropriate therapy.
What we do
Initial treatment of CLL
Early–intermediate stage with high–very-high-risk CLL
Newly diagnosed patients have a prognostic evaluation, and qualifying patients with non-progressive high-risk early–intermediate stage disease (≥1 of: 17p—, 11q—, unmutated IGHV/use of VH3-21 + expression of ZAP-70 ± CD38) are offered participation in a combination monoclonal antibody based therapy trial. For high-risk patients not participating in this study, the standard of care is active monitoring every 3 months. At each visit patients are monitored for disease progression and complications. Patients receive ongoing education about CLL and its complications, with emphasis on early recognition and management of infection, appropriate vaccinations, skin and other malignancy prevention/screening, and monitoring for disease progression. Our clinic is also designed to facilitate rapid patient access for evaluation of new clinical problems. Patients have direct telephonic access to a CLL-dedicated registered nurse to triage and manage problems and arrange clinic visits as required.
Initial treatment of progressive disease
Patients with high-risk CLL who have disease progression according to standard criteria, are biologically fit, and do not have detectable defects of TP53 are usually treated with CIT. Our current practice is to consider FCR for most fit patients under the age of 60 years and PCR (or dose-reduced FCR [62]) for most patients over the age of 65 years. Patients between 60 and 65 years old are carefully evaluated for biological fitness and organ function, and treatment decisions made in consultation with the patient with due consideration of their personal opinions about the risks of treatment. Our patients are also offered participation in experimental treatment options whenever possible. Patients with defective TP53 are treated with regimens containing alemtuzumab or high-dose corticosteroids with the aim of achieving the best possible remission, and then proceeding to RIC allotransplant whenever possible. Our current experimental treatment option utilizes pentostatin, alemtuzumab, and low-dose rituximab (PAR) (NCT00669318), and standard of care is high-dose methylprednisolone and rituximab [55] in patients with bulky disease (palpable nodes >5 cm in diameter, spleen palpable >6 cm below costal margin), and alemtuzumab and rituximab [46] for patients with less bulky disease.
Relapsed/refractory CLL
Patients without features of very-high risk disease are treated with CIT if biologically fit. Patients with progressive purine refractory disease or TP53 defective CLL are treated on an experimental protocol if possible. Our current experimental options are PAR (NCT00669318) and everolimus and alemtuzumab (NCT00935792). Standard of care therapies are high-dose methyl-prednisolone and rituximab, and alemtuzumab and rituximab. In all sufficiently fit patients with a matched donor, the goal of treatment is to achieve a response that will allow the patient to proceed to RIC allotransplant.
Biologically frail patients
Patients without TP53 defects who are unlikely to tolerate standard CIT are usually treated with regimens that contain corticosteroids, cyclophosphamide, and rituximab such as R-C(V)P [63,64]. We often omit the vincristine in this regimen in older patients at high risk of peripheral neuropathy. An alternative regimen is PR [51]. In frail patients with TP53 defective CLL, treatment options include monotherapy with alemtuzumab or ofatumumab, or intermediate-dose corticosteroids.
Future developments
Improving evaluation of prognosis in CLL
Although prognostic methods and treatments for CLL have improved dramatically in the recent past, they clearly still require both major improvements and the development of consensus for incorporation into clinical practice. In the interim, clinical care could be improved by optimal use of the available resources by practitioners evaluating and managing patients with CLL. Our current evaluation of high-risk CLL relies extensively on clinical factors (e.g. stage) and the extent and duration of clinical responses to initial therapy. We believe that prognostic evaluation can be markedly improved by the routine use of prognostic profiles at diagnosis and before initial and subsequent treatment, to identify patients with high-risk CLL who need different treatment approaches. The best current example of this is the use of FISH to detect patients with 17p— and molecular methods to detect TP53 dysfunction. We and others are committed to studies designed to continue the development of more accurate prognostic methods for use in clinical practice.
Improving initial management of patients with very-high-risk CLL
Our current standard of care for those patients with non-progressive disease is regular active monitoring and prevention or early treatment of the complications of CLL. However, we believe that this cohort should also be investigated with interventions that could delay or even prevent disease progression. Phase II clinical trials suggest that combination monoclonal antibody therapy is effective and well tolerated in this cohort. We now need to determine in a phase III trial whether these interventions are beneficial in these patients.
Improvement of treatment options in patients with high-risk CLL
We are convinced that improving treatment will require a more complete understanding of the biology of the CLL B-cell and its interaction with the microenvironment. Initial investigations have already yielded important insights into membrane adhesion markers, cell signaling, novel receptors, and apoptosis pathway proteins that could be manipulated to improve treatment. Thus, we can now test drugs that interrupt CXCR4–CXCRL12 interactions, antibodies to unique receptor sites such as CD37, anti-CD20 antibodies with improved complement activation potential, inhibitors of the BCR directed signaling that target AKT and mTOR (mammalian target of rapamycin), and mimetics that bind to proapoptotic proteins to block their function (Table II). Even if these new agents are not highly effective as single agents, their use can provide information to design better agents, or they could be useful in combination therapies by reducing CLL cell resistance to currently used therapies.
Table II.
Examples of novel drugs for the therapy of CLL.
| Drug | Class |
|---|---|
| Lenalidomide | Immune modulator (IMID) |
| Alvocidib (flavopiridol) | Cyclin dependent kinase (CDK) inhibitor |
| Ofatumumab | Human anti-CD20 monoclonal antibody |
| Veltuzumab | Humanized anti-CD20 monoclonal antibody |
| HCD122 | Human anti-CD40 monoclonal antibody |
| TRU-016 | Anti-CD37 IgG fusion protein |
| Obatoclax | BCL2 inhibitor |
| ABT-263/ ABT-737 | BCL2 and BCLXL inhibitor |
| CAL-101 | PI3K inhibitor |
| Fostamatinib | SYK inhibitor |
| Everolimus | mTOR inhibitor |
| AiX | AKT inhibitor |
| PGG β-glucan | Complement receptor 3 agonist |
| 17-DMAG | HSP90 inhibitor |
| Dasatinib | Tyrosine kinase inhibitor |
| Plerixafor | CXCL12 inhibitor |
CLL, chronic lymphocytic leukemia; IgG, immunoglobulin G; PI3K, phosphatidylinositol 3-kinase; mTOR, mammalian target of rapamycin.
CLL is characterized by the early development of profound defects in the immune system which are exacerbated by therapy [8]. Interventions that correct these deficiencies could be very useful in the treatment of CLL by decreasing the major morbidity caused by infections, autoimmune complications, and second malignancies, and by increasing the patient’s own ability to mount an immune response against their CLL. We thus need to continue efforts to better understand the immune defects in CLL, and use this information to develop methods that can improve immune function. Approaches for ex vivo modification of T cells that can then be infused into patients with CLL to induce a graft versus leukemia effect is one exciting potential approach that has shown promise in phase I trials for hematological malignancies [65]. Related to this approach is the potential development of an anti-leukemia vaccine where the leukemic cells could be fused with autologous dendritic cells, with the subsequent development of cytotoxic T cells against a wide array of tumor antigens [66]. The recent demonstration that lenalidomide is able to reverse the defects in immunologic synapse formation in patients with CLL represents an additional novel maneuver to reverse the profound immune T cell and natural killer (NK) defects in patients with CLL [67]. We thus believe that there are considerable opportunities for manipulation of the defective immune system in patients with CLL that could be of therapeutic benefit.
Acknowledgments
This article was supported in part by research funding from the University of Iowa/Mayo Clinic Lymphoma SPORE P50 CA 97274 to C.S.Z. Clive S. Zent is the principal investigator of studies at Mayo Clinic funded by Genzyme, Genentech, Novartis, and GlaxoSmithKline. Neil E. Kay is the principal investigator of studies at Mayo Clinic funded by Hospira, Celgene, Cephalon, Genentech, GlaxoSmithKline, Novartis, and Supergen.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. Available from: http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 2.Seftel MD, Demers AA, Banerji V, et al. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33:1463–1468. doi: 10.1016/j.leukres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Mauro F, Foa R, Cerretti R, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000;95:2786–2792. [PubMed] [Google Scholar]
- 5.Kyasa MJ, Parrish RS, Schichman SA, Zent CS. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. Am J Hematol. 2003;74:1–8. doi: 10.1002/ajh.10369. [DOI] [PubMed] [Google Scholar]
- 6.Zent CS, Ding W, Schwager SM, et al. The prognostic significance of cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) Br J Haematol. 2008;141:615–621. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno C, Hodgson K, Ferrer G, et al. Autoimmune cytopenias in chronic lymphocytic leukemia: prevalence, clinical associations, and prognostic significance. Blood. 2010;116:4771–4776. doi: 10.1182/blood-2010-05-286500. [DOI] [PubMed] [Google Scholar]
- 8.Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23:145–153. doi: 10.1016/j.beha.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leuk Lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- 10.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robak E, Robak T. Skin lesions in chronic lymphocytic leukemia. Leuk Lymphoma. 2007;48:855–865. doi: 10.1080/10428190601137336. [DOI] [PubMed] [Google Scholar]
- 12.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 13.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–205. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin T, Davis Z, Gardiner A, Oscier D, Stevenson F. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 15.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 16.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 17.Dewald GW, Brockman SR, Paternoster SF, et al. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia. Br J Haematol. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 18.Dohner H, Fischer K, Bentz M, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85:1580–1589. [PubMed] [Google Scholar]
- 19.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 20.Dohner H, Stilgenbauer S, James MR, et al. 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89:2516–2522. [PubMed] [Google Scholar]
- 21.Fegan C, Robinson H, Thompson P, Whittaker JA, White D. Karyotypic evolution in CLL: identification of a new subgroup of patients with deletions of 11q and advanced or progressive disease. Leukemia. 1995;9:2003–2008. [PubMed] [Google Scholar]
- 22.Tobin G, Thunberg U, Johnson A, et al. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted Vlambda2–14 gene use and homologous CDR3s: implicating recognition of a common antigen epitope. Blood. 2003;101:4952–4957. doi: 10.1182/blood-2002-11-3485. [DOI] [PubMed] [Google Scholar]
- 23.Nolz JC, Tschumper RC, Pittner BT, Darce JR, Kay NE, Jelinek DF. ZAP-70 is expressed by a subset of normal human B-lymphocytes displaying an activated phenotype. Leukemia. 2005;19:1018–1024. doi: 10.1038/sj.leu.2403726. [DOI] [PubMed] [Google Scholar]
- 24.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 25.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 26.Zent CS, Call TG, Hogan WJ, Shanafelt TD, Kay NE. Update on risk-stratified management for chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47:1738–1746. doi: 10.1080/10428190600634036. [DOI] [PubMed] [Google Scholar]
- 27.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 28.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 29.Zent CS. Chronic lymphocytic leukemia in the elderly: who should be treated? Am Soc Clin Oncol Ed. 2010:268–271. [Google Scholar]
- 30.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Hermelink HK, Montserrat E, Catovsky D, Campo E, Harris NL, Stein H. Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumors of haematopoietic and lymphoid tissue. Lyon: IARC; 2008. pp. 180–187. [Google Scholar]
- 32.Nowakowski GS, Dewald GW, Hoyer JD, et al. Interphase fluorescence in situ hybridization with an IGH probe is important in the evaluation of patients with a clinical diagnosis of chronic lymphocytic leukaemia. Br J Haematol. 2005;130:36–42. doi: 10.1111/j.1365-2141.2005.05548.x. [DOI] [PubMed] [Google Scholar]
- 33.Morice WG, Kurtin PJ, Hodnefield JM, et al. Predictive value of blood and bone marrow flow cytometry in B-cell lymphoma classification: comparative analysis of flow cytometry and tissue biopsy in 252 patients. Mayo Clin Proc. 2008;83:776–785. doi: 10.4065/83.7.776. [DOI] [PubMed] [Google Scholar]
- 34.Dronca RS, Jevremovic D, Hanson CA, et al. CD5-positive chronic B-cell lymphoproliferative disorders: diagnosis and prognosis of a heterogeneous disease entity. Cytometry B Clin Cytom. 2010;78(Suppl 1):S35–S41. doi: 10.1002/cyto.b.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jevremovic D, Dronca RS, Morice WG, et al. CD5+ B-cell lymphoproliferative disorders: beyond chronic lymphocytic leukemia and mantle cell lymphoma. Leuk Res. 2010;34:1235–1238. doi: 10.1016/j.leukres.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Shanafelt TD, Geyer SM, Bone ND, et al. CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: a prognostic parameter with therapeutic potential. Br J Haematol. 2008;140:537–546. doi: 10.1111/j.1365-2141.2007.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austen B, Skowronska A, Baker C, et al. Mutation status of the residual ATM allele is an important determinant of the cellular response to chemotherapy and survival in patients with chronic lymphocytic leukemia containing an 11q deletion. J Clin Oncol. 2007;25:5448–5457. doi: 10.1200/JCO.2007.11.2649. [DOI] [PubMed] [Google Scholar]
- 38.Asslaber D, Pinon JD, Seyfried I, et al. MicroRNA-34a expression correlates with MDM2 SNP309 polymorphism and treatment-free survival in chronic lymphocytic anemia. Blood. 2010;115:4191–4197. doi: 10.1182/blood-2009-07-234823. [DOI] [PubMed] [Google Scholar]
- 39.Kujawski L, Ouillette P, Erba H, et al. Genomic complexity identifies patients with aggressive chronic lymphocytic leukemia. Blood. 2008;112:1993–2003. doi: 10.1182/blood-2007-07-099432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruzzi JF, Macapinlac H, Tsimberidou AM, et al. Detection of Richter’s transformation of chronic lymphocytic leukemia by PET/CT. J Nucl Med. 2006;47:1267–1273. [PubMed] [Google Scholar]
- 42.Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:171–178. doi: 10.1080/10428190802688517. [DOI] [PubMed] [Google Scholar]
- 43.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 44.Tam CS, Shanafelt TD, Wierda WG, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114:957–964. doi: 10.1182/blood-2009-03-210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Best OG, Gardiner AC, Davis ZA, et al. A subset of Binet stage A CLL patients with TP53 abnormalities and mutated IGHV genes have stable disease. Leukemia. 2009;23:212–214. doi: 10.1038/leu.2008.260. [DOI] [PubMed] [Google Scholar]
- 46.Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113:2110–2118. doi: 10.1002/cncr.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 48.Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B-chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 50.Stilgenbauer S, Eichhorst BF, Busch R, et al. Biologic and clinical markers for outcome after fludarabine (F) or F and cyclophosphamide (FC) - comprehensive analysis of the CLL4 trial of the GCLLSG. Blood. 2008;112(Suppl 1):Abstract 2089. [Google Scholar]
- 51.Kay NE, Wu W, Kabat B, et al. Pentostatin and rituximab therapy for previously untreated patients with B-cell chronic lymphocytic leukemia. Cancer. 2010;116:2180–2187. doi: 10.1002/cncr.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thornton PD, Matutes E, Bosanquet AG, et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann Hematol. 2003;82:759–765. doi: 10.1007/s00277-003-0710-5. [DOI] [PubMed] [Google Scholar]
- 53.Stilgenbauer S, Döhner H. Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med. 2002;347:452–453. doi: 10.1056/NEJM200208083470619. [DOI] [PubMed] [Google Scholar]
- 54.Faderl S, Thomas DA, O’Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 55.Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma. 2007;48:2412–2417. doi: 10.1080/10428190701724801. [DOI] [PubMed] [Google Scholar]
- 56.Castro JE, Sandoval-Sus JD, Bole J, Rassenti L, Kipps TJ. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia. 2008;22:2048–2053. doi: 10.1038/leu.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettitt AR, Matutes E, Oscier D. Alemtuzumab in combination with high-dose methylprednisolone is a logical, feasible and highly active therapeutic regimen in chronic lymphocytic leukaemia patients with p53 defects. Leukemia. 2006;20:1441–1445. doi: 10.1038/sj.leu.2404265. [DOI] [PubMed] [Google Scholar]
- 58.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 60.Sher T, Miller KC, Lawrence D, et al. Efficacy of lenalidomide in patients with chronic lymphocytic leukemia with high-risk cytogenetics. Leuk Lymphoma. 2010;51:85–88. doi: 10.3109/10428190903406806. [DOI] [PubMed] [Google Scholar]
- 61.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]
- 63.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 64.Bowen DA, Call TG, Shanafelt TD, et al. Treatment of autoimmune cytopenia complicating progressive chronic lymphocytic leukemia/small lymphocytic lymphoma with rituximab, cyclophosphamide, vincristine, and prednisone. Leuk Lymphoma. 2010;51:620–627. doi: 10.3109/10428191003682767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Har-Noy M, Zeira M, Weiss L, Fingerut E, Or R, Slavin S. Allogeneic CD3/CD28 cross-linked Th1 memory cells provide potent adjuvant effects for active immunotherapy of leukemia/lymphoma. Leuk Res. 2009;33:525–538. doi: 10.1016/j.leukres.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 66.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/ CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 67.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]