Abstract
Background
Cardiovascular risk factors and diseases are important aetiological factors in depression, particularly late-life depression. Brain changes associated with vascular disease and depression can be detected using magnetic resonance imaging. Using diffusion tensor imaging (DTI), we investigated whether the Framingham stroke risk profile (FSRP), a well validated risk prediction algorithm, is associated with changes in white-matter connectivity. We hypothesised that depressed participants would show reduced white-matter integrity with higher FSRP, and non-depressed controls (matched for mean vascular risk) would show minimal co-variance with white-matter changes.
Methods
36 participants with major depression (age 71.8±7.7 years, mean FSRP 10.3±7.6) and 25 controls (age 71.8±7.3 years, mean FSRP 10.1±7.7) were clinically interviewed and examined, followed by 60-direction DTI on a 3.0 Tesla scanner. Image analysis was performed using FSL tools (www.fmrib.ox.ac.uk/fsl) to assess the correlation between FSRP and fractional anisotropy (FA). Voxelwise statistical analysis of the FA data was carried out using Tract Based Spatial Statistics. The significance threshold for correlations was set at p<0.05 using threshold-free cluster-enhancement. Partial correlation analysis investigated significant correlations in each group.
Results
Participants in the depressed group showed highly significant correlations between FSRP and FA within: body of corpus callosum (r=−0.520, p=0.002), genu of corpus callosum (r= −0.468, p=0.005), splenium of corpus callosum (r=−0.536, p=0.001) and corticospinal tract (r=−0.473, p=0.005). In controls, there was only one significant correlation in the body of corpus callosum (r=−0.473, p=0.023).
Conclusions
FSRP is associated with impairment in white-matter integrity in participants with depression; these results suggest support for the vascular depression hypothesis.
Keywords: DTI, Diffusion tensor, connectivity, vascular, depressed
Introduction
The “vascular depression” hypothesis proposes that cardiovascular disease is an important aetiological factor in depression, particularly late-life depression (Alexopoulos et al., 1997; Sheline et al., 2010). This is supported by clinical observations which confirm that stroke and cardiovascular disease increase the likelihood of depression (Thomas et al., 2004), and that specific cardiovascular risk factors may predispose to depression. Neuroimaging findings have been important in the development of the vascular depression theory. Magnetic Resonance Imaging (MRI) has shown that white-matter hyperintensities are more common in patients with late-life depression compared to controls (Herrmann et al., 2008) and a vascular basis for white-matter hyperintensities has been suggested (Chen et al., 2006; Smith et al., 2009; Thomas et al., 2002; Thomas et al., 2003). White-matter hyperintensities are particularly prevalent within frontal regions (Dalby et al., 2010; Sheline et al., 2008).
Further investigation of white-matter pathology may yield important information on the effects of raised cardiovascular risk on brain structure. Changes in fibre tracts on a macro- or microscopic scale have been associated with vascular disease and can have major implications for brain function (Roman, 1996). In this paper we describe a diffusion tensor imaging (DTI) protocol, which has been able to detect subtle changes in white-matter (Sexton et al., 2009). This method measures the direction of water diffusion within brain tissue. Restricted diffusion is expected within white-matter tracts (anisotropic diffusion), where water molecules are constrained by myelin sheaths and the parallel organisation of fibres. Measuring the direction of diffusion allows investigation of orientation, as well as integrity of white-matter. The sensitivity of this method is important, given that any changes to structural white-matter connectivity are likely to be subtle, given the absence of major cardio-vascular disease in our sample.
There are emerging studies which consider the effect of specific cardiovascular risk factors on brain structure. Hypertension appears to confer a vulnerability to depression which may be mediated through micro-vascular brain injury (Hajjar et al., 2011) and reduced white-matter integrity in frontal-striatal regions (Hoptman et al., 2009). The Framingham Stroke Risk Profile (FSRP) has been associated with the volume of white-matter hyperintensities in those with (Smith et al., 2010) and without depression (Jeerakathil et al., 2004), although previous research has not focussed on associations between FSRP and changes in white-matter connectivity. The FSRP is widely used to predict individual risk of stroke (D’Agostino et al., 2008; D’Agostino et al., 1994; Wilson et al., 1998) and uses data that can be readily acquired in clinical settings. Given the strength of evidence linking vascular disease and depression (Alexopoulos, et al., 1997), it is plausible that the FSRP will also predict structural brain changes in those with depression.
This paper describes an exploratory case-control study which considers the correlation between elevated cardiovascular risk as identified by the FSRP and white-matter connectivity measured using DTI. Reductions in fractional anisotropy (FA) in late-life depression are well documented (Sexton, et al., 2009). If vascular pathology contributes to reductions in white-matter integrity, in accordance with the vascular hypothesis of depression, we would expect a correlation between FSRP and regions where FA is reduced in depression. In the same regions in controls, where white-matter integrity is relatively preserved, vascular risk is not expected to be significantly correlated with FA.
Clinically, “vascular depression” is particularly relevant to those with late-life depression, an area of increasing importance whilst the proportion of the population over the age of 65 is rising significantly (Christensen et al., 2009; Office-for-National-Statistics, 2008). With a greater prevalence of people with chronic cardiovascular disease (Crimmins, 2004), better understanding of the effects of such disease on brain structure and mental health will be vital in preventing and treating depression.
Hypothesis
Depressed participants with high FSRP will show reduced white-matter integrity, whereas controls matched for vascular risk will show minimal white-matter changes. Specifically, depressed participants will show reduced white-matter integrity with higher FSRP; controls matched for mean vascular risk and overall variance will show minimal covariance with white-matter changes.
Methods
Sample
The sample included 36 participants with major depression (age 71.83 ± 7.71 years) recruited through primary and secondary care services in Oxfordshire and Buckinghamshire and 25 controls (age 71.76±7.30 years) recruited from the community. The mean 10-year Framingham Stroke Risk score was 10.33±7.64 in cases and 10.08±7.74 in controls; other baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the sample
| Control | Cases | p-value | |
|---|---|---|---|
| Number of Participants | 25 | 36 | |
| Gender (F: M) | 16 : 9 | 24 : 12 | 0.83 |
| Age | 71.8 ± 7.3 | 71.8 ± 7.7 | 0.97 |
| FSIQ (NART) | 122.4 ± 5.1 | 119.9 ± 7.9 | 0.17 |
| Cognitive Impairment | |||
| - ACE-R | 95.2 ± 5.0 | 91.5 ± 6.3 | 0.02* |
| - MMSE | 29.5 ± 0.7 | 29.0 ± 1.4 | 0.09 |
| Framingham Stroke Risk | 10.08 ± 7.74 | 10.33 ± 7.64 | 0.97 |
| Age at Onset | N/A | 45.39 ± 18.97 | N/A |
| Severity | |||
| - HAM-D | N/A | 4.2 ± 4.8 | N/A |
| Current Medication | |||
| - Number of Medications | N/A | 1.4 ± 0.8 | N/A |
| - Medication Free | 2 | ||
| - Anticonvulsants | 1 | ||
| - Antidepressants | 33 | ||
| - Antipsychotics | 7 | ||
| - Anxiolytic | 5 | ||
| - Lithium | 4 |
Values presented are: mean ± standard deviation
indicates statistical significance (p < 0.05).
Inclusion and exclusion criteria
For inclusion in this study all participants were required to be greater than 60 years old, with no potentially confounding co-morbid medical, psychiatric or neurological conditions. Those recruited to the depressed group met the criteria for DSM-IV major depression (Diagnostic and statistical manual of mental disorders : DSM-IV, 1994), ascertained through clinical interview with a psychiatrist, but were not necessarily currently depressed. Controls were required to have no history of memory impairment or psychiatric illness, determined by administering the Structured Clinical Interview for DSM-IV Axis 1 Disorders Non-Patient Edition (First et al., 2007). Participants with implanted metallic devices were excluded, as required by standard MRI safety protocols.
Clinical Interview
All participants underwent clinical interview and examination. The clinical assessment was based on a semi-structured proforma designed for this study. Participants with depression were interviewed to determine age at onset of depression, defined as the age at which an individual experienced their first episode of major depression. Personal testimony was corroborated with information from hospital notes, where available. The severity of depressive symptoms experienced was further classified according to the 17-item HAM-D (Hamilton, 1967) and the 15-item Geriatric Depression Scale (GDS) (Yesavage et al., 1982). Medication status was ascertained through personal testimony and hospital notes, classified under the following headings: antidepressant, antipsychotic, anticonvulsant, lithium salts and anxiolytics.
The FSRP was calculated for all participants and was based upon the following predictors: age, systolic blood pressure, diabetes mellitus, cigarette smoking, prior cardiovascular disease, atrial fibrillation, left ventricular hypertrophy and use of hypertensive medication (D’Agostino, et al., 1994). These were obtained from personal testimony, together with hospital notes. Clinical examination with a standard sphygmomanometer was used to assess blood pressure.
Cognitive impairment was assessed in all participants using standardised neuropsychological measures including the Addenbrooke’s cognitive examination revised (ACE-R) (Mioshi et al., 2006), which includes the mini-mental state examination (MMSE) (Folstein et al., 1975). The Full-Scale Intelligence Quotient (FSIQ) was estimated from the number of errors on the National Adult Reading Test (McGurn et al., 2004) and the number of years of full time education was determined from personal testimony.
MRI acquisition
All participants underwent a 60-direction DTI on a 3.0 Tesla Trio Siemens scanner, performed at the University of Oxford Centre for Clinical Magnetic Resonance Imaging (OCMR), with a 12-channel head-coil. Whole-brain DTI was acquired using an echoplanar imaging sequence (TR = 7900/7800 ms, TE = 98/82 ms, field of view = 240 mm, voxel size = 2.5 mm isotropic, b value = 1000, number of directions = 60, number of acquisitions = 2). T2-weighted images were also acquired to characterise WMH (TR = 6000 ms, TE = 91 ms, field of view = 220 mm, voxel size = 0.7 × 0.7 × 4.0 mm).
Ethical approval
This study received a favourable ethical opinion from the Local Research Ethics Committee. All participants gave written, informed consent prior to participation in this study.
Image analysis
Image analysis was performed using FSL tools (www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004) to assess the correlation between fractional anisotropy (FA) and FSRP. First, we performed voxelwise statistical analysis of the FA data using Tract Based Spatial Statistics (TBSS) (Smith et al., 2006) across the whole TBSS skeleton. DTI data were checked manually to remove artefacts before eddy current correction was run to correct for eddy current distortions and head motion. FA images were created by fitting a tensor model to the raw diffusion data using FMRIB’s diffusion toolbox, and then brain-extracted using Brain Extraction Tool (BET) (Smith, 2002). These data were aligned to common space using FMRIB’s non-linear image registration tool (FNIRT) (Andersson et al., 2007a; Andersson et al., 2007b). The mean FA image was created and thinned to create a mean FA skeleton representing the centres of all tracts common to the group. Each participant’s aligned FA data was then projected onto this skeleton before applying voxelwise cross-subject statistics.
Statistics were performed using “randomise” - a permutation-based inference tool for nonparametric statistical thresholding, with age included as a confound regressor (Nichols and Holmes, 2002). The significance threshold for correlations was set at p< 0.05, using threshold-free cluster-enhancement (Smith and Nichols, 2009). Group differences in FA were investigated across the whole skeleton; tracts of interest were based on the ICBM-DTI-81 white-matter labels atlas and the JHU white-matter tractography atlas within the FSL atlas tool.
Second, we performed partial correlation analysis between FSRP and mean values of DTI, on a tract-by-tract basis, including only the voxels that were significantly different between groups. Partial correlation analysis used age and gender as covariates, and investigated significant differences between groups in each tract of interest in (i) depressed participants, (ii) control participants. Group differences between depressed and control groups have been reported elsewhere (Sexton et al., 2011).
Results
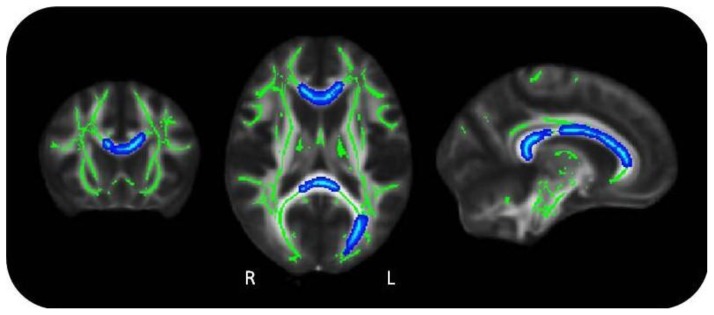
Using TBSS, participants in the depressed group showed significant correlations between FSRP and FA (Figure 1) across widespread regions of the brain, indicating significant impairment in white-matter integrity, in line with the original hypothesis. For controls there was no correlation between FSRP and FA.
Figure 1. Correlations between Framingham stroke risk profile and fractional anisotropy, in depressed group.
Green: mean FA skeleton; Blue: reduction in FA (p<0.05)
Using partial correlation analysis, participants in the depressed group showed significant correlations between FSRP and FA (r= −0.673, p<0.001), whereas in controls, there was no significant relationship (r= −0.373, p=0.08) (Table 2). This was highly significant (p<0.01) within the body of corpus callosum (r=−0.520, p=0.002), genu of corpus callosum (r= −0.468, p=0.005), splenium of corpus callosum (r=−0.536, p=0.001), corticospinal tract (r=−0.473, p=0.005) and also significant (p<0.05) within the cingulum (r= −0.373, p=0.03). In the control group, there was one significant correlation (p<0.05) within the body of corpus callosum (r=−0.473, p=0.023).
Table 2.
Correlations between Framingham stroke risk profile and fractional anisotropy, for voxels significantly different between groups
| Tract of Interest | Number of Voxels | Depressed | Control | ||
|---|---|---|---|---|---|
|
| |||||
| r | p | r | p | ||
| All | 6078 | −0.673 | <0.001** | −0.373 | 0.080 |
| Cingulum | 17 | −0.373 | 0.030* | −0.268 | 0.217 |
| Corpus Callosum | |||||
| - Body | 1190 | −0.520 | 0.002** | −0.473 | 0.023* |
| - Genu | 881 | −0.468 | 0.005** | −0.385 | 0.070 |
| - Splenium | 935 | −0.536 | 0.001** | −0.240 | 0.270 |
| Corticospinal Tract | 311 | −0.473 | 0.005** | −0.025 | 0.259 |
| Inferior Longitudinal Fasciculus | 20 | −0.304 | 0.080 | −0.157 | 0.474 |
p < 0.05,
p < 0.01
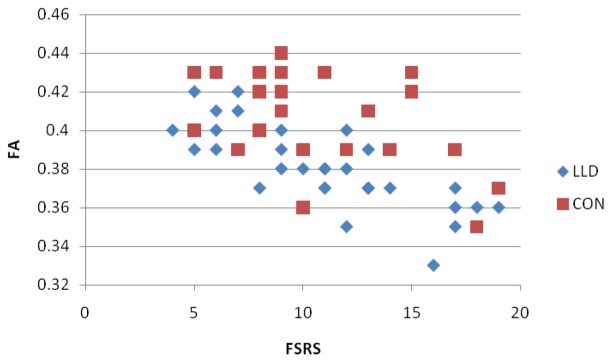
Figure 2 illustrates the mean FA in voxels significantly correlated with FSRP across the depressed group. This shows that whilst the regression slope was similar for both groups, controls showed greater variance in FA values.
Figure 2.
Correlations between Framingham stroke risk profile (FSRP) and fractional anisotropy (FA)
Discussion
The FSRP was associated with reduced white-matter integrity in participants with depression. Our results suggest that white-matter tracts, including, corpus callosum, corticospinal tract and cingulum, warrant further investigation in order to investigate the mechanisms underlying vascular depression. These white-matter tracts with significant correlations were examined in more detail to compare FSRP and FA between depressed and control groups. Greater variability in FA values was evident in controls (Figure 2), a particularly interesting finding given that both groups were matched for vascular risk, as measured by FSRP. This leads to the hypothesis that the greater white-matter integrity in controls provides resilience against the effects of vascular risk, and the subsequent development of depression. Though there was one area of regional significance in controls (body of corpus callosum), this was less significant than in the depressed group.
High vascular burden of disease is more likely with advancing age, thus “vascular depression” is especially relevant to those with late-onset depression. Vascular disease may precipitate both relapse in those who have had depression in the past, and first-onset of the disease in later life. The knowledge that vascular illness and elevated risk factors might have a specific effect on brain structure is important in understanding depression, and raises further questions about the links between depression and cognitive impairment. Both conditions not only share common risk factors, but also structural changes (such as hippocampal atrophy (McKinnon et al., 2009) and reduced brain volume (Dotson et al., 2009)). Also, neuropsychological deficits in executive function and processing speed are well documented in depression (Herrmann et al., 2007). Impaired white-matter integrity within pre-frontal regions would be a plausible explanation linking structural and functional changes in both conditions (Buckner, 2004; Park and Reuter-Lorenz, 2009; Shimony et al., 2009).
A key strength of this study was the MRI acquisition process which entailed two repeats of sixty-direction DTI. This exceeds the suggested minimum of thirty directions estimated as needed for robust estimation of FA (Jones, 2004) and is higher than many published studies (Sexton, et al., 2009). There are, however, limitations to this study particularly in the recruitment of the sample which was not systematically ascertained. Furthermore, most of the depressed participants had early-onset disease (n=23), before the age of 60, rather than late-onset disease (n=13). Although age at onset was not the focus of this study, the literature suggests that the impact of vascular risk on mood is greater for those developing depression over the age of 60, and it will be of interest to explore the effect of age at onset in more detail within a larger sample.
Participants were not excluded on the basis of medication. Whilst this means that our results are more representative of those who are routinely seen in specialist mental health care settings, it is also possible that medication may lead to structural brain changes that could influence our results. Given the age of our sample, where many have co-morbid health problems, and the nature of the sample (recruitment of those with major depressive disorder), excluding people on the basis of medication would have not only made recruitment much more difficult, but would also have introduced new bias to the study.
Framingham scores and spread were similar in both groups, yet there was no correlation with FA in the control group. There are several possible explanations for this, relating to both cases and controls. It may be that those people becoming “cases” have pre-existing impairments in white-matter integrity which provide a biological vulnerability to depression, not uniquely related to vascular disease. Another explanation is that depression itself might induce changes within white-matter structure. Whilst this cross-sectional study does not provide information about the causality of vascular brain changes, this could be addressed through future longitudinal studies. On the other hand, controls may have specific protective factors which offer them resilience against white-matter change, and developing depression. These are most likely to be biological, for example, functional differences with increased recruitment of other parts of the brain, particularly in frontal regions (Buckner, 2004; Park and Reuter-Lorenz, 2009). Medication may also be a protective factor (greater use of statins could be one possibility). Finally, it may mean that the level of cardiovascular risk as measured by FSRP does not correlate accurately with structural brain changes, and that alternative measures of vascular risk need to be investigated. Although this study was not designed to address these questions, they provide interesting scope for future research.
In summary, this exploratory study combines the FSRP, a well validated, multi-factorial, cardiovascular risk algorithm, with white-matter changes quantified with DTI. Our results suggest that even in the absence of major vascular disease, elevated FSRPs in those with depression are associated with significant changes in white-matter connectivity, to a degree not observed within the control group. This is apparent in widespread regions of the brain, most significantly within the corpus callosum and corticospinal tract. Greater understanding of the mechanisms by which vascular risk may contribute to pathophysiology in depression is important, in order to prevent and treat this common illness. These results support the vascular depression hypothesis, and should lead to further research within larger, systematically (and preferably prospectively) acquired cohorts.
Acknowledgments
Many thanks to all those who have helped with this study, particularly, clinical staff who referred patients, Dr Kevin Bradley, Consultant Radiologist, who reviewed brain scans and most importantly to all participants and their relatives. Funding was provided from the Gordon Edward Small’s Charitable Trust (Scottish Charity Register: SC008962). CLA had support from Oxford University Clinical Academic Graduate School (OUCAGS@medsci.ox.ac.uk).
Footnotes
Conflict of interest
None
Description of authors’ roles
CLA undertook clinical interviews and analysed the data. CES co-ordinated the study, undertook recruitment and data collection, and analysesd the data. UGK and LMM undertook recruitment and data collection. CEM was involved in design, supervised the project and advised on analysis. KPE designed the study, undertook clinical interviews and supervised data collection and analysis. CLA wrote the first draft. CES, UGK, LMM, MK, ASM, CEM and KPE reviewed the manuscript, were involved in re-writing and approved the final version.
References
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007a. from www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007b. from www.fmrib.ox.ac.uk/analysis/techrep.
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Chen CS, Chen CC, Kuo YT, Chiang IC, Ko CH, Lin HF. Carotid intima-media thickness in late-onset major depressive disorder. International Journal of Geriatric Psychiatry. 2006;21(1):36–42. doi: 10.1002/gps.1420. [DOI] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. Trends in the health of the elderly. Annual Review of Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- Dalby RB, Chakravarty MM, Ahdidan J, Sorensen L, Frandsen J, Jonsdottir KY, et al. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychological Medicine. 2010;40(8):1389–1399. doi: 10.1017/S0033291709991656. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders : DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. Journal of Psychiatry & Neuroscience. 2009;34(5):367–375. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Quach L, Yang F, Chaves PH, Newman AB, Mukamal K, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the cardiovascular health study. Circulation. 2011;123(8):858–865. doi: 10.1161/CIRCULATIONAHA.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal Social and Clinical Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Herrmann L, Lemasurier M, Ebmeier K. White matter hyperintensities in late life depression: A systematic review. Journal of Neurology Neurosurgery and Psychiatry. 2008;79(6):619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychological Medicine. 2007;37(12):1693–1702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, et al. Blood pressure and white matter integrity in geriatric depression. Journal of Affective Disorders. 2009;115(1–2):171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35(8):1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. [Comparative Study] Magnetic Resonance in Medicine. 2004;51(4):807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- McGurn B, Starr JM, Topfer JA, Pattie A, Whiteman MC, Lemmon HA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62(7):1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry & Neuroscience. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s Cognitive Examination revised (ACE-R): A brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry. 2006;21(11):1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office-for-National-Statistics. National Population Projections. 27 2008. 2008-based from http://www.statistics.gov.uk/downloads/theme_population/pp2no27.pdf.
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC. From UBOs to Binswanger’s disease. Impact of magnetic resonance imaging on vascular dementia research. Stroke. 1996;27(8):1269–1273. doi: 10.1161/01.str.27.8.1269. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Allan CL, Bradley K, Herrmann LL, Kalu UG, McDermott L, et al. Network Disruption in Late-Life Depression Examined with MultiModal Magnetic Resonance Imaging. 2011. In Submission. [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biological Psychiatry. 2009;66(9):814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial.[Erratum appears in Arch Gen Psychiatry. 2010 Oct;67(10):1043 Note: Welsh-Boehmer, Kathleen [corrected to Welsh-Bohmer, Kathleen]] Archives of General Psychiatry. 2010;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. American Journal of Psychiatry. 2008;165(4):524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. [Research Support, N.I.H., Extramural] Biological Psychiatry. 2009;66(3):245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Babyak MA, Doraiswamy PM, Hinderliter A, Hoffman BM, et al. Intima-media thickness and age of first depressive episode. Biological psychology. 2009;80(3):361–364. doi: 10.1016/j.biopsycho.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Babyak MA, Watkins LL, Hinderliter A, Hoffman BM, et al. Cerebrovascular risk factors and cerebral hyperintensities among middle-aged and older adults with major depression. American Journal of Geriatric Psychiatry. 2010;18(9):848–852. doi: 10.1097/JGP.0b013e3181dba0fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Kalaria RN, O’Brien JT. Depression and vascular disease: what is the relationship? [Review] Journal of Affective Disorders. 2004;79(1–3):81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O’Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Archives of General Psychiatry. 2002;59(9):785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O’Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. [Research Support, Non-U.S. Gov’t] International Journal of Geriatric Psychiatry. 2003;18(1):7–13. doi: 10.1002/gps.720. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]