Abstract
Falls are experienced annually by approximately one third of community dwellers over the age of 65, and while neuro-cognitive deficits have been shown to increase falls risk, the specific nature of these deficits remain unspecified. Here we examined whether visual-spatial attention may be a core neuro-cognitive system showing abnormal function in fallers. Using a between-groups design, we recorded event-related potentials in a canonical spatial cuing task performed by two groups of senior (aged 65+ years old) participants: those with a recent history of falls and those with no such history. In terms of attentional control systems in cortex, we found no significant differences in function between groups. However, in terms of attentional facilitation of cortical processing, we found that fallers manifest specific abnormalities in the sensory/perceptual processing of targets in the left visual field. Our findings thus suggest that fallers have specific deficits in visuocortical systems associated with attentional enhancement of events on the left side of visual space.
Keywords: falls, cognitive impairment, visual-spatial attention, aging
INTRODUCTION
Falls in seniors is a major health care concern due to the injuries and injury-related death associated with falling. Surprisingly, factors other than just peripheral musculoskeletal problems contribute to falls risk. Basic deficits in cognitive function have been shown to be associated with falls (Clark, Lord, & Webster, 1993; Tinetti, Speechley, & Ginter, 1988) although the specific nature of these cognitive impairments have remained unclear. Our paper here hypothesizes that one specific aspect of cognition that may be related to falls risk is visual-spatial attention.
Why might visual-spatial attention be involved? There are at least three key pieces of evidence that suggest that visual-spatial attention is an important aspect of cognition to explore as a factor involved in falls risk.
First, visual-spatial attention has been linked to motor functions in normals (Handy et al., 2005). While visual-spatial attention has traditionally been associated with the ventral, or “what” pathway (Posner, 1980), research has recently began to focus on its role in the dorsal, or “how” pathway using vision to guide actions (Handy et al., 2003, 2005; Handy & Tipper, 2007). This suggests that problems that lead to falls, such as trouble planning and guiding movements, may be caused by underlying impairments in visual-spatial attention.
Second, deficits in visual-spatial processing are frequently the first symptom to appear in older populations as an indicator of age-related illness. Specifically, deficits in spatial abilities are often the first non-memory cognitive function to be impaired in age-related neurological disorders associated with increased falls risk, such as Alzheimer’s disease (Bagurdes et al., 2008; Drago et al., 2008). For example, Parasuraman et al. (1992) found visual-spatial deficits in patients with dementia of the Alzheimer type (DAT) using a spatial cueing paradigm. Additionally, Alzheimer’s patients show deficits in the perception of motion (Rizzo & Nawrot, 1998), which is integral to safe movement through the environment.
Third, fallers may have a narrowed focus of attention compared to non-fallers. In a study by Liu-Ambrose et al. (2008), fallers were shown to have less interference than non-fallers induced by peripheral flankers in the Erikson Flanker task. These results were interpreted as fallers having a more narrowed, or direct, focus of attention, leading to less distraction from the peripheral flanking arrows. Collectively, these three pieces of evidence point towards visual-spatial attention as a clear candidate for cognitive deficits associated with falls risk in seniors.
There are two separate aspects of visual-spatial attention that may be impaired in fallers: attentional control and attentional facilitation. Attentional control is the orienting or directing of attention to a particular location in space (Green & McDonald, 2008). On the other hand, attentional facilitation is the increase in the visual sensory-evoked cortical response for a stimulus in an attended location (Mangun, Hillyard, & Luck, 1993). Given these two aspects of visual-spatial attention, the main question of our study is whether either or both attentional control and attentional facilitation are impaired in seniors with a recent history of falls. Specifically, do fallers show deficits in the ability to orient attention to begin with, or if they are able to direct their attention, is there a deficit in the perceptual/sensory benefit for the cued location usually observed among normals?
In the current study, attentional control and attentional facilitations were assessed in fallers and non-fallers using event-related potentials (ERPs) in a spatial cueing paradigm (Posner, 1980). Attentional control was assessed by examining the ERP components elicited by attention-directing cues. The anterior directing attention negativity (ADAN) reflects correlates of directing attention (Green & McDonald, 2008), while the early attentional directing negativity (EDAN) reflects comprehension of attentional cues (Harter et al., 1989; Hopf & Mangun, 2000; van Velzen et al., 2002). Second, attentional facilitation was assessed by examining the ERP components elicited by visual targets. Sensory aspects of attentional facilitation are measured by an increase in the amplitude of the P1 and N1 components while cognitive aspects, such as expectancy, are measured by an increase in amplitude of the P3, Nd1, and Nd2 components for unexpected targets relative to expected targets. Given the importance of identifying falls risk factors, our primary aim was to determine whether there are impairments in fallers in terms of attentional control, attentional factilitation, or both aspects of visual-spatial attention.
METHODS
Subjects
Participants were a subset of senior women, aged 65–75 years, who participated in a 12-month prospective study examining the role of exercise on executive functioning. Women were used exclusively in this study due to differences in cognitive responses to exercise between genders (Colcombe & Kramer, 2003). Additionally, women are at greater risk for falls (Lord, Sherrington, & Menz, 2001). The incidence of falls was monitored throughout the 12-month study via monthly calendars.
Ten community-dwelling women who had experienced ≥ 2 minimal displacement non-syncopal falls in the previous six months prior to the study, aged 65–74 years (M = 69.8, SD = 3.16) participated in the study. One faller was left-handed and all had normal or corrected-to-normal vision. Fallers had on average 3 falls (SD = 1.25), ranging between 2 and 6 falls.
In addressing the question of possible visual-spatial attentional deficits in seniors with a history of falls, it is important to distinguish between impairments due to aging in general versus impairments specifically correlating with falls risk. Visual-spatial attention is relatively well preserved with age (Curran et al., 2001; Kok, 2000; Lorenzo-Lopez et al., 2002), although some notable differences between seniors and young controls have been found. Due to these reported age-related differences, our study included an age-matched control group of non-fallers as a normative reference. Ten community-dwelling controls, aged 66–74 years (M = 69.0, SD = 2.67) participated in the study. To be included in the “non-fallers” control group, individuals must not have experienced any minimal displacement falls (with or without syncope) in the previous six months prior to this study.
General inclusion criteria for all participants included an MMSE score ≥ 24 and visual acuity of at least 20/40 with or without corrective lenses. General exclusion criteria for all participants included those with neurodegenerative disease (e.g., Alzheimer’s disease) and stroke, those who were currently taking psychotropic drugs, and those with a history indicative of carotid sinus sensitivity (i.e., syncopal falls). All participants provided written informed consent at the beginning of the study.
Descriptive measures
To reduce the number of possible confounding variables in the association between impaired visual attention and a recent history of falls, several descriptive measures were obtained for all participants (Table 1). Global cognitive state was assessed using the Montreal Cognitive Assessment (MOCA), where the maximum score is 30 and higher scores indicate better performance. The Geriatric Depression Scale (GSD) was used to screen for depression, where a score of 11 and above indicates severe depression. General mobility was assessed by the Timed Up and Go Test (TUG), which instructs participants to rise from a standard chair with arms, walk a distance of three meters, turn, walk back to their chair and sit down again. Faster times indicate better performance. Physiological falls risk was assessed by the Physiological Profile Assessment (PPA) (Lord, Sherrington, & Menz 2001) which assesses vision, proprioception, strength, reaction time, and balance. A PPA z-score below 0 indicates low risk for falling, 0–1 indicates mild risk, 1–2 indicates moderate risk, and 2 and above indicates high risk. Cognitive performance of three central executive functions were assessed: 1) set shifting, assessed using Trail Making Test B; 2) updating (working memory), assessed using the digits forward and back tests; and 3) response inhibition, assessed using the Stroop Colour Word Test. Faster times on both Trail Making Test B and Stroop indicate better performance. Digits forward and back tests are measured by number of digits correctly completed.
Table 1.
Descriptive Measures for Non-Fallers and Fallers
| Measure | Non-Fallersa
|
Fallersa
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Ageb | 69.00 | 2.67 | 69.80 | 3.16 |
| MOCA | 24.60 | 2.63 | 26.50 | 1.90 |
| GDS | 0.50 | 1.58 | 1.00 | 2.16 |
| TUGc | 6.44 | 1.58 | 5.99 | 0.60 |
| ABC | 91.38 | 14.32 | 94.16 | 5.96 |
| PPA | 0.04 | 0.56 | −0.66 | 0.92 |
| Digits forward | 8.70 | 0.95 | 8.40 | 1.84 |
| Digits backward | 4.20 | 2.53 | 4.50 | 1.90 |
| Trail Bc | 89.66 | 45.94 | 67.43 | 17.64 |
| Stroopc | 79.16 | 9.19 | 82.71 | 18.36 |
n = 10 for each group.
Years.
Seconds.
Apparatus and Stimuli
Stimuli were presented on an 18 inch colour monitor placed 100 cm from the subject. At the beginning of each trial, a fixation cross was presented in the centre of the screen for 1000 ms. Next, an arrow (1.26° × 0.46°) was presented at fixation and cued either the left or the right target location. The cue remained on the screen for the rest of the trial. The target, which was an “X” (0.92° × 0.92°), appeared 1000 ms (randomly jittered between 900 and 1100 ms) after the on-set of the cue in either in the left visual field or the right visual field (target was 4.57° from the top of the screen 11.31° from the bottom of the screen, and 4.86° from the left/right edge of the screen) and remained on the screen until a response was made. The arrow predicted the target location with 80% accuracy. After the response, the next trial began immediately, with the presentation of the next fixation cross.
Procedure
The task requires subjects to indicate via button presses whether the target appeared in the right visual field or left visual field, as quickly and accurately as possible. Participants were instructed to press one button with their left hand if the target appeared on the left, and another button with their right hand if the target appeared on the right. There were 12 blocks all together, each with 76 trials (60 cued, 12 uncued, 4 catch). Each block lasted approximately 4 minutes. Subjects were told to keep their eyes on the central fixation point for the duration of the experiment.
Electrophysiological recording and Analysis
During task performance, electroencephalograms (EEG) were recorded from 32 active electrodes (Bio-Semi Active 2 system) evenly distributed over the head. All EEG activity was recorded relative to two scalp electrodes located over medial-frontal cortex (CMS/DRL), using a second order low pass filter of .05 Hz, with a gain of .5 and digitized on-line at a sampling rate of 256 samples-per-second. To ensure proper eye fixation and allow for the correction and/or removal of events associated with eye movement artifacts, vertical and horizontal electro-oculograms (EOGs) were also recorded, the vertical EOG from an electrode inferior to the right eye, and the horizontal EOG from an electrode on the right outer canthus. Off-line, computerized artifact rejection was used to eliminate trials during which detectable eye movements (> 1°), blinks, muscle potentials, or amplifier blocking occurred. For each subject, ERPs were averaged into 3,000 ms epochs, beginning 1,500 ms before stimulus onset. Subsequently, all ERPs were algebraically re-referenced to the average of the left- and right-mastoid signals, and filtered with a low-pass Gaussian filter (10 Hz half-amplitude cutoff) to eliminate high-frequency artifacts in the waveforms. The resulting ERPs (on average, 456 cued and 432 uncued trials per subject) were then used to produce grand-averaged waveforms. Statistical quantification of ERP data was based on mean amplitude measures relative to a -200 to 0 pre-stimulus baseline.
In terms of statistical analysis, repeated-measures mixed-model ANOVAs were used, using unpooled error terms in order to account for potential violations of sphericity for factors having more than 2 levels (Handy, Nagamatsu, Mickelborough, & Liu-Ambrose, In press).
Behavioural analysis
Behavioural data (reaction times and accuracy) were analyzed using an ANOVA with factors of group (fallers vs. non-fallers), visual field (left vs. right), and cueing (cued vs. uncued).
Electrophysiological analysis
The two aspects of visual-spatial attention that we examined were attentional control and attentional facilitation. Based on previous work examining visual-spatial attention in seniors, delayed latencies for the P1, N1, and P3 components, as well as differences in ERP morphology, such as attenuated P1 and N1 amplitudes, have been established as normative for seniors relative to young adult controls (Curran et al., 2001). Therefore, time ranges and electrode sites for each component were chosen according to standard windows and locations for examining these components in seniors.
Attentional control can be separated into the control of covert attentional orienting, which is measured by the ADAN (Anterior directing attention negativity, Seiss et al., 2007) component to the cue, and the appreciation of the meaning of the symbolic cue, which is measured by the EDAN (Early directing attention negativity, Seiss et al., 2007) component to the cue. Both the ADAN and the EDAN were examined for sites that are ipsilateral versus contralateral to the cued visual field, with a greater negativity expected at electrodes contralateral to the direction of the cue compared to electrodes ipsilateral to the cued direction. Therefore, for each component, effects involving factors of laterality and between-groups differences were examined, with results involving other factors being tangential to the focus of our study. Attentional control was analyzed using a mixed-model repeated-measures ANOVA with factors of group (fallers vs. non-fallers), visual field (left vs. right), and laterality (ipsilateral vs. contralateral to the cued visual field).
Attentional facilitation can be further separated into sensory aspects of target responses, measured as an increase in amplitude of the P1 and N1 components to targets, and cognitive aspects of target responses, measured as an increase in amplitude of the P3, Nd1, and Nd2 components to the targets. For the P1 and N1, between-groups effects were analyzed via a mixed-model repeated-measures ANOVA that had factors of group (fallers vs. non-fallers), visual field (left vs. right), cueing (cued vs. uncued), and laterality (ipsilateral vs. contralateral to the visual field of the target). The P1 and N1 components were examined for targets which were cued versus uncued, with an increased amplitude expected for attended targets relative to unattended targets. The analysis for cognitive components was the same as for sensory/perceptual components, excluding the factor of laterality. The P3, Nd1, and Nd2 components were examined for cued relative to uncued targets. As these components reflect expectancies, the amplitudes of the P3, Nd1, and Nd2 are larger for unattended targets versus attended targets. Results presented for attentional facilitation involved effects of cueing and between-groups effects, with other factors being extraneous to the focus of our study.
RESULTS
Descriptive measures
The mean scores and standard deviations for each of the descriptive measures are presented in Table 1. Independent samples t-tests were done for each descriptive measure (SPSS 12.0) and indicated that fallers and non-fallers did not significantly differ on any of the descriptive variables, all p values > .05. Specifically, the fallers and non-fallers were equally matched on age, MOCA, depression level, mobility, physiological falls risk, balance, set shifting, updating, and response inhibition.
Behaviour
Mean reaction times and accuracy scores are shown in Table 2 as a function of group (fallers vs. non-fallers) and attentional condition (cued vs. uncued). There were no significant differences in the reaction times or accuracy of fallers and non-fallers, F(1,18) = 0.11, p = 0.74. A significant main effect of cueing was found, F(1,18) = 13.89, p < 0.01, indicating reaction times were faster for cued relative to uncued trials. A significant main effect of visual field was also found, F(1,18) = 8.20, p = 0.01, indicating that participants were faster responding to targets in the right visual field compared to targets in the left visual field.
Table 2.
Behavioural Results for Non-Fallers and Fallers
| Condition | Non-Fallersa
|
Fallersa
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Cuedb | ||||
| Left | 0.46 | 0.06 | 0.43 | 0.10 |
| Right | 0.43 | 0.06 | 0.43 | 0.10 |
| Uncuedb | ||||
| Left | 0.50 | 0.05 | 0.50 | 0.12 |
| Right | 0.48 | 0.07 | 0.47 | 0.10 |
| Accuracyc | ||||
| Left | 0.60 | 1.07 | 1.00 | 1.56 |
| Right | 1.00 | 1.41 | 1.60 | 1.07 |
n = 10 for each group.
Reaction times measured in seconds.
Number errors.
Electrophysiology
Attentional Control
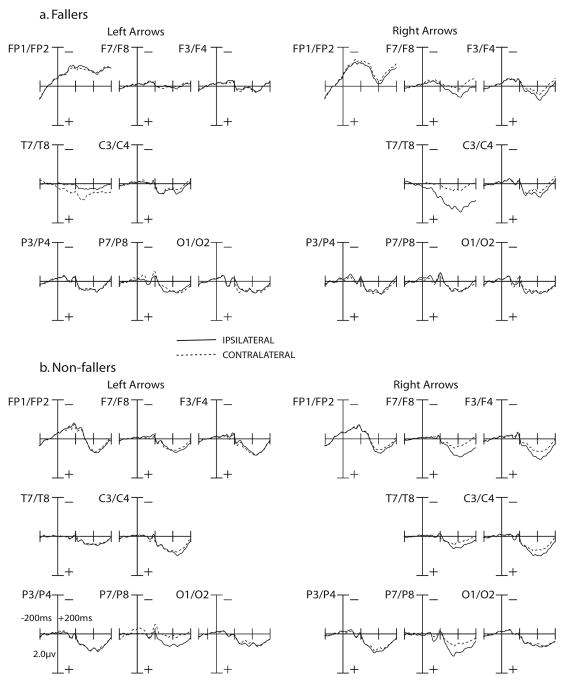
The plots for the ADAN component are presented in Figure 1 and mean amplitudes are presented in Table 3. The ADAN was examined at a time window of 310–440 ms post-cue at electrodes FP1, FP2, F7, F8, F3, F4, C3, and C4 (Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007; Talsma et al., 2005; van Velzen & Eimer, 2003). Between-group differences approached significance, F(1,18) = 3.41, p = .08. Specifically, there was a trend, where non-fallers tended to have a higher overall mean amplitude for the ADAN component, regardless of condition. Across all participants, the ADAN amplitude to cues was more negative in contralateral sites compared to ipsilateral sites to the visual field that was cued. This was confirmed by a significant main effect of laterality, F(1,18) = 20.86, p < .001. There was also a significant visual field by laterality interaction, F(1,18) = 6.13, p = .02, where the difference in amplitude between ipsilateral and contralateral sites was significantly greater in the right visual field than the left visual field.
Fig. 1.
Grand-averaged ERP responses to cues for the ADAN and EDAN components in fallers (top) and non-fallers (bottom), as a function of laterality (ispilateral vs. contralateral to cued visual field). Time window is out to 600 ms post-cue, with a 200 ms pre-cue baseline. Amplitude measured in uV. There were no significant differences between fallers and non-fallers for the ADAN and EDAN components.
Table 3.
Mean Peak Amplitudes for Attentional Control for Non-Fallers and Fallers
| Conditiona | Non-Fallersb
|
Fallersb
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| ADAN | ||||
| Ipsilateral | 0.69 | 0.61 | 0.09 | 1.08 |
| Contralateral | 0.56 | 0.56 | −0.02 | 1.00 |
|
| ||||
| EDAN | ||||
| Isilateral | 0.42 | 0.54 | 0.18 | 1.02 |
| Contralateral | 0.33 | 0.46 | 0.12 | 0.87 |
Peak amplitudes measured in uV.
n = 10 for each group
The plots for the EDAN component are presented in Figure 1 and mean amplitudes are presented in Table 3. The EDAN component was examined at a time window of 200–400 ms post-cue at electrodes FP1, FP2, F7, F8, F3, F4, T7, T8, C3, C4, P3, P4, P7, P8, O1, and O2 (Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007; Talsma et al., 2005; van Velzen & Eimer, 2003). There were no significant between-groups differences, F(1,18) = 1.22, p = .28. The EDAN amplitude was more negative for cues in contralateral sites relative to ipsilateral sites to the cued visual field. This was confirmed via a main effect of laterality, F(1,18) = 7.32, p = .01. Additionally, there was a significant visual field by laterality interaction, F(1,18) = 4.60, p = .05. In the right visual field, ipsilateral sites showed a larger EDAN amplitude than contralateral sites, but in the left visual field, the opposite pattern was observed with larger amplitudes in contralateral sites relative to ipsilateral sites.
Attentional Facilitation
Sensory/perceptual components
The plots for the P1 component are presented in Figure 2 and mean amplitudes are presented in Table 4. The P1 to targets was analyzed looking at a time window of 100–150 ms post-stimulus at electrode sites OL and OR (Mangun & Hillyard, 1991). Across both groups of participants, when sites were ipsilateral to targets, the P1 amplitude was larger for cued trials compared to uncued trials, whereas when sites were contralateral to targets, the P1 amplitude was larger for uncued trials, replicating the P1 results in seniors from Curran et al. (2001). This was confirmed by a significant cueing by laterality interaction, F(1,18) = 6.37, p = .02. Between fallers and non-fallers, there was an effect of cueing by visual field. Specifially, the P1 amplitudes were different between fallers and non-fallers in contralateral sites to targets in the left visual field. This observation was supported by a significant group by visual field by cueing by laterality interaction, F(1,18) = 4.79, p = .04. This interaction was followed up by a within-groups analysis looking at fallers and non-fallers separately. Fallers showed a larger P1 amplitude in ispsilateral sites for cued targets relative to uncued targets and larger P1 amplitude in contralateral sites for uncued targets relative to cued targets in both visual fields, as confirmed by a significant cueing by laterality interaction, F(1,9) = 10.41, p = .01. In contrast, non-fallers showed a difference in the cueing by laterality interaction for the left versus right visual field. In the left visual field, the P1 amplitude was larger in both ipsilateral and contralateral sites to the visual field of the target for cued targets compared to uncued targets. In the right visual field, the P1 amplitude was larger in ipsilateral sites for cued targets relative to uncued targets, but larger in contralateral sites for uncued targets compared to cued targets. This was confirmed by a trend towards a visual field by cueing by laterality interaction, F(1,9) = 4.44, p = .06 in non-fallers.
Fig. 2.
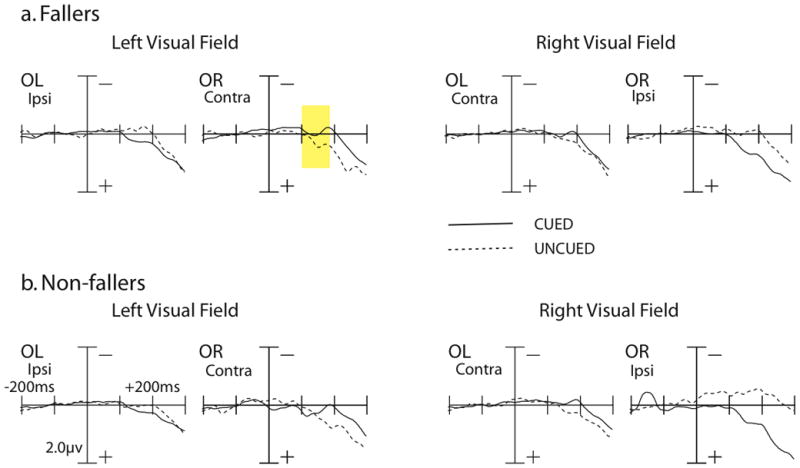
Grand-averaged ERP responses to targets for the P1 and N1 time windows. Data are shown as a function of visual field (left vs. right) and cueing (cued vs. uncued) for fallers (top) and non-fallers (bottom). Time window is out to 300 ms post-cue, with a 200 ms pre-cue baseline. Amplitude measured in uV. For the P1 component, fallers showed a larger amplitude for uncued trials relative to cued trials in the left visual field for contralateral sites (highlighted in yellow). In contrast, non-fallers showed a larger P1 amplitude for cued trials relative to uncued trials. There were no significant differences between fallers and non-fallers for the N1 component.
Table 4.
Mean Peak Amplitudes for Attentional Facilitation for Non-Fallers and Fallers
| Conditiona | Non-Fallersb
|
Fallersb
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| P1 | ||||
| Cued ipsilateral | ||||
| Left | 0.03 | 0.12 | 0.10 | 0.21 |
| Right | 0.26 | 0.60 | 0.22 | 0.39 |
| Cued contralateral | ||||
| Left | 0.30 | 0.85 | −0.04 | 0.35 |
| Right | −0.10 | 0.25 | −0.01 | 0.36 |
| Uncued ipsilateral | ||||
| Left | 0.02 | 0.34 | −0.16 | 0.39 |
| Right | −0.39 | 0.73 | −0.07 | 0.56 |
| Uncued contralateral | ||||
| Left | 0.18 | 0.83 | 0.16 | 0.37 |
| Right | −0.01 | 0.26 | 0.05 | 0.25 |
|
| ||||
| N1 | ||||
| Cued ipsilateral | ||||
| Left | 0.23 | 0.24 | 0.27 | 0.35 |
| Right | 0.62 | 1.24 | 0.65 | 0.63 |
| Cued contralateral | ||||
| Left | 0.10 | 0.85 | −0.14 | 0.39 |
| Right | −0.13 | 0.30 | −0.02 | 0.55 |
| Uncued ipsilateral | ||||
| Left | 0.01 | 0.32 | −0.17 | 0.60 |
| Right | −0.51 | 0.88 | −0.10 | 0.75 |
| Uncued contralateral | ||||
| Left | 0.60 | 0.75 | 0.42 | 0.58 |
| Right | 0.25 | 0.27 | 0.22 | 0.38 |
|
| ||||
| P3 | ||||
| Cued | 1.58 | 0.09 | 1.00 | 1.07 |
| Uncued | 2.44 | 1.07 | 3.01 | 1.46 |
|
| ||||
| Nd1 | ||||
| Cued | 0.30 | 0.38 | 0.48 | 0.58 |
| Uncued | 0.59 | 0.49 | 0.62 | 0.78 |
|
| ||||
| Nd2 | ||||
| Cued | 0.98 | 0.65 | 1.31 | 0.94 |
| Uncued | 1.48 | 0.82 | 1.69 | 1.06 |
Peak amplitdues measured in uV.
n = 10 for each group.
The plots of the N1 components can be seen in Figure 2 and mean amplitudes are presented in Table 4. The N1 was analyzed looking at a time window of 150–200 ms post-stimulus at electrode sites OL and OR (Mangun & Hillyard, 1991). No between-groups differences were found for the N1 component, F(1,18) = 0.00, p = .97. In the right visual field, N1 amplitudes were larger for cued trials relative to uncued trials whereas in the left visual field, N1 amplitudes were larger for uncued trials relative to cued trials, as confirmed via a significant visual field by cueing interaction, F(1,18) = 6.13, p = .02. When sites were ipsilateral to the targets, the N1 amplitude was larger for cued trials compared to uncued trials. When sites were contralateral to targets, however, the N1 amplitude was larger for uncued trials. This was supported by a significant cueing by laterality interaction, F(1,18) = 54.28, p < .001. These results suggest normal modulations of the N1 component for both fallers and non-fallers.
Cognitive/post-perceptual components
The plots for the P3 component can be seen in Figure 3 and mean amplitudes are presented in Table 4. The P3 component was examined at a time window of 350–450 ms post-stimulus at electrode sites FZ, CZ, and PZ (Eimer, 1996; Eimer, 1998). No significant between-groups differences were found, F(1,19) = 1.11, p = .31. Normal modulations of the P3 component were found for both groups (Curran et al., 2001; Eimer, 1996; Eimer, 1998), with larger amplitudes for the P3 component for uncued relative to cued trials, indicated by a significant main effect for cueing, F(1,18) = 39.90, p < .001.
Fig. 3.
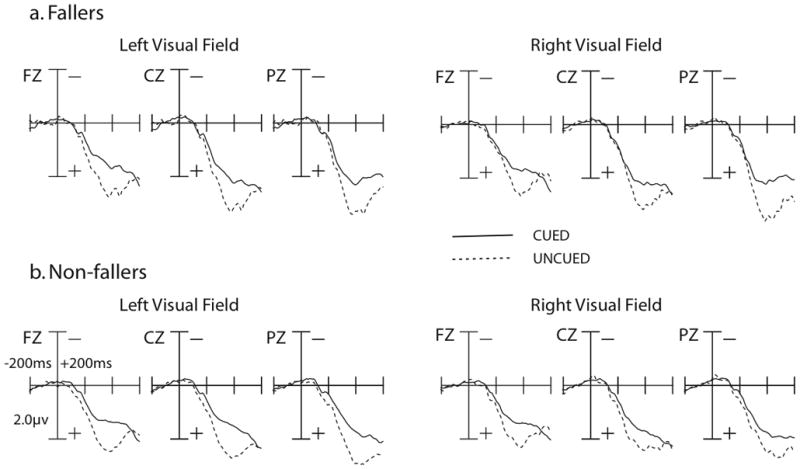
Grand-averaged ERP responses to targets for the P3, Nd1, and Nd2 components in fallers (top) and non-fallers (bottom). Time window is out to 600 ms post-cue, with a 200 ms pre-cue baseline. Amplitude measured in uV. There were no significant differences between fallers and non-fallers for the P3, Nd1, and Nd2 components.
The plots for the Nd1 component can be seen in Figure 3 and mean amplitudes are presented in Table 4. The Nd1 component was examined at a time window of 150–200 ms post-stimulus at electrode sites FZ, CZ, and PZ (Eimer, 1996; Eimer, 1998). No significant between-groups differences were found, F(1,18) = 0.24, p = .63, but both fallers and non-fallers showed normal Nd1 modulations (Curran et al., 2001; Eimer, 1996; Eimer, 1998). Specifically, uncued trials showed a larger Nd1 amplitude than cued trials, confirmed via a significant main effect of cueing, F(1,18) = 12.66, p < .01.
The plots for the Nd2 component can be seen in Figure 3 and mean amplitudes are presented in Table 4. The Nd2 component was examined at a time window of 220–300 ms post-stimulus at electrode sites FZ, CZ, and PZ (Eimer, 1996; Eimer, 1998). No significant between-groups differences were found, F(1,18) = 0.53, p = .48, although both groups showed normal Nd2 modulations (Curran et al., 2001; Eimer, 1996; Eimer, 1998). A main effect of cueing, F(1,18) = 23.84, p < .001 was found, where uncued trials showed a larger Nd2 amplitude than cued trials.
DISCUSSION
The goal of our study was to examine whether seniors with a history of falls show deficits in visual spatial attention relative to age-matched controls. In this regard, two aspects of visual-spatial attention were assessed: attentional control, which concerns the ability to orient attention to a particular location in visual space, and attentional facilitation, which concerns whether attentional orienting actually affects or modulates sensory/perceptual sensitivity at the attended location.
In terms of attentional control, fallers and non-fallers showed no significant differences in function in that both groups were able to direct their attention towards the cued location. This was indicated by the presence of ADAN and EDAN components in the ERPs elicited by cues. However, in terms of attentional facilitation, fallers showed impairments in the normal ability of attention to modulate visual sensory processing. Specifically, both groups showed increases in the amplitude of the P1 ERP component for attended vs. unattended targets in the right visual field. In contrast, for targets in the left visual fields, only non-fallers showed the normal attention-related increase in P1 amplitude. There were no group differences in terms of cognitive aspects of attention, such as expectancy, as indicated by normal modulation of the P3, Nd1, and Nd2 components in both fallers and non-fallers. Our results thus suggest that the difference between fallers and non-fallers is not in generating an attentional orienting response to begin with or later cognitive processing of the targets, but rather, in their ability for attention to facilitate or enhance visual processing in the left visual field.
That fallers may show impairments in spatial attention-related facilitation is consistent with our recent finding that fallers appear to have a narrowed focus of attention at fixation (Liu-Ambrose et al., 2008). To the point, we found that fallers showed reduced response interference in an Eriksen flanker task relative to age-matched controls, data suggesting that there was a reduction in attentional processing of distractors distal to the target at fixation. Our current findings expand our understanding of spatial attention deficits in fallers by demonstrating that this population also appears to have a reduced ability to facilitate perceptual processing when attention is oriented to the left side of visual space. Given this conclusion, at least two key questions follow.
First, why might visual-spatial attention only be impaired in the left visual field of fallers? Several converging lines of evidence suggest that the left visual field is particularly susceptible to attentional deficits from neurological conditions or disorders. For example, patients with unilateral visual neglect are more likely to manifest neglect in the left visual field relative to the right (Bublak, Redel, & Finke, 2006; Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990). Why? Visual-spatial attention studies with split-brain patients suggest that the attentional bias in the right hemisphere is the result of the two hemispheres working independently to orient attention (Mangun et al., 1994). While the right hemisphere appears capable of orienting attention to both sides of visual space, the left hemisphere orients exclusively to the right visual field. As a consequence, whereas damage to the left hemisphere leaves the right hemisphere still capable of orienting to both the left and right side of space, damage to the right hemisphere leaves the left hemisphere only orienting to the right side of space. The importance of understanding this relationship between spatial attention and cerebral hemispheres is that our data here would thus suggest that the basis for neurocognitive deficits in fallers may be right hemisphere specific.
Second, if fallers have impaired visual-spatial attention in the left visual field, how might this lead to falls? We suggest that attentional deficits may lead to falls in both direct and indirect ways. First, these deficits may lead to falls directly by causing one to fail to notice something immediately relevant for falls-avoidance. For example, it has been hypothesized that falls risk may be associated with abnormalities in attentional abilities in the lower visual field (Di Fabio et al., 2005), indicating that decreased attention to objects located on the ground, such as a step, may pose as potential fall hazards. While our study investigated attention in the left versus right visual fields, future studies will examine attention in the upper versus lower visual fields to further consider the role of visual-spatial attention in falls.
At the same time, indirect links between visual-spatial attention and falls may stem from a lack of motor coordination with the hands and vision. Visual-spatial attention has been shown to be integral for the planning of object-related actions, such as grasping objects (Handy et al., 2005). There are hand-related objects in the environment that aid in successful movement and vision is integral for their proper implementation. For example, an impairment in the ability to use vision to accurately judge the distance of a handrail may result in a fall, or the inability to properly organize one’s hand configuration to grasp a handrail to either steady oneself when negotiating stairs or catch oneself when actually starting to fall. While it is clear that there are both possible direct and indirect factors linking falls and visual-spatial attention, further studies are necessary in order to determine the exact mechanisms leading to falls.
In closing, there are two additional issues worth noting regarding how we have interpreted our results. First, although a between-group difference in the ADAN ERP component approached significance (P = 0.08), we interpreted this result as suggesting that there were no between-group differences in attentional control. While we recognize that the absence of significance may be power-related due to small sample sizes within each group, the pattern of results for attentional control were nevertheless inconsistent with the between-groups effect we found for attentional facilitation. Specifically, differences in attentional facilitation between fallers and non-fallers were in the left visual field. If fallers did have impairments in attentional control, we would expect to see a similar pattern of results. Instead, fallers showed a difference in overall amplitude for the ADAN, rather than visual field or laterality differences. Based on this inconsistency between the patterns of results, we have thus reported normal attentional control for fallers.
Second, there were notable differences in attentional facilitation effects as identified via P1 vs. reaction time measures. In particular, we report that fallers have impaired attentional facilitation in the left visual field as indicated by the P1 ERP component, yet there were no corresponding differences between fallers and non-fallers, as measured by reaction times. That is, both groups showed normal attentional effects in reaction times, with responses faster for cued relative to uncued targets. In hindsight, this result is perhaps not surprising. For one, behavioural effects of attention have been previously found without corresponding effects in the P1 (e.g., Handy & Khoe, 2005), indicating that attention can differently affect reaction times and visual sensory gain. For another, the finding is consistent with the hypothesis that the two measures may reflect different underlying processes. For example, sensory gain effects captured in the P1 may be more important for vision-for-action whereas reaction time effects may be more central to vision-for-perception (e.g., Handy et al., 2003; Handy et al., 2005). Indeed, that fallers––who have problems in the motor domains––showed selective deficits in sensory gain is certainly consistent with this possibility.
Acknowledgments
The authors thank Dr. Olav Krigolson with his help with programming for this experiment and Lindsay Katarynych for her assistance with participant recruitment and scheduling. Supported by grants from NSERC, MSFHR, CIHR (MOB – 93373) to Dr. Handy and MSFHR and CIHR (MOB – 93373) to Dr. Liu-Ambrose.
Footnotes
ETHICS
The reported research was approved by the Clinical Research Ethics Board (CREB) at The University of British Columbia.
References
- Bagurdes LA, Mesulam MM, Gitelman DR, Weintraub S, Small DM. Modulation of the spatial attention network by incentives in healthy aging and mild cognitive impairment. Neuropsychologia. 2008;46:2943–2948. doi: 10.1016/j.neuropsychologia.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Bublak P, Redel P, Finke K. Spatial and non-spatial attention deficits in neurodegenerative diseases: Assessment based on Bundesen’s theory of visual attention (TVA) Restorative Neurology and Neuroscience. 2006;24:287–301. [PubMed] [Google Scholar]
- Clark RD, Lord SR, Webster IW. Clinical parameters associated with falls in an elderly population. Gerontology. 1993;39:117–123. doi: 10.1159/000213521. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Di Fabio RP, Zampieri C, Henke J, Olson K, Rickheim D, Russell M. Influence of elderly executive cognitive function on attention in the lower visual field. Gerontology. 2005;51:94–107. doi: 10.1159/000082194. [DOI] [PubMed] [Google Scholar]
- Drago V, Foster PS, Ferri R, Arico D, Lanuzza B, Heilman KM. Distractibility and Alzheimer’s Disease: The “neglected” phenomenon. Journal of Alzheimer’s Disease. 2008;15:1–10. doi: 10.3233/jad-2008-15101. [DOI] [PubMed] [Google Scholar]
- Eimer M. ERP modulations indicate the selective processing of visual stimuli as a result of transient and sustained spatial attention. Psychophysiology. 1996;33:13–21. doi: 10.1111/j.1469-8986.1996.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Eimer M. Mechanisms of visuospatial attention: Evidence from event-related brain potentials. Visual Cognition. 1998;5:257–286. [Google Scholar]
- Green JJ, McDonald JJ. Electrical neuroimaging reveals timing of attentional control activity in human brain. Plos Biology. 2008;6(4):730–738. [Google Scholar]
- Handy TC, Borg JS, Turk DJ, Tipper CM, Grafton ST, Gazzaniga MS. Placing a tool in the spotlight: Spatial attention modulates visuomotor responses in cortex. NeuroImage. 2005;26:266–276. doi: 10.1016/j.neuroimage.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Handy TC, Grafton ST, Shroff NM, Ketay S, Gazzaniga MS. Graspable objects grab attention when the potential for action is recognized. Nature Neuroscience. 2003;6:421–427. doi: 10.1038/nn1031. [DOI] [PubMed] [Google Scholar]
- Handy TC, Khoe W. Attention and sensory gain control: A peripheral visual process? Journal of Cognitive Neuroscience. 2005;17:1936–1949. doi: 10.1162/089892905775008715. [DOI] [PubMed] [Google Scholar]
- Handy TC, Nagamatsu LS, Mickelborough MJS, Liu-Ambrose TYL. Statistical strategies for translational ERP studies. In: Handy TC, editor. Brain Signal Analysis: Advances in Bioelectric and Biomagnetic Methods. Cambridge, MA: MIT Press; (In press) [Google Scholar]
- Handy TC, Tipper CM. Attentional orienting to graspable objects: What triggers the response? NeuroReport. 2007;18:941–944. doi: 10.1097/WNR.0b013e3281332674. [DOI] [PubMed] [Google Scholar]
- Harter MR, Miller SL, Price NJ, LaLonde ME, Keyes AL. Neural processes involved in directing attention. Journal of Cognitive Neuroscience. 1989;1:223–237. doi: 10.1162/jocn.1989.1.3.223. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR. Shifting visual attention in space: An electrophsiological analysis using high spatial resolution mapping. Clinical Neurophysiology. 2000;111:1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Jongen EM, Smulders FT, Van der Heiden JS. Lateralized ERP components related to spatial orienting: Discriminating the direction of attention from processing sensory aspects of the cue. Psychophysiology. 2007;44(6):968–986. doi: 10.1111/j.1469-8986.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biological Psychology. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Liu-Ambrose TYL, Nagamatsu LS, Leghari MA, Handy TC. Does impaired cerebellar function contribute to risk of falls in seniors? A pilot study using functional magnetic resonance imaging. Journal of the American Geriatric Society. 2008;56:2153–2155. doi: 10.1111/j.1532-5415.2008.01984.x. [DOI] [PubMed] [Google Scholar]
- Lord S, Sherrington C, Menz H. A physiological profile approach for falls prevention. In: Lord S, editor. Falls in older people. Risk factors and strategies for prevention. Cambridge: Cambridge University Press; 2001. pp. 221–238. [Google Scholar]
- Lorenzo-Lopez L, Doallo S, Vizoso C, Amenedo E, Holguin SR, Cadaveira F. Covert orienting of visuospatial attention in the early stages of aging. NeuroReport. 2002;13(11):1459–1462. doi: 10.1097/00001756-200208070-00022. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual spatial priming. Journal of Experimental Psychology - Human Perception and Performance. 1991;17(4):1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA, Luck SJ. Electrocortical substrates of visual selective attention. Attention and Performance. 1993;14(14):219–243. [Google Scholar]
- Mangun GR, Luck SJ, Plager R, Loftus W, Hillyard SA, Handy T, Clark VP, Gazzaniga MS. Monitoring the visual world: Hemispheric asymmetries and subcortical processes in attention. Journal of Cognitive Neuroscience. 1994;6:265. doi: 10.1162/jocn.1994.6.3.267. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain and Cognition. 1990;12(2):240–266. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Nawrot M. Perception of movement and shape in Alzheimer’s disease. Brain. 1998;121:2259–2270. doi: 10.1093/brain/121.12.2259. [DOI] [PubMed] [Google Scholar]
- Seiss E, Gherri E, Eardley AF, Eimer M. Do ERP components triggered during attentional orienting represent supramodal attentional control? Psychophysiology. 2007;44(6):987–990. doi: 10.1111/j.1469-8986.2007.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D, Slagter HA, Nieuwenhuis S, Hage J, Kok A. The orienting of visuospatial attention: An event-related brain potential study. Cognitive Brain Research. 2005;25(1):117–129. doi: 10.1016/j.cogbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New England Journal of Medicine. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Van Velzen J, Eimer M. Early posterior ERP components do not reflect the control of attentional shifts toward expected peripheral events. Psychophysiology. 2003;40(5):827–831. doi: 10.1111/1469-8986.00083. [DOI] [PubMed] [Google Scholar]