Abstract
BACKGROUND
Cognitive decline among seniors is a pressing health care issue. Specific exercise training may combat cognitive decline. We compared the effect of once-weekly and twice-weekly resistance training with twice-weekly balance and tone exercise training on the performance of executive cognitive functions in senior women.
METHODS
In this single-blinded randomised trial, 155 community-dwelling women aged 65 to 75 years old living in Vancouver, Canada were randomly allocated to once-weekly resistance training (n=54), twice-weekly resistance training (n=52), or to twice-weekly balance and tone training (i.e., control group) (n=49). Primary outcome measure was performance on the Stroop Test, an executive cognitive test of selective attention and conflict resolution. Secondary outcomes of executive cognitive functions included set shifting as measured by the Trail Making Tests (Part A & B) and working memory as assessed by verbal digits forward and backward tests. Gait speed, muscular function, and whole brain volume were also secondary outcome measures.
RESULTS
Both resistance training groups significantly improved their performance on the Stroop Test compared with those in the balance and tone group (p≤0.03). Specifically, task performance improved by 12.6% and 10.9% in the once-weekly and twice-weekly resistance training groups respectively; it deteriorated by 0.5% in the balance and tone group. Enhanced selective attention and conflict resolution was significantly associated with increased gait speed. Also, both resistance training groups demonstrated reductions in whole brain volume compared with the balance and tone group at the end of the study (p≤0.03).
CONCLUSIONS
Twelve months of once-weekly or twice-weekly resistance training benefited the executive cognitive function of selective attention and conflict resolution among senior women.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT00426881
Keywords: Resistance Training, Executive Functions, Older Adults
INTRODUCTION
Cognitive decline among seniors is a pressing health care issue. Effective pharmacologic treatment of mild cognitive impairment and dementia remains a major medical challenge 1. Hence, effective primary prevention strategies of cognitive decline would greatly benefit individuals and society.
Observational studies suggest that physical activity may limit age-associated cognitive decline 2, 3. However, those studies did not distinguish between the two main types of physical activity --- aerobic and resistance training. Intervention studies have shown that aerobic exercise training enhances brain and cognitive function 4. Whether resistance training has similar benefits on cognitive function in seniors has received little investigation 5.
We had three reasons to examine whether resistance training improves cognitive function in seniors. First, a meta-analysis highlighted that the greatest benefit of aerobic exercise on cognition occurred when it was paired with resistance training 6. There are plausible biological mechanisms whereby resistance training might ameliorate cognitive function independently of aerobic exercise 7. Second, a six-month trial 8 indicated that resistance training benefitted memory performance and verbal concept formation among seniors. This raised the possibility that a broader spectrum of cognitive functions may also show improvement with resistance training. Third, no study to date has examined the minimum frequency of resistance training (i.e., once-weekly or twice-weekly) required for cognitive benefits. However, frequency of training may influence long-term exercise adherence. If a relatively standard resistance training program had cognitive benefits, and there was evidence for a minimally effective dose (frequency and duration), this would add substantially to physicians’ options of exercise prescription for seniors.
We aimed to compare the effect of once-weekly and twice-weekly resistance training with twice-weekly balance and tone exercise training on the performance of executive cognitive functions in senior women. We focused on executive cognitive functions because they are highly associated with the ability to perform instrumental activities of daily living 9 and mobility 10.
METHODS
Study Design
We conducted a randomised, controlled 52-week prospective study of exercise from May 2007 to April 2008 with three measurement periods (baseline, mid-point, and trial completion). The assessors were blinded to the participants’ assignments. However, the success of blinding was not formally assessed throughout the trial.
Participants
The sample consisted solely of women because cognitive response to exercise differs between the sexes 6. From February 2007 to April 2007, we recruited using print advertisements and television features. Individuals were screened by a standardized telephone interview. Women who lived in Vancouver, Canada, were eligible for study entry if they: 1) were aged 65 to 75 years; 2) were living independently in their own home; 3) scored ≥ 24 on the Mini-Mental State Examination (MMSE); and 4) had a visual acuity of at least 20/40, with or without corrective lenses. We excluded those who: 1) had a current medical condition for which exercise is contraindicated; 2) had participated in resistance training in the last six months; 3) had a neurodegenerative disease and/or stroke; 4) had depression; 5) did not speak and understand English fluently; 6) were taking cholinesterase inhibitors; 7) were on oestrogen replacement therapy; or 8) were on testosterone therapy.
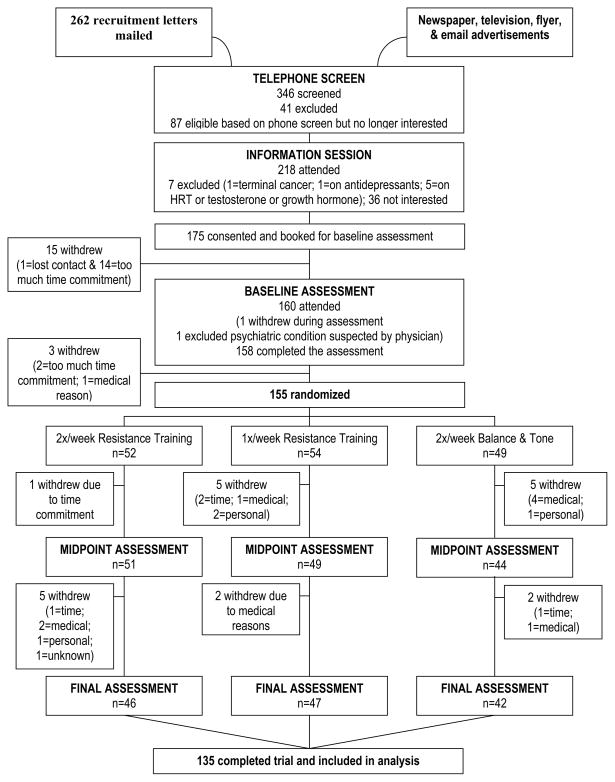
Figure 1, the CONSORT flow diagram, shows the number of participants in the treatment arms at each stage of the study. Ethical approval was obtained from the Vancouver Coastal Health Research Institute and the University of British Columbia’s Clinical Research Ethics Board. All participants provided written informed consent.
Figure 1.
CONSORT flow chart.
Descriptive Variables
At baseline, participants underwent a physician assessment to confirm current health status and eligibility for the study. We used the 15-item Geriatric Depression Scale (GDS) 11 to screen for depression. Current level of physical activity was determined by the Physical Activities Scale for the Elderly (PASE) self-report questionnaire 12. General mobility was assessed by the Timed Up and Go Test (TUG) 13.
Primary Outcome Measure
This study focused on three executive cognitive functions: selective attention and conflict resolution, set shifting, and working memory. Our primary outcome measure was the specific executive cognitive function of selective attention and conflict resolution, as measured by the Stroop Test 14. We previously demonstrated that it responds to exercise training 15 and used those observed changes in our sample size calculation.
For the Stroop Test, there were three conditions. First, participants were instructed to read out words printed in black ink (e.g., BLUE). Second, they were instructed to read out the colour of coloured-X’s. Finally, they were shown a page with colour-words printed in incongruent coloured inks (e.g., the word “BLUE” printed in red ink). Participants were asked to name the ink colour in which the words are printed (while ignoring the word itself). There were 80 trials for each condition and we recorded the time participants took to read each condition. The ability to selectively attend and control response output was calculated as the time difference between the third condition and the second condition. Smaller time differences indicate better selective attention and conflict resolution.
Secondary Outcome Measures
Secondary measures of executive cognitive functions were set shifting and working memory. Also, to understand the wider range of effects resistance training may have on senior women, we assessed gait speed, quadriceps muscular function, and whole brain volume.
Set Shifting
We used the Trail Making Tests (Part A & B) to assess set shifting 16. Part A assesses psychomotor speed and requires the participant to draw lines that connect encircled numbers sequentially, such as drawing a line from 1 to 2, 2 to 3, 3 to 4, etc. Part B consists of encircled numbers and letters. Participants were instructed to draw a line as quickly and as accurately as possible from 1 to A, A to 2, 2 to B, B to 3, and so on, until they completed the task. We recorded the amount of time (in seconds) it took to complete each task. To index set shifting, we calculated the difference between Part B and Part A completion time. Smaller difference scores indicate better set shifting.
Working Memory
We used the verbal digits forward and verbal digits backward tests to index the central executive component of working memory 17. Both tests consist of seven pairs of random number sequences that the assessor reads aloud at the rate of one per second. The sequence begins with three digits and increases by one at a time up to a length of nine digits. The test includes two sequences of each length and testing ceases when the participant fails to recollect any two with the same length. The score recorded, ranging from 0 to 14, is the number of successful sequences. For the verbal digits forward test, the participant’s task is to repeat each sequence exactly as it is given. For the verbal digits backward test, the participant’s task is to repeat each sequence in the reversed order. The difference between the verbal digits forward test score and the verbal digits backward test score was used as an index of the central executive component of working memory. Smaller difference scores indicate better working memory.
Gait Speed
Gait speed is a significant and independent predictor of falls and fracture risk in older women 18. Participants were asked to walk at their usual pace along a 4-meter path. Gait speed (m/s) was calculated from the mean of two trials. The test-retest reliability of gait speed in our laboratory is 0.95 (ICC) 19.
Muscular Function
In a sub-set of participants who were eligible (i.e., no significant pre-existing knee joint, hip, and back condition), isotonic quadriceps strength (1RM) and peak muscle power was assessed using the Keiser air-pressured digital resistance leg press machine (Keiser Sports Health Equipment, Fresno, CA, USA). The study physician screened all participants for eligibility. Two assessors completed all assessments of 1 RM and peak muscle power; they attended two 30-minute training sessions prior to the baseline measurement period.
The initial load for quadriceps 1RM assessment was the participant’s own body mass. Participants pushed against the leg press on a 3 second count and also returned to the start position on a 3 second count. Load increased by 10% increments until participants were no longer able to lift the load through their available range of motion. The load (N) of the last successfully completed leg press was recorded and used for statistical analysis.
Following the completion of the quadriceps 1RM testing, eligible participants were given a 15-minute break. They then underwent quadriceps muscle power assessments where they completed leg press extensions at 6 relative loads of their 1RM (i.e., 40%, 50%, 60%, 70%, 80%, and 90%). Participants performed the concentric portion of the leg press repetition as rapidly as possible and then slowly lowered the load over 3 seconds. Beginning at 40% of 1RM, participants performed three repetitions at each relative 1RM load. There was a 30-second rest between repetitions. The Keiser air-software air-pressured digital resistance leg press machine recorded the power (W) produced. The peak quadriceps muscle power obtained by each participant was used for statistical analysis.
Whole Brain Volume
For those that met the inclusion criteria for MRI scanning and consented, whole brain volume was measured via T1-weighted structural MRI images obtained using a Philips Achieva 3T scanner. Calculations of whole brain volume and their percentage change across study time points were made using the SIENA (or Structural Image Evaluation, using Normalization, of Atrophy) method 20. SIENA is a longitudinal method that works on comparing pairs of scans within-subjects and is available as part of the FSL software package 21. SIENA has been shown to have an overall error rate of approximately 0.2% of the absolute brain volume 20, 22, 23. SIENA is designed to be fully-automatic, but careful evaluation of its intermediate output is essential to ensuring accurate results. To minimize error, we performed visual checks of intermediate output from three critical processes: brain extraction, spatial alignment, and tissue segmentation.
Randomization
The randomization sequence was generated by www.randomization.com and was concealed until interventions were assigned. This sequence was held independently and remotely by the Research Coordinator. Participants were enrolled and randomised by the Research Coordinator to one of three groups: once-weekly resistance training (1x RT), twice-weekly resistance training (2x RT), or twice-weekly balance and tone (BAT).
Sample Size
The required sample size for this study was calculated based on predictions of 12-month changes in the Stroop Test. Specifically, we predicted 6% improvement for the 1x RT and a 12% improvement for the 2x RT. We also estimated 10% deterioration in the BAT group (i.e., control group). These estimates were based on our previous work 15 that demonstrated a home-based program of strength and balance retraining exercises significantly improved Stroop Test performance. Assuming a 20% attrition rate and using an alpha level of < 0.05, 52 participants per group ensured a power of 0.80.
Exercise Intervention
Both the RT and the BAT classes began one month after the baseline assessments were completed (i.e., May 2007). Classes were held at held at two locations, the local YMCA and the Centre for Hip Health and Mobility research centre. All classes were led by certified fitness instructors who received additional training and education from the study investigators. The classes were 60 minutes in duration, with a 10-minute warm-up, 40 minutes of core content, and a 10-minute cool-down. To ensure that programs were delivered faithfully and consistently across sites, a research assistant who was not involved in delivering the study’s classes conducted quality assessments every month using a standard form. Attendance was recorded daily by the assistants. Compliance, expressed as the percentage of the total classes attended, was calculated from these attendance sheets.
Specific strategies were implemented to promote participant engagement. These included: 1) semi-monthly newsletters that featured personal accomplishments of the participants, healthy recipes contributed by the participants, and study updates; 2) three social events (e.g., Winter Holiday Tea); 3) personalized birthday cards; 4) following-up on participants who missed two consecutive classes without reason; and 5) providing support and suggestions to overcome barriers to participation.
Resistance Training
The protocol for the RT program was progressive and high-intensity in nature. Both a Keiser® Pressurized Air system and free weights were used to provide the training stimulus. The Keiser-based exercises consisted of biceps curls, triceps extension, seated row, latissmus dorsi pull downs, leg press, hamstring curls, and calf raises. The intensity of the training stimulus was at a work range of six to eight repetitions (two sets). The training stimulus was subsequently increased using the 7RM method – when two sets of six to eight repetitions were completed with proper form and without discomfort. Other key strength exercises included mini-squats, mini-lunges, and lunge walks. The number of sets completed and the load lifted for each exercise was recorded for each participant at every class.
Balance and Tone
The BAT program consisted of stretching exercises, range of motion exercises, basic core-strength exercises including kegals (i.e., exercises to strengthen the pelvic floor muscles), balance exercises, and relaxation techniques. Key balance exercises included Tai Chi-based forms (i.e., Crane, Tree Pose), tandem stand, tandem walking, and single leg stance (eyes open and closed). Other than bodyweight, no additional loading (e.g., hand weights, resistance bands, etc.) was applied to any of the exercises. There is no evidence that these exercises improve cognitive function 4. This group served to control for confounding variables such as physical training received by traveling to the training centres, social interaction, and changes in lifestyle secondary to study participation.
Adverse Effects
Participants were questioned about the presence of any adverse effects, such as musculoskeletal pain or discomfort, at each exercise session. All instructors also monitored participants for symptoms of angina and shortness of breath during the exercise classes.
Statistical Analysis
All analyses were “full analysis set” 24 (defined as the analysis set which is as complete and as close as possible to the intention-to-treat ideal of including all randomised participants). Data were analyzed using SPSS (Windows Version 17.0).
Between-group differences in selective attention and conflict resolution at mid-point and trial completion were compared by multiple linear regression analysis. In the models, baseline scores, experimental group, baseline MMSE score, baseline waist circumference 25, 26, diagnosis of diabetes (yes/no) 26–28, and visual edge contrast sensitivity score 29 were included as covariates. Two planned simple contrasts were performed when there were significant main group effects. These contrasts were employed to assess differences between: 1) the 1x RT group and the BAT group; and 2) the 2x RT group and the BAT group. In addition, difference contrasts were employed within each RT group to assess when cognitive benefits of resistance training were evident. The overall alpha was set at p<0.05.
We analyzed our secondary outcome measures of executive cognitive functions in the same manner as our primary outcome measure with the exception that visual edge contrast sensitivity was not included as a covariate in the model for working memory.
For models of gait speed, quadriceps 1RM, and peak quadriceps muscle power, baseline scores and experimental group were included as covariates. For models of percent change in whole brain volume, presence of diabetes was included as a covariate. Finally, Pearson correlations were computed to determine whether changes in selective attention and conflict resolution between the beginning and the end of the intervention period were related to changes in gait speed.
RESULTS
Descriptive Variables, Exercise Adherence and Physical Activity Levels
The mean age of the cohort was 69.6 ± 2.9 years and the exercise compliance over the one year was 67.9%. The 1x RT group had an average compliance of 71.0%, 70.3% for the 2x RT group, and 62.0% for the BAT group. Baseline demographic and characteristics of the 135 participants who completed the 12-month trial are shown in Table 1. Physical activity levels (PASE scores) did not differ significantly between the groups at mid-point (p=0.98) or at trial completion (p=0.68).
Table 1.
Baseline characteristics of trial participants (N=155).
| Variable * | BAT (n=49) | 1x RT (n=54) | 2x RT (n=52) | Total (N=155) |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (yr) | 70.0 (3.3) | 69.5 (2.7) | 69.4 (3.0) | 69.6 (3.0) |
| Height (cm) | 161.0 (6.9) | 160.9 (7.0) | 162.8 (6.5) | 161.6 (6.8) |
| Weight (kg) | 67.0 (11.5) | 69.2 (16.2) | 72.1 (16.8) | 69.5 (15.2) |
| Education | ||||
| Less than Grade 9† | 1.0 (2) | 1.0 (1.9) | 1.0 (1.9) | 3.0 (1.9) |
| Grade 9 to 12 without Certificate or Diploma† | 2.0 (4.1) | 3.0 (5.6) | 4.0 (7.7) | 9.0 (5.8) |
| High School Certificate or Diploma† | 6.0 (12.2) | 9.0 (16.7) | 10.0 (19.2) | 25.0 (16.1) |
| Trades or Professional Certificate or Diploma† | 14.0 (28.6) | 10.0 (18.5) | 6.0 (11.5) | 30.0 (19.4) |
| University Certificate or Diploma† | 7.0 (14.3) | 12.0 (22.2) | 9.0 (17.3) | 28.0 (18.1) |
| University Degree† | 19.0 (38.8) | 19.0 (35.2) | 22.0 (42.3) | 60.0 (38.7) |
| MMSE Score (max. 30 pts) | 28.8 (1.2) | 28.5 (1.3) | 28.6 (1.5) | 28.6 (1.3) |
| Falls in the Last 12 Months (yes/no)† | 16 (34) | 13 (24.5) | 20 (38.5) | 49 (32.2) |
| Geriatric Depression Scale (max. 15 pts) | 0.5 (1.8) | 0.3 (1.1) | 0.9 (2.3) | 0.6 (1.8) |
| Functional Comorbidity Index (max. 18 pts) | 2.2 (1.7) | 1.8 (1.7) | 2.3 (1.6) | 2.1 (1.7) |
| Lawton and Brody (max. 8 pts) | 8.0 (0) | 8.0 (0.1) | 7.9 (0.5) | 8.0 (0.3) |
| Physical Activity Scale for the Elderly | 126.1 (51.0) | 116.2 (61.4) | 121.2 (60.4) | 121.0 (57.7) |
| Timed Up and Go Test (sec) | 6.8 (1.4) | 6.6 (1.4) | 6.6 (1.4) | 6.6 (1.4) |
|
| ||||
| Edge Contrast Sensitivity (dB) | 22.4 (1.6) | 22.1 (2.0) | 22.1 (1.4) | 22.2 (1.7) |
yr = year; kg = kilogram; MMSE = Mini-Mental State Examination; sec = seconds; dB = decibel units.
Count = number of “yes” cases within each group. % = percent of “yes” within each group.
Primary Outcome Measure
Table 2 shows the baseline, mid-point and one year retest results for the executive cognitive functions. The regression analyses revealed no significant between-group differences at mid-point of the trial. However, at the end of the trial there was a significant between-group difference in selective attention and conflict resolution (p=0.01). Planned simple contrasts indicated that both 1x RT and 2x RT had improved Stroop performance compared with the BAT group at trial completion (p≤0.03). Specifically, task performance improved by 12.6% and 10.9% in the 1x RT and 2x RT groups respectively, while the BAT group demonstrated 0.5% deterioration. Within each RT group, difference contrasts demonstrated that Stroop Test performance was not significantly different from baseline to mid-point (p=0.79), but was significantly different from mid-point to trial completion (p=0.001)
Table 2.
Mean values (SDs) for outcome measures.
| Variable * | Baseline | Mid-Point | Final |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| 2x RT | n=52 | n=51 | n=46 |
| Stroop CW – Stroop C (sec) | 45.02 (15.8) | 46.08 (17.2) | 40.88 (14.9)** |
| Trail B – Trail A (sec) | 49.53 (36.6) | 41.66 (30.8) | 38.72 (33.6) |
| Digit Forward – Digit Backward | 3.40 (2.4) | 4.12 (2.9) | 3.82 (2.1) |
| Gait Speed (m/sec) | 1.16 (0.2) | 1.34 (0.2) | 1.41 (0.2) |
| 1 RM (N)† | 314.97 (66.9) | 380.61 (84.0) | 388.20 (82.4) |
| Peak Muscle Power (W)† | 624.88 (194.2) | 707.24 (171.6)** | 708.56 (161.2)** |
| % Δ Whole Brain Volume from Baseline§ | N/A | −0.02 (0.60) | −0.43 (0.65)** |
| 1x RT | n=54 | n=49 | n=47 |
| Stroop CW – Stroop C (sec) | 47.37 (26.2) | 46.46 (25.8) | 39.49 (14.1)** |
| Trail B – Trail A (sec) | 41.35 (26.5) | 36.13 (27.8) | 34.05 (27.4) |
| Digit Forward – Digit Backward | 3.52 (2.0) | 3.83 (2.3) | 3.38 (1.9) |
| Gait Speed (m/sec) | 1.17 (0.2) | 1.36 (0.2) | 1.37 (0.2) |
| 1 RM (N)† | 323.94 (63.2) | 371.00 (86.4) | 386.48 (97.0) |
| Peak Muscle Power (W)† | 679.30 (184.2) | 633.37 (192.3) | 622.26 (204.4) |
| % Δ Whole Brain Volume from Baseline§ | N/A | −0.04 (0.48) | −0.32 (0.54)** |
| BAT | n=49 | n=44 | n=42 |
| Stroop CW – Stroop C (sec) | 43.98 (15.1) | 49.23 (19.1) | 43.77 (18.9) |
| Trail B – Trail A (sec) | 47.12 (41.3) | 43.37 (28.8) | 35.98 (21.9) |
| Digit Forward – Digit Backward | 3.25 (2.5) | 4.35 (2.8) | 4.00 (1.9) |
| Gait Speed (m/sec) | 1.17 (0.2) | 1.33 (0.2) | 1.37 (0.2) |
| 1 RM (N)† | 338.18 (70.4) | 363.91 (77.4) | 356.33 (85.3) |
| Peak Muscle Power (W)‡ | 660.30 (229.7) | 649.52 (208.8) | 552.08 (194.0) |
| % Δ Whole Brain Volume from Baseline§ | N/A | 0.13 (0.67) | 0.00 (0.63) |
Stroop CW = Stroop colour-words condition; Stroop C = Stroop coloured-X’s condition; N = newtons; W = watts; m/sec = meters per second.
Significantly different from the BAT group at p<0.05.
For Stroop Test, 95% CI of difference between 1x RT and BAT = −13.8 to −2.5 and 95% CI of difference between 2x RT and BAT = −12.2 to −0.8.
For peak muscle power, 95% CI of difference between 2x RT and BAT at mid-point = 22.2 to 151.3 and 95% CI of difference between 2x RT and BAT at trial completion = 81.7 to 230.0.
For % Δ in whole brain volume, 95% CI of difference between 1x RT and BAT = −0.76 to −0.04 and 95% CI of difference between 2x RT and BAT = −0.89 to −0.12.
2x RT baseline n=31, mid-point n=26, final n=25; 1x RT baseline n=30, mid-point n=28, final n=27; BAT baseline n=27, mid-point n=21, final n=24.
2x RT baseline n=30, mid-point n=23, Final n=25; 1x RT baseline n=29, mid-point n=26, final n=27; BAT baseline n=27, mid-point n=21, final n=24.
2x RT % Δ in whole brain volume from baseline to mid-point n=18, % Δ in whole brain volume from baseline to final n=18; 1x RT % Δ in whole brain volume from baseline to mid-point n=28, % Δ in whole brain volume from baseline to final n=28; BAT % Δ in whole brain volume from baseline to mid-point n=20, % Δ in whole brain volume from baseline to final n=18.
Secondary Outcome Measures
The regression analyses revealed no significant between-group differences at mid-point and trial completion in set shifting and working memory.
There were no significant between-group differences at mid-point and trial completion in gait speed and quadriceps 1RM. However, there were significant between-group differences in peak muscle power at mid-point (p<0.01) and trial completion (p<0.001). Planned simple contrasts indicated that 2x RT increased peak muscle power at mid-point (p<0.01) and trial completion (p<0.001) compared with the BAT group. Specifically, at trial completion, peak muscle power increased by 13.4% in the 2x RT group, but decreased by 8.4% and 16.3% for the 1x RT and the BAT group, respectively.
There were also between-group differences in percent change of whole brain volume at trial completion (p≤0.03). Both 1x RT and 2x RT demonstrated reductions in whole brain volume compared with the BAT group at the end of the study (p≤0.03). Specifically, there was a 0.32% and a 0.43% reduction in whole brain volume for the 1x RT and 2x RT groups, respectively. In contrast, there was 0% change in whole brain volume for the 2x BAT group.
Change in Executive Cognitive Function and Change in Gait Speed
Improvement in selective attention and conflict resolution over the 12-month intervention period was significantly associated with improvement in gait speed (r=0.24; p<0.01).
Adverse Events
Results of the Chi Square test indicated significant group differences (p=0.02) in the proportion of participants reporting adverse events. Specifically, musculoskeletal complaints (e.g., knee joint discomfort, bursa irritation in the lateral hip) developed in 14 women (29.8%) in the 1x RT group, five (10.9%) in the 2x RT group, and four (9.5%) in the BAT group. All documented musculoskeletal complaints either resolved or diminished within 4 weeks of onset. There was also one fall in the BAT group; this fall did not result in injury.
COMMENT
In 65 to 75 year old community-dwelling women, 12 months of progressive resistance training once- or twice-weekly improved selective attention and conflict resolution, relative to twice-weekly balance and toning exercises. We also found that resistance training twice-weekly improved peak quadriceps muscle power, and that resistance training once- or twice-weekly led to small but significant reductions in whole brain volume. To our knowledge, this is the first study to demonstrate that engaging in progressive resistance training as little as once a week can significantly benefit executive cognitive function in community-dwelling senior women.
Our study provides novel data relating the frequency and duration of resistance training with cognitive benefits in women. We observed a cognitive benefit after 12 months of training but not at the six-month time point. Cassilhas 8 reported cognitive benefits after six months of resistance training in men. There were differences in the frequency of resistance training between the two studies (i.e., once-weekly and twice-weekly training in our study versus thrice weekly in the Brazilian study); also different cognitive functions may have different change trajectories with resistance training. Sex may also be a moderating factor. Our study included women only and the participants trained less frequently than those in Cassilhas’ study 8. Finally, differences in the control groups may have contributed to the lack of between-group differences in cognitive performance at six months. The Brazilian study’s 8 control group trained only once-weekly; our Canadian control group trained twice-weekly.
We also demonstrated that enhanced selective attention and conflict resolution was associated with increased gait speed. To our knowledge, this study is the first to demonstrate this relationship. Our current finding adds weight to previous observations of a strong relationship between gait speed and cognitive function 30. The implication for clinicians is that improved gait speed is a predictor of substantial reduction in mortality 31.
The design of our control group (i.e., BAT) may have also contributed to the lack of between-group differences at six and 12 months in quadriceps 1 RM. Our control group included balance training in their twice-weekly program. Previous studies have demonstrated that balance training exercises can improve muscle strength 32, 33. In addition, in our own previous investigation of different types of exercise training (i.e., resistance training, agility training, and stretching (i.e., control) exercises) in senior women with low bone mass, we did not find any significant between-group differences in measures of quadriceps strength and mobility 34.
We highlight that although both resistance training groups enhanced selective attention and conflict resolution by the end of the trial, there were more musculoskeletal adverse events in the once-weekly resistance training group than the twice-weekly resistance training group and the twice-weekly balance and tone group. Hence, the possible increased risk for musculoskeletal injury with once-weekly resistance training must be weighed against its benefit of reduced training time compared with twice-weekly resistance training.
An unexpected result was the reduced whole brain volume for the two resistance training groups. Although reduced brain volumes are commonly associated with impaired function 35, those groups who improved cognitive executive function and muscular function had brain volume reductions. There are precedents that parallel our apparently paradoxical finding 36, 37. In a beta-amyloid immunization trial among those with probable AD, immunization led to significant clinical benefit, reduced beta-amyloid load, and reduced brain volume 36. The investigators hypothesized that removal of beta-amyloid and/or other protein constituents from brain tissue may have caused cerebral fluid shifts, resulting in brain volume reductions on MRI. However, we are very cautious in our interpretation of the whole brain volume results and emphasize that this facet of the study, although not the first report of such a phenomenon, needs further investigation.
Because our participant sample included women aged 65 to 75 years only, the findings may not generalize to men or to women of other ages. Also, although we observed reduced whole brain volumes, the study was not designed to image which specific brain regions demonstrated volumetric changes.
CONCLUSION
We provide novel randomized controlled trial evidence that a pragmatic resistance training program can enhance the executive cognitive function of selective attention and conflict resolution, while simultaneously improving muscular function in senior women. This has important clinical implications as cognitive impairment is a major health problem that currently lacks a clearly effective pharmaceutical therapy and resistance training is not widely-adopted by seniors. The doses of resistance training we used in this study fall within those recommended by the 2008 Physical Activity Guidelines for seniors (U.S. Department of Health and Human Services; http://www.health.gov/paguidelines).
Table 3.
Mean change (SDs) for outcome measures.
| Variable * | Mean Change at Mid-Point from Baseline (SD) ** | Mean Change at Final from Baseline (SD) ** |
|---|---|---|
| 2x RT | n=51 | n=46 |
| Stroop CW – Stroop C (sec) | −0.96 (15.13) | 5.01 (13.75) |
| Trail B – Trail A (sec) | 10.27 (40.25) | 10.96 (36.92) |
| Digit Forward – Digit Backward | −0.67 (2.94) | −0.47 (2.24) |
| Gait Speed (m/sec) | 0.19 (0.17) | 0.24 (0.16) |
| 1 RM (N)† | 60.27 (51.18 | 69.80 (74.75) |
| Peak Muscle Power (W)† | 74.68 (118.55) | 72.42 (108.12) |
| % Δ Whole Brain Volume§ | −0.02 (0.60) | −0.43 (0.65) |
| 1x RT | n=49 | n=47 |
| Stroop CW – Stroop C (sec) | 0.28 (28.37) | 6.22 (22.31) |
| Trail B – Trail A (sec) | 4.91 (26.12) | 7.3 (30.36) |
| Digit Forward – Digit Backward | −0.43 (2.63) | 0.06 (2.54) |
| Gait Speed (m/sec) | 0.18 (0.19) | 0.19 (0.19) |
| 1 RM (N)† | 42.15 (57.26) | 44.22 (67.10) |
| Peak Muscle Power (W)† | −27.54 (105.66) | −78.61 (151.03) |
| % Δ Whole Brain Volume§ | −0.04 (0.48) | −0.32 (0.54) |
| BAT | n=44 | n=42 |
| Stroop CW – Stroop C (sec) | −4.27 (15.15) | 0.26 (17.12) |
| Trail B – Trail A (sec) | 2.17 (39.27) | 8.64 (32.15) |
| Digit Forward – Digit Backward | −0.93 (3.42) | −0.64 (2.70) |
| Gait Speed (m/sec) | 0.17 (0.16) | 0.22 (0.18) |
| 1 RM (N)† | 24.73 (53.44) | 18.15 (70.06) |
| Peak Muscle Power (W)‡ | −24.27 (132.56) | −90.60 (144.58) |
| % Δ Whole Brain Volume§ | 0.13 (0.67) | 0.00 (0.63) |
Stroop CW = Stroop colour-words condition; Stroop C = Stroop coloured-X’s condition; N = newtons; W = watts; m/sec = meters per second.
Mean change for all cognitive measures = baseline value minus mid-point value or baseline value minus final value. Positive change indicates improvement. Mean change for all performance measures = mid-point value minus baseline value or final value minus baseline value. Positive change indicates improvement.
2x RT baseline n=31, mid-point n=26, final n=25; 1x RT baseline n=30, mid-point n=28, final n=27; BAT baseline n=27, mid-point n=21, final n=24.
2x RT baseline n=30, mid-point n=23, Final n=25; 1x RT baseline n=29, mid-point n=26, final n=27; BAT baseline n=27, mid-point n=21, final n=24.
2x RT % Δ in whole brain volume from baseline to mid-point n=18, % Δ in whole brain volume from baseline to final n=18; 1x RT % Δ in whole brain volume from baseline to mid-point n=28, % Δ in whole brain volume from baseline to final n=28; BAT % Δ in whole brain volume from baseline to mid-point n=20, % Δ in whole brain volume from baseline to final n=18.
Acknowledgments
The authors would like to thank the Vancouver South Slope YMCA management and members who enthusiastically supported the study by allowing access to participants for the training intervention. The authors are indebted to Dr. Karim Khan for the medical assessments and guidance and Ms. Lindsay Katarynych for coordinating this study. We thank the instructors for their commitment to the participants’ health and safety.
Drs. Liu-Ambrose, Ashe, and Handy are MSFHR Scholars.
Funding: The Vancouver Foundation (BCMSF, Operating Grant to TLA), Natural Sciences and Engineering Research Council of Canada (NSERC, Operating Grant to TCH), and the Michael Smith Foundation for Health Research (MSFHR, Establishment Grant to TLA) provided funding for this study. The Canada Foundation for Innovation funded essential infrastructure used in this study (New Opportunities Fund to TLA).
Sponsor’s Role: None.
Footnotes
Data from this manuscript were presented at the following scientific meetings:
1. 2009 American College of Sports Medicine, Seattle, Washington, USA, on May 28, 2009 (podium presentation).
2. 19th World IAGG Congress of Gerontology and Geriatrics, on July 7, 2009 (podium presentation).
Data Access and Responsibility: TLA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions:
TLA: Study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript, and critical review of manuscript. LSN, TCH: Acquisition of data, analysis and interpretation of data, preparation of manuscript, and critical review of manuscript. PG, BLB, MCA: Acquisition of data, interpretation of data, and critical review of manuscript.
All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest: All authors have nothing to declare.
References
- 1.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase Inhibitors in Mild Cognitive Impairment: A Systematic Review of Randomised Trials. PLoS Med. 2007;4:e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 3.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and Dementia in Physically Capable Elderly Men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 4.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu-Ambrose T, Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43:25–7. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 7.Liu-Ambrose T, Donaldson M. Exercise and Cognition in Older Adults: Is there a Role for Resistance Training Programs? Br J Sports Med. 2008 doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401–7. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 9.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining Executive Control in Normal Aging Predicts Change in Functional Status: The Freedom House Study. Journal of the American Geriatrics Society. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 10.Iersel MBv, Kessels RPC, Bloem BR, Verbeek ALM, Olde Rikkert MGM. Executive Functions Are Associated With Gait and Balance in Community-Living Elderly People. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- 11.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–11. [PubMed] [Google Scholar]
- 12.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999;52:643–51. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 13.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 14.Graf P, Uttl B, Tuokko H. Color- and picture-word Stroop tests: performance changes in old age. J Clin Exp Neuropsychol. 1995;17:390–415. doi: 10.1080/01688639508405132. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Ambrose T, Donaldson MG, Ahamed Y, et al. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc. 2008;56:1821–30. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 16.Spreen O, Strauss E. A compendium of neurological tests. 2. New York: Oxford University Press, Inc; 1998. [Google Scholar]
- 17.Wechsler D. Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; Harcourt Brace Jovanovich: 1981. [Google Scholar]
- 18.Dargent-Molina P, Favier F, Grandjean H, et al. Fall-related factors and risk of hip fracture: The EPIDOS prospective study. Lancet. 1996;348:145–149. doi: 10.1016/s0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu-Ambrose T, Khan KM, Donaldson MG, Eng JJ, Lord SR, McKay HA. Falls-related self-efficacy is independently associated with balance and mobility in older women with low bone mass. J Gerontol A Biol Sci Med Sci. 2006;61:832–8. doi: 10.1093/gerona/61.8.832. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Rao A, De Stefano N, et al. Longitudinal and cross-sectional analysis of atrophy in Alzheimer’s disease: cross-validation of BSI, SIENA and SIENAX. Neuroimage. 2007;36:1200–6. doi: 10.1016/j.neuroimage.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Camara O, Schnabel JA, Ridgway GR, et al. Accuracy assessment of global and local atrophy measurement techniques with realistic simulated longitudinal Alzheimer’s disease images. Neuroimage. 2008;42:696–709. doi: 10.1016/j.neuroimage.2008.04.259. [DOI] [PubMed] [Google Scholar]
- 24.ICH Expert Working Group. ICH Harmonised Tripartite Guideline: Statistical Principals in Clinical Trials. Statistics in Medicine. 1999;18:1905–1942. [PubMed] [Google Scholar]
- 25.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–6. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 27.Roriz-Filho SJ, Sá-Roriz TM, Rosset I, et al. (Pre)diabetes, brain aging, and cognition. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–8. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gussekloo J, de Craen AJ, Oduber C, van Boxtel MP, Westendorp RG. Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ Study. Am J Geriatr Psychiatry. 2005;13:781–6. doi: 10.1176/appi.ajgp.13.9.781. [DOI] [PubMed] [Google Scholar]
- 30.Soumare A, Tavernier B, Alperovitch A, Tzourio C, Elbaz A. A Cross-Sectional and Longitudinal Study of the Relationship Between Walking Speed and Cognitive Function in Community-Dwelling Elderly People. J Gerontol A Biol Sci Med Sci. 2009 doi: 10.1093/gerona/glp077. [DOI] [PubMed] [Google Scholar]
- 31.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–34. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 32.Li JX, Xu DQ, Hong Y. Changes in muscle strength, endurance, and reaction of the lower extremities with Tai Chi intervention. Journal of Biomechanics. 2009;42:967–971. doi: 10.1016/j.jbiomech.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Xu DQ, Hong Y, Li JX. Tai Chi exercise and muscle strength and endurance in older people. Med Sport Sci. 2008;52:20–9. doi: 10.1159/000134281. [DOI] [PubMed] [Google Scholar]
- 34.Liu-Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, McKay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trial. J Am Geriatr Soc. 2004;52:657–65. doi: 10.1111/j.1532-5415.2004.52200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson NE, Moore MM, Dame A, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–33. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- 36.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 37.Sparks DL, Lemieux SK, Haut MW, et al. Hippocampal volume change in the Alzheimer Disease Cholesterol-Lowering Treatment trial. Cleve Clin J Med. 2008;75 (Suppl 2):S87–93. doi: 10.3949/ccjm.75.suppl_2.s87. [DOI] [PubMed] [Google Scholar]