Abstract
A da Vinci Robotic Surgical System was purchased for The Heart Hospital Baylor Plano in fall 2011 and a program for robotic-assisted thoracic surgery commenced at the facility. Successive thoracic patients were offered and accepted a robotic-assisted operation. No patient was excluded because of age, height, weight, or comorbidities. The first 20 patients are summarized herein. Of the first 10 operations, only one was a lobectomy. As the program staff gained experience, seven of the latter 10 procedures were lobectomies. The average length of stay was 2.6 days (longest, 4 days). The average operating room time was 147 minutes overall and 200 minutes for lobectomies. The longest operating room time was 337 minutes in a patient who underwent a right middle lobectomy that was converted to a video-assisted thoracic surgery. Two patients developed atrial fibrillation, one of whom had a pacemaker and a history of paroxysmal atrial fibrillation. One patient developed a bronchopleural fistula on the first postoperative day, following a coughing episode. One patient was readmitted 6 days after hospital discharge with a pneumothorax, which was successfully treated with a small-bore catheter. In conclusion, robotic-assisted thoracic surgery has many advantages. Decreased complications can lead to improved outcomes, and hospitals can achieve cost savings as a result of reduced length of stay.
Despite advances in the care of patients with lung cancer, the disease remains prevalent globally and has a high mortality rate (1). With widespread adoption of computed tomographic lung cancer screening, the incidence of resectable disease will increase as patients are diagnosed at an earlier stage (2). Surgical resection has traditionally involved spreading the ribs by thoracotomy. Minimally invasive techniques performed by video-assisted thoracic surgery (VATS) have avoided rib spreading and have resulted in reduced pain and faster recovery (3). Despite improved outcomes, VATS has had limited acceptance, being used for 6% to 20% of lobectomies performed in the United States (4, 5). Limited acceptance of VATS may be due to difficult maneuverability of instruments and two-dimensional imaging.
The use of robotic surgical systems has rapidly increased and has become the standard in certain specialties. Robotic-assisted prostatectomy increased from 5% in 1998 to 85% in 2010 (6). Similar increases have been observed in gynecologic surgery. Robotic surgical systems offer a minimally invasive approach with improved instrumentation and three-dimensional visibility.
Thoracic robotics is in its infancy. Previously, most thoracic operations at The Heart Hospital Baylor Plano (THHBP) were VATS. The potential for improved outcomes with robotic surgical systems prompted the purchase of a da Vinci system. Results from our first 20 patients are reported herein.
METHODS
The da Vinci Robotics Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) was purchased in fall 2011 by THHBP (Figure 1). Our staff and surgeons were trained according to the manufacturer's suggested pathway. We developed a dedicated team for the operating room. Successive thoracic patients were offered and accepted a robotic-assisted operation. No patient was excluded because of age, height, weight, or comorbidities. All patients were informed that this is a novel technique.
Figure 1.

The da Vinci four-arm robot.
The technique used has been previously described by Cerfolio and colleagues (6). Four robotic arms as well as an assistant port were used for most patients (Figure 2). The assistant performs an active role in robotic-assisted thoracic surgery, including the stapling of all vessels, fissures, and bronchi.
Figure 2.
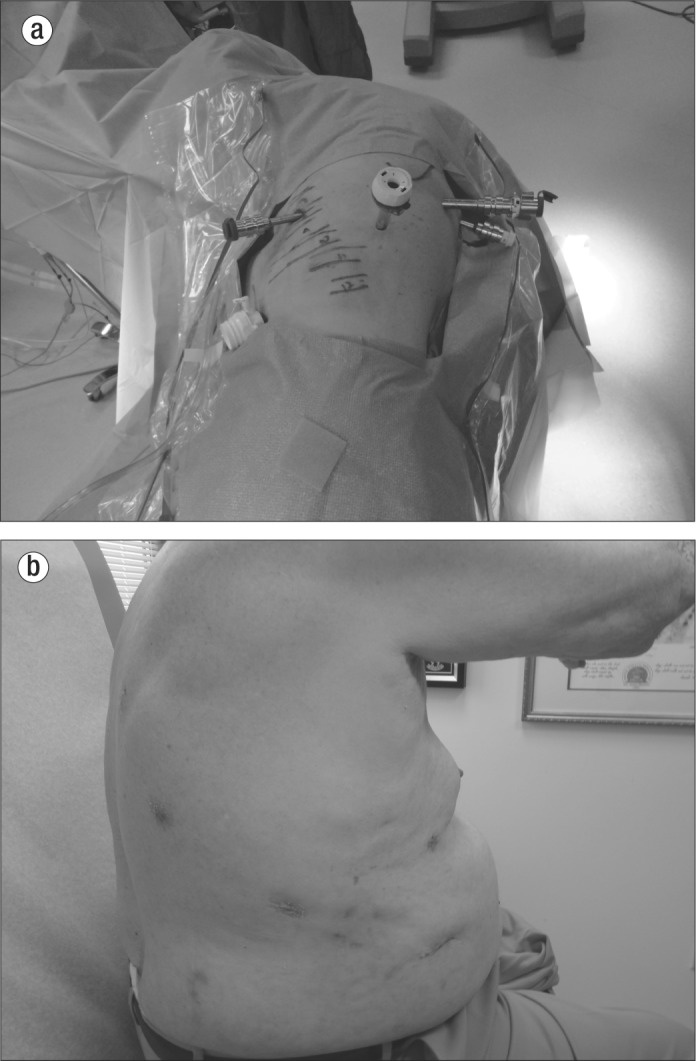
(a) Ports in a typical patient. (b) Port sites in a patient 3 weeks postoperatively.
All lymph node stations were sampled for lobectomies (stations 2R, 4R, 7, 8, and 9 for right lobectomies and stations 5, 6, 7, 8, and 9 for left lobectomies). Patients were tracked for operating room time, length of stay, and postoperative complications.
RESULTS
The first robotic-assisted thoracic operations were performed on November 11, 2011. The Table demonstrates the operations performed. Only one of the first 10 operations was a lobectomy. As the program staff gained experience, seven of the latter 10 operations were lobectomies. Every lobe was removed: the right upper lobe (n = 3), right middle lobe (n = 1), right lower lobe (n = 1), right middle and lower lobe (n = 1), left upper lobe (n = 1), and left lower lobe (n = 1). All but one of the lobes were cancerous. The left upper lobectomy was for Mycobacterium avium-intracellularae with bronchiectasis.
Table.
Characteristics, outcomes, and complications of robotic thoracic procedures in 20 consecutive patients
| Operation | Age (years) | Diagnosis | Time in OR (min) | Length of stay (days) | Complication |
|---|---|---|---|---|---|
| Right upper lobectomy | 50 | NSCLC stage IB | 216 | 2 | None |
| Left lower lobectomy | 50 | NSCLC stage IV | 144 | 2 | None |
| Left upper lobectomy | 57 | Mycobacterium avium-intracellulare | 333 | 4 | Bronchopleural fistula |
| Right upper lobectomy | 72 | NSCLC stage IV | 204 | 4 | Atrial fibrillation |
| Right middle/lower lobectomy | 72 | NSCLC stage IA | 238 | 4 | Readmission for pneumothorax |
| Right upper lobectomy | 77 | NSCLC stage IIB | 160 | 3 | None |
| Right middle lobectomy | 81 | NSCLC stage IB | 337 | 3 | None |
| Right lower lobectomy | 81 | NSCLC stage IIB | 219 | 3 | Atrial fibrillation |
| Wedge resection, right upper lobe | 51 | Bronchioalveolar carcinoma | 63 | 2 | None |
| Wedge resection, left upper lobe | 60 | Granuloma | 99 | 1 | None |
| Wedge resection, right middle lobe | 60 | Interstitial fibrosis | 74 | 4 | None |
| Wedge resection, right upper lobe | 60 | Bronchioalveolar carcinoma | 88 | 2 | None |
| Wedge resection right, lower lobe | 68 | Benign intrapulmonary lymph node | 118 | 2 | None |
| Wedge resection, left upper lobe | 71 | Bronchioalveolar carcinoma | 82 | 4 | None |
| Wedge resection, right upper lobe | 76 | Metastatic melanoma | 100 | 2 | None |
| Wedge resection, right middle/lower lobe | 76 | Granuloma | 109 | 1 | None |
| Excision of posterior mediastinal mass | 47 | Schwannoma | 86 | 2 | None |
| Excision of anterior mediastinal mass | 66 | Bronchogenic cyst | 125 | 3 | Mucous plug |
| Repair of bronchopleural fistula | 77 | Air leak after reperformed CABG/AVR | 108 | 3 | None |
| Sympathectomy | 55 | Raynaud's syndrome | 37 | 1 | None |
AVR indicates aortic valve replacement; CABG, coronary artery bypass grafting; NSCLC, non–small cell lung cancer; OR, operating room.
The outcomes are depicted in the Table. The average length of stay was 2.6 days. The longest length of stay was 4 days, for a patient who developed postoperative atrial fibrillation (AF). One of the two patients who developed AF had a history of paroxysmal AF and had a pacemaker in place.
The average operating room time was 147 minutes for all 20 patients and 200 minutes for those undergoing lobectomies. The longest operating room time was 337 minutes, for a patient who underwent a right middle lobectomy that was converted to a VATS due to right-lung inflation during the robotic procedure.
Complications developed in five patients. Two patients developed AF. One patient developed a bronchopleural fistula after a coughing episode on the first postoperative day. The fistula occurred as a result of a staple tearing through the bronchus and required open repair. It is suspected that the staple height used for this bronchus was insufficient. A patient with an anterior mediastinal mass developed a mucous plug in the left main stem bronchus, resulting in collapse of the left lung on the first postoperative day. The patient underwent fiber optic bronchoscopy with evacuation of a mucous plug, which resulted in expansion of the lung. This patient was discharged home on her third postoperative day. One patient was readmitted because of a pneumothorax 6 days after hospital discharge. The patient was treated with a small-bore catheter and subsequently recovered.
DISCUSSION
Recent studies have shown favorable results regarding robotic systems for thoracic operations. Cerfolio et al demonstrated reduced morbidity rates (27% vs. 38%) and mortality rates (0% vs. 3%) with robotic assistance compared with rib-and-nerve–sparing thoracotomy (7). In addition to improved morbidity and mortality, chest tubes were removed in less time (1.5 days vs. 3 days), and the length of hospital stay was reduced (2 days vs. 4 days). Verbal pain score 3 weeks postoperatively was also reduced.
Another recent multiinstitutional retrospective review of robotic-assisted lobectomy demonstrated excellent results (8). Morbidity and mortality were low. Long-term survival was also acceptable and compared well with VATS and thoracotomy.
Our experience confirms the observations of other investigators (6–11) that patients undergoing robotic procedures have less pain, with reduced atelectasis. There are also fewer air leaks and less chest tube drainage, resulting in earlier removal of chest tubes. Moreover, patients are discharged earlier and require fewer transfusions.
Surgeons have better visualization with robotic systems. A more complete lymph node dissection can be performed, resulting in a more comprehensive oncologic procedure. The surgeon has greater, more intuitive control of the instruments compared with VATS.
There are, however, potential disadvantages to robotic-assisted surgery. For instance, the financial investment required is not always available in the budgets of health care facilities. Also, robotic-assisted surgery requires change on the part of the surgeon and the operating team; these staff members must be willing to alter the course of their profession. Moreover, the limited availability of a robotic system may be problematic, although most hospitals now have a robot to support their urology and gynecology services.
In conclusion, the early results of the robotic-assisted thoracic surgical program initiated at THHBP suggest that surgery can be safely performed during the learning curve. Outcomes were acceptable, and patient satisfaction was high. Although in its infancy, the field of thoracic robotics is undergoing exponential growth, and such procedures may eventually become a standard of care.
Acknowledgments
The author wishes to thank Jeremy D. Brown, BS, MS, and Kelli R. Trungale, MLS, ELS, for their editorial assistance.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team. Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, Gareen IF, Gatsonis C, Goldin J, Gohagan JK, Hillman B, Jaffe C, Kramer BS, Lynch D, Marcus PM, Schnall M, Sullivan DC, Sullivan D, Zylak CJ. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, Bains M, Downey RJ, Rusch VW. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138(1):11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from the Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135(2):247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Gopaldas RR, Bakaeen FG, Dao TK, Walsh GL, Swisher SG, Chu D. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg. 2010;89(5):1563–1570. doi: 10.1016/j.athoracsur.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg. 2011;91(6):1729–1736. doi: 10.1016/j.athoracsur.2011.01.104. [DOI] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142(4):740–746. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Park BJ, Melfi F, Mussi A, Maisonneuve P, Spaggiari L, Da Silva RK, Veronesi G. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143(2):383–389. doi: 10.1016/j.jtcvs.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg. 2011;23(1):36–42. doi: 10.1053/j.semtcvs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Louie BE, Farivar AS, Aye RW, Vallières E. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg. 2012;93(5):1598–1604. doi: 10.1016/j.athoracsur.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 11.Suri RM, Antiel RM, Burkhart HM, Huebner M, Li Z, Eton DT, Topilsky T, Sarano ME, Schaff HV. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann Thorac Surg. 2012;93(3):761–769. doi: 10.1016/j.athoracsur.2011.11.062. [DOI] [PubMed] [Google Scholar]


