Abstract
Background
Many studies have been published on the association between single nucleotide polymorphisms (SNP) near the IL28B gene and response to the combined treatments of pegylated-interferon (PegIFN) and ribavirin (RBV) in chronic HCV-infected patients, but without identical conclusions. The aim of this study was to assess impact of the IL28B polymorphisms on the effect of HCV standard treatment using meta-analysis based method.
Methods
Association studies between polymorphisms of rs12979860 or rs8099917 and response to PegIFN/RBV treatment in chronic HCV patients were retrieved from PubMed. Data of qualified studies on sustained virological response (SVR) in different genotypes were extracted and analyzed using meta-analysis method in Stata 10 software.
Results
Thirty-four papers, containing 46 independent studies, were included in the analysis. In the HCV G1/4 patients without treatment history, individuals carrying rs12979860 CC genotype were more likely to achieve SVR (OR 3.97, 95%CI 3.29–4.80) compared to those carrying CT/TT genotypes. Similar results were observed in the HCV G1/4 patients with unsuccessful or unknown treatment history (OR 3.76, 95%CI 2.67–5.28) or in the patients co-infected with human immunodeficiency virus (OR 5.20, 95%CI 3.04–8.90). However, associations could not be observed in HCV G2/3 patients. For rs8099917, similar results were obtained for genotype TT compared to genotypes TG/GG, indicating that TT genotype was significantly associated with better treatment response in patients infected with genotype 1 or 4 HCV, but not genotype 2 or 3 HCV.
Conclusion
Polymorphisms of rs12979860 and rs8099917 near IL28B only associate with the treatment response to PegIFN/RBV in patients infected with HCV genotype 1 or 4 but not with genotype 2 or 3, irrespective of the previous treatment history or HIV co-infected status. Therefore, identification of IL28B genotypes is necessary only in patients infected with relatively difficult-to-treat genotype 1 or 4 HCV.
Introduction
About 170 million people are affected with hepatitis C virus (HCV) throughout the world and 70% of them develop chronic infection which may progress to cirrhosis and hepatocellular carcinoma [1]. The current recommended treatment for chronic HCV infection is a combination of pegylated interferon (PegIFN α-2a or PegIFN α-2b) plus body-weighted ribavirin (RBV) for a duration of 24 weeks or 48 weeks depending on the HCV viral genotypes. However, only about 50% genotype 1 or 4 patients treated and 80% genotype 2 or 3 patients treated could respond completely and achieve sustained virological response (SVR) [2]. Moreover, side effects from the therapy such as influenza-like symptoms, psychiatric symptoms and hematological abnormalities, could result in the dose reduction or even the premature discontinuation of the treatment [3]. To avoid these potential adverse events in patients who do not benefit from the treatment and to reduce the cost of therapy, it is necessary to predict an individual's response before or at the early stage of the treatment. Several factors, viral or host, such as HCV genotypes, baseline viral load, liver fibrosis, and mutations of interferon sensitivity determining region (ISDR), have been reported to be linked to the treatment outcomes [3]–[5]. However, these factors still could not fully predict the therapy response.
In 2009, three studies which were published almost at the same time reported that single nucleotide polymorphisms (SNP) near IL28B gene region were associated with the treatment effect of pegylated-interferon and ribavirin (PegIFN/RBV) in HCV-infected patients using genome-wide association study (GWAS) method [4], [6], [7]. Thereafter, a number of studies were published on the association between SNPs near IL28B and the clearance of HCV with or without treatments in HCV infected subjects in different ethnicities and HCV genotypes [8]–[12]. The most studied two SNPs, rs12979860 and rs8099917, are located upstream to the IL28B gene [4]. The CC genotype of rs12979860 or TT of rs8099917 was considered to be associated with a better treatment response. However, results were not consistent from different studies. The aim of this study was to summarize the associations between SNPs (rs12979860 and rs8099917) near IL28B gene and outcome of the combination therapy of PegIFN plus RBV in chronic HCV infected patients from public data.
Methods
Study search and selection
Studies on the associations between SNPs of rs12979860 or rs8099917 and the treatment response in HCV infected patients were retrieved from PubMed using the following strategy: (“IL28B” OR “IL-28B” OR “interleukin 28B” OR “interleukin-28B” OR “interferon lambda 3” OR “IFN lambda 3” OR “rs12979860” OR “rs8099917”) AND (“Hepatitis C” OR “HCV”). No language or time restrictions were applied and database searching for the last time was November 20, 2011. Papers retrieved were reviewed by two reviewers (Jia and Ding) independently following the including and excluding criteria (File S1). References of retrieved publications were also screened manually to search potential articles fitted the criteria.
Research studies met the following inclusion criteria were included in the analysis. (1) chronic HCV-infected patients with detectable HCV RNA before treatment and received the combined therapy of peginterferon and ribavirin only; (2) the clear outcomes defined as sustained virological response (SVR), i.e. undetectable HCV RNA 24 weeks or 6 months after the cessation of treatment, or non-SVR, which was detectable HCV RNA at the end of follow-up or premature discontinuation of treatment because of the poor response; (3) relevant data of therapeutic effect stratified by HCV genotypes as different HCV genotypes respond differently to the therapy.
Studies were excluded if patients suffered from other complicated liver diseases such as hepatocellular carcinoma or underwent liver transplantation. If more than one articles reported overlapping subjects, the one with more detailed descriptions of baseline information and effect data was selected. When results were stratified by races or HCV genotypes in the article, each part was considered as an independent study. However, HCV genotypes were classified into two categories, G2/3 (genotype 2 or 3) and G1/4 (genotype 1, 4 and others), according to response to the combined treatment [2], [13]. When results of treatment per protocol (TPP) analysis and intention to treatment (ITT) analysis were both reported, only ITT data were extracted for the analysis.
The following information was extracted from each study using the assessment form for data collection (File S1): the first author, date of publication, area of the study, demographic data of subjects, HCV genotype, duration of therapy, and the effect data of each IL28B genotype.
Data analysis
Meta-analysis was used to summarize associations across studies between IL28B genotypes and SVR of the combined treatment. Though studies performed in Asian populations reported a strong linkage disequilibrium between rs12979860 and rs8099917 [14]–[16], results from Caucasians showed a weaker linkage disequilibrium [4], [17], [18]. Therefore, we summarized effects of two SNPs separately. For each SNP, a recessive model was applied (for rs12979860, CC vs CT+TT; and for rs8099917, TT vs TG+GG). OR (odds ratio) of each study with its 95% CI (confidence interval) was calculated and displayed in a forest plot. Heterogeneity across studies was tested by Cochran's Q statistic and quantified by I2, a transformation of Q that estimates the percentage of variation in effect size that is due to heterogeneity. However, the pooled ORs were summarized using random effect model (DerSimonian and Laird method) even in absence of heterogeneity, given the diversity of the study design.
If patients fail in previous interferon-based treatment or are co-infected with human immunodeficiency virus (HIV), they respond differently to the therapy compared to patients with HCV mono-infection and treatment naïve [3]. Therefore, separate analyses were performed to reduce the potential heterogeneity. Furthermore, in treatment-naïve patients, summarized ORs stratified by races were calculated separately in G1/4 patients and G2/3 patients. Potential publication bias was assessed by Egger method and Peter method separately. All analyses were carried out in Stata software (version 10, Package sbe24_3 and sbe19_6) and a two-tailed P value less than 0.05 was considered as a statistical significance.
Results
Literature retrieved
The process of literature selection is showed in figure 1. Two hundred and forty-nine articles met the keyword selection and the full texts of 77 were gone through thoroughly after initially screening titles and abstracts. Forty three of 77 were excluded according to the inclusion and exclusion criteria. Table 1 shows the detailed information of 34 articles, including 46 independent studies [4], [8], [14]–[17], [19]–[46]. Since only one study was conducted in Hispanic patients [4], we incorporated it into the Caucasian group. Among those studies, 30 were on patients with HCV G1/4 and the summarized SVR rate was 45.3% (3600/7944). In contrast, the SVR rate in the rest 16 studies on patients infected with HCV G2/3 was much higher (80.1%, 1831/2287), as expected.
Figure 1. Flow chart summarizing the process of study selection.
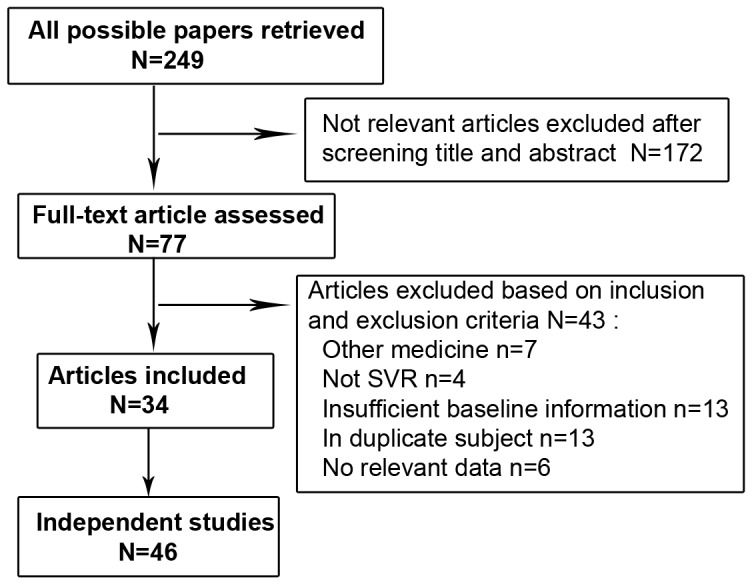
Table 1. Information of studies included in the Meta-analysis.
| No | Author | Publishing date | Region | Race | Genotype | Treatment duration | Gender (% for male) | Age | SVR | Total |
| Treatment-naïve patients | ||||||||||
| 1 | Ge et al [4] | Aug-09 | USA | African | 1 | 48W | 62.8% | 50.1±6.5a | 45 | 191 |
| 2 | Caucasian | 1 | 48W | 62.0% | 47.5±7.2 | 488 | 871 | |||
| 3 | Hispanic | 1 | 48W | 61.3% | 45.3±9.2 | 38 | 75 | |||
| 4 | Hayes et al [14] | Nov-10 | Japan | Asian | 1b | 48W | 56.5% | 58(51–65)c | 366 | 812 |
| 5 | Kawaoka et al [15] | Sep-10 | Japan | Asian | 2 | 24W | N/A | N/A | 99 | 130 |
| 6 | Kurosaki et al [19] | Sep-10 | Japan | Asian | 1b | ≥24W | 50.4% | 57.1±9.9 | 194 | 496 |
| 7 | Mangia et al [8] | Jun-10 | Italy | Caucasian | 2/3 | 12/24W | 57.8% | ≥40:77.6% | 201 | 268 |
| 8 | Scherzer et al [20] | Jan-11 | Austria | Caucasian | 3 | 24W | 63.9% | 36.2±9.3 | 53 | 69 |
| 9 | Yu et al [21] | Jan-11 | Taiwan | Asian | 2 | 24W | 54.8% | 52.8±11.1 | 429 | 482 |
| 10 | Moghaddam et al [17] | Mar-11 | Norway,Denmark | Caucasian | 3 | 14–24W | 59.1% | >40:39.5% | 226 | 281 |
| 11 | Fattovich et al [22] | Mar-11 | Italy | Caucasian | 1 | 48W | 64.5% | 46±11 | 65 | 121 |
| 12 | Caucasian | 2/3 | 24W | 55.3% | 46±11 | 144 | 159 | |||
| 13 | Lin et al [16] | Mar-11 | Taiwan | Asian | 1 | 24W | 64.4% | 51.4±11.1 | 131 | 191 |
| 14 | Bitetto et al [23] | Apr-11 | Italy | Caucasian | 1/4/5 | 48W | 51.7% | 47 (18–77)c | 47 | 110 |
| 15 | Caucasian | 2/3 | 24W | 87 | 101 | |||||
| 16 | Lindh et al [24] | Jun-11 | Denmark, Finland, Norway and Sweden | Caucasian | 2/3 | 12/24W | 59.8% | 42 (20–72)c | 237 | 341 |
| 17 | Mangia et al [25] | May-11 | Italy | Caucasian | 1 | 24/48/72W | 57.7% | 50.2 | 230 | 454 |
| 18 | de Rueda et al [26] | Jun-11 | Spain | Caucasian | 1/4 | 48W | 58.2% | >40:61.9% | 198 | 373 |
| 19 | Caucasian | 2/3 | 48W | 41 | 50 | |||||
| 20 | Ladero et al [27] | Jun-11 | Spain | Caucasian | 1 | 48W | 62.7% | 48 (20–74)c | 51 | 110 |
| 21 | Sarrazin et al [28] | Jul-11 | Germany | Caucasian | 1 | 24–72W | 55.2% | 43 (18–73)c | 291 | 542 |
| 22 | Suppiah et al [29] | Sep-11 | Australia | Caucasian | 1 | 48W | 62.5% | SVR:40.9±10.8;N-SVR:45.7±9.3 | 389 | 848 |
| 23 | Sandra et al [30] | Sep-11 | Austria | Caucasian | 1 | 48W | 55.0% | 46.4±12.2 | 51 | 113 |
| 24 | De Nicola et al [31] | Sep-11 | Italy | Caucasian | 4 | 48W | 83.6% | 46 (27–63)c | 50 | 103 |
| 25 | Howell et al [32] | Oct-11 | USA | African | 1 | 48W | 53.8% | 49.0±7.0 | 45 | 173 |
| 26 | Caucasian | 1 | 48W | 60.6% | 47.2±8.5 | 101 | 188 | |||
| 27 | Umemura et al [33] | Jul-11 | Japan | Asian | 1 | 48W | 46.2% | 58 (17–74)c | 22 | 52 |
| 28 | Scherzer et al [34] | Nov-11 | Austria | Caucasian | 1/4 | 24/48/72W | 64.7% | 44.4±10.8 | 182 | 328 |
| Patients with unsuccessful or unknown treatment history | ||||||||||
| 29 | Miyaaki et al [35] | Nov-11 | Japan | Asian | 1b | 48W | 55.2% | 56.8±9.3 | 29 | 67 |
| 30 | Lindh et al [36] | Nov-10 | Sweden | Caucasian | 1 | 24–72W | 60.9% | 45.4d | 63 | 106 |
| 31 | Hayashi et al [37] | Jun-11 | Japan | Asian | 1b | 48W | 52.5% | 55.9±10.3 | 138 | 299 |
| 32 | Halfon et al [38] | Oct-11 | Israel | Caucasian | 1 | 48W | 70.7% | 47±12 | 77 | 156 |
| 33 | Caucasian | 2/3 | 24W | 31 | 42 | |||||
| 34 | Lyoo et al [39] | Sep-11 | South Korea | Asian | 1 | 48W | 56.9% | 52.4±9.7 | 42 | 65 |
| 35 | Sakamoto et al [40] | Feb-11 | Japan | Asian | 2 | 24W | 49.6% | 64 (20–73)b | 98 | 129 |
| 36 | Sinn et al [41] | Mar-11 | Korea | Asian | 1 | 48W | 53.4% | 56.6±9.2 | 35 | 55 |
| 37 | Asian | 2 | 24W | 52 | 63 | |||||
| 38 | O'Brien et al [42] | Jul-11 | USA | Caucasian | 1 | 48W | 75.4% | 49 (45–53)b | 92 | 646 |
| HIV/HCV co-infected patients | ||||||||||
| 39 | Pineda et al [43] | Sep-10 | Spain | Caucasian | 1/4 | 48W | 84.4% | 42 (38–44)c | 33 | 103 |
| 40 | Caucasian | 2/3 | 24/48W | 44 | 51 | |||||
| 41 | Aparicio et al [44] | Oct-10 | Spain | Caucasian | 1/4 | N/A | 66.9% | SVR:48.4±0.7; Non-SVR:47.2±0.6 | 38 | 114 |
| 42 | Caucasian | 3 | 29 | 46 | ||||||
| 43 | Rallón et al [45] | Apr-11 | Spain | Caucasian | 1/4 | 48/72W | 75.0% | 42 (38–45)b | 57 | 135 |
| 44 | Caucasian | 2/3 | 24/48W | 47 | 60 | |||||
| 45 | Labarga et al [46] | May-11 | Spain | Caucasian | 1/4 | 48W | 82.3% | 43d | 12 | 47 |
| 46 | Caucasian | 2/3 | 48W | 13 | 15 | |||||
Age were described as
mean ± standard deviation,
median (interquartile range),
median (range) and
mean age.
N/A: not available.
Treatment-naïve patients
Studies on the HCV G1/4 and the HCV G2/3 treatment-naïve patients were analyzed separately both for rs12979860 and rs8099917. The summarized SVR rate was 48.5% (2984/6151) for G1/4 group and 80.6% (1517/1881) for G2/3 group.
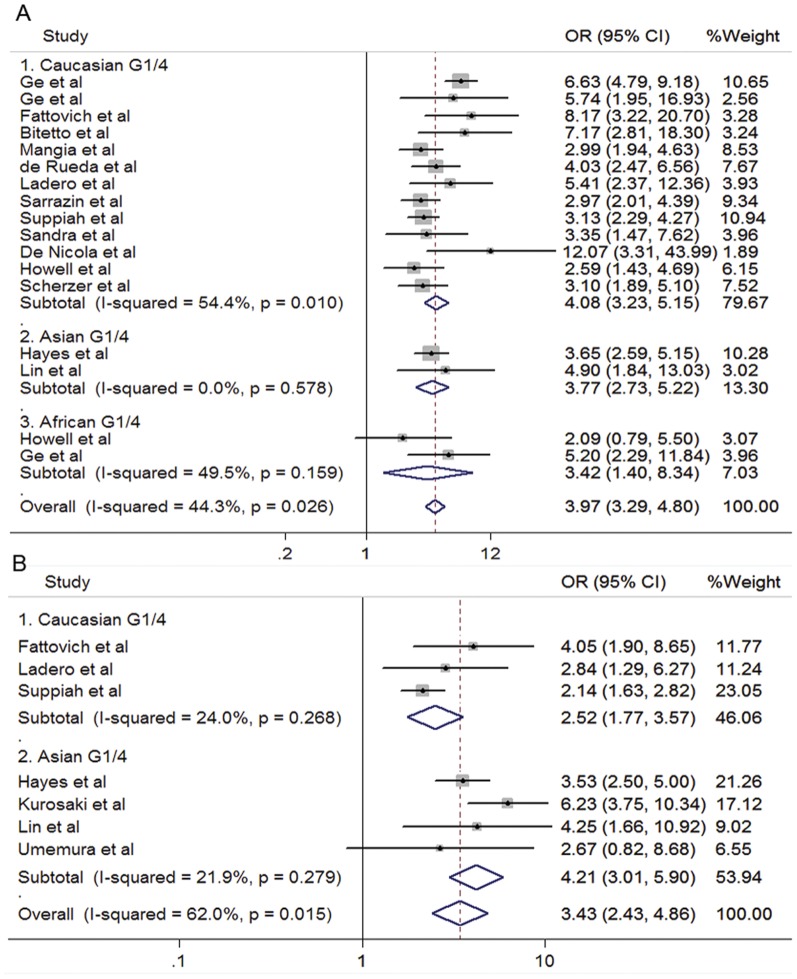
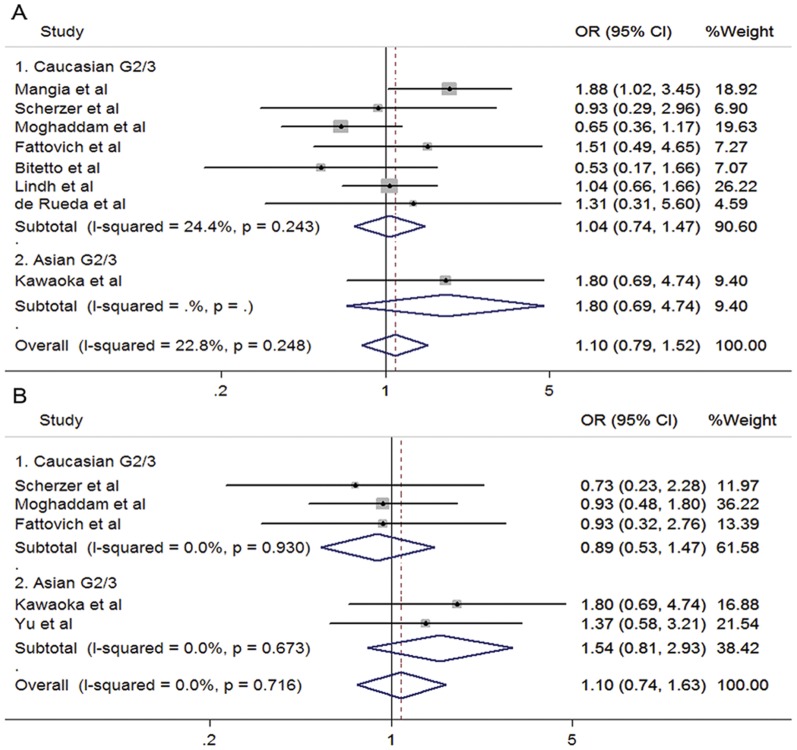
Seventeen studies evaluated the association between response of combined therapy and SNP of rs12979860 in HCV G1/4 patients (Figure 2). When data from Caucasian were combined, the CC genotype showed a favorable effect compared to CT/TT (SVR: 73.1% vs 40.5%; OR 4.08, 95%CI 3.23–5.15; P<0.001). The favorable effects of CC genotype were also observed in Asian (SVR: 57.8% vs 24.8%; OR 3.77, 95%CI 2.73–5.22; P<0.001) and African patients (SVR: 48.0% vs 21.0%; OR 3.42, 95%CI 1.40–8.34; P = 0.007). The summarized OR from all studies indicated that rs12979860 CC genotype was associated with an increased possibility of SVR in the treatment-naïve patients with HCV genotype 1 or 4 infection, when they received the PegIFN/RBV treatment (OR 3.97, 95%CI 3.29–4.80, P<0.001; Figure 2). For patients of HCV G2/3, associations could not be observed either in Caucasian (SVR: 78.5% vs 77.5%; OR 1.04, 95%CI 0.74–1.47; P = 0.811) or Asian patients (SVR: 78.3% vs 66.7%; OR 1.80, 95%CI 0.69–4.74; P = 0.231). Neither was a significant association found in the pooled estimation (OR 1.10, 95%CI 0.79–1.52, P = 0.572; Figure 3).
Figure 2. Forrest plots for association between polymorphisms and response to PegIFN/RBV in treatment-naïve HCV G1/4 patients.
A. rs12979860 (CC vs CT/TT). B. rs8099917 (TT vs TG/GG).
Figure 3. Forrest plots for association between polymorphisms and response to PegIFN/RBV in treatment-naïve HCV G2/3 patients.
A. rs12979860 (CC vs CT/TT). B. rs8099917 (TT vs TG/GG).
We next grouped studies on Caucasian patients with complete SVR data for each rs12979860 genotype (Table 2). There was no Asian aggregation due to the limited number of studies. The SVR rates declined as the frequencies of C allele within HCV G1/4 or G2/3 group decreased. And the SVR rates were always higher in HCV G2/3 patients than in G1/4 patients for each rs12979860 genotype. For rs12979860 genotype distribution, the frequency of favorable CC genotype was higher in HCV G2/3 whereas the unfavorable CT or TT genotype was reversed (Table 2). The pooled estimations from these Caucasian studies were similar to those from all studies. The ORs were 4.09 (95% CI 2.98–5.62; P<0.001) for HCV G1/4 group and 1.03 (95% CI 0.70–1.51; P = 0.893) for HCV G2/3 group respectively.
Table 2. SVR rates and frequency for rs12979860 genotypes in Caucasian treatment-naïve patients with complete data.
| Reference | CC | CT | TT | |
| SVR | ||||
| G1/4 (51.2%) | [4], [22], [23], [25], [27], [28], [29], [32] | 72.7% (793/1090) | 41.6% (684/1646) | 34.4% (156/454) |
| G2/3 (77.8%) | [8], [17], [20], [22], [23], [24] | 78.3% (403/515) | 78.6% (453/576) | 71.9% (92/128) |
| Genotype frequency | ||||
| G1/4 | [4], [22], [23], [25], [27], [28], [29], [32] | 34.2% (1090/3190) | 51.6% (1646/3190) | 14.2% (454/3190) |
| G2/3 | [8], [17], [20], [22], [23], [24] | 42.2% (515/1219) | 47.3% (576/1219) | 10.5% (128/1219) |
Similar results were observed for rs8099917 TT genotype in chronic hepatitis C patients infected with HCV genotype 1 or 4. Compared to TG/GG genotypes, individuals bearing rs8099917 TT genotype were more likely to clear virus after PegIFN/RBV treatment both in Caucasian and Asian groups (Caucasian: 56.8% vs 35.8%, OR 2.52, 95%CI 1.77–3.57, P<0.001; Asian: 54.9% vs 21.3%, OR 4.21, 95%CI 3.01–5.90, P<0.001; Figure 2). The summarized OR for all studies was 3.43 with 95% CI from 2.43 to 4.86 (P<0.001, Figure 2). Again, no significant association was observed in patients infected with HCV genotype 2 or 3 (OR 1.10, 95%CI 0.74–1.63, P = 0.648; Figure 3).
Patients with unsuccessful or unknown treatment history
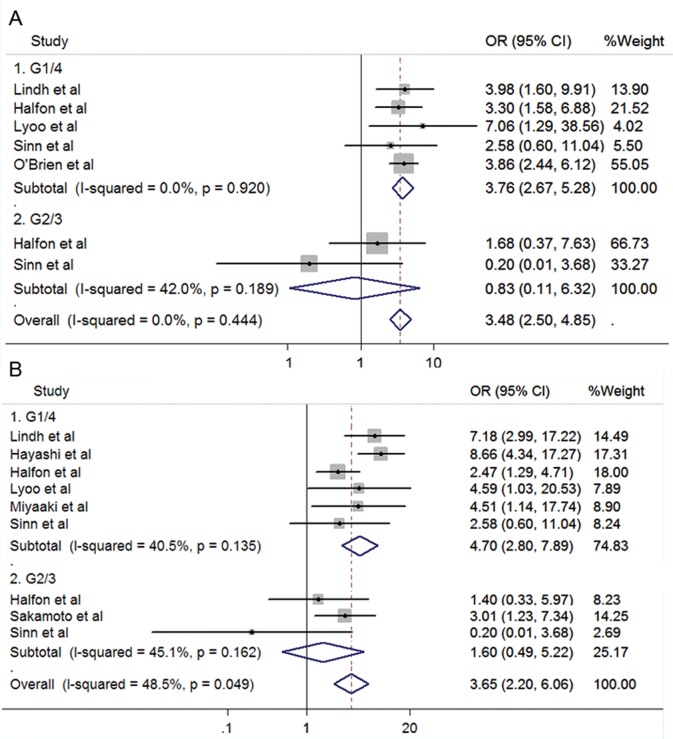
Studies were grouped together if they contained at least one patient failed to a previous interferon-based treatment or if no information of treatment history was given. This group was to show the impact of SNPs in the complex clinical practice since treatment response was reported to be different in patients with treatment history [2]. As expected, the summarized SVR rates were slightly lower than those in treatment-naïve patients, 34.1% (476/1394) in HCV G1/4 and 77.4% (181/234) in HCV G2/3 group. However, associations between SNPs and treatment effects were similar to that in the treatment-naïve group. The positive associations were observed in HCV G1/4 group carrying rs12979860 CC (OR 3.76, 95%CI 2.67–5.28, P<0.001) or rs8099917 TT genotype (OR 4.70, 95%CI 2.80–7.89, P<0.001) respectively, whereas no significant associations was found in HCV G2/3 group (Figure 4).
Figure 4. Forrest plots for association between polymorphisms and response to PegIFN/RBV in patients possibly with treatment history.
A. rs12979860 (CC vs CT/TT). B. rs8099917 (TT vs TG/GG).
HCV/HIV co-infected patients
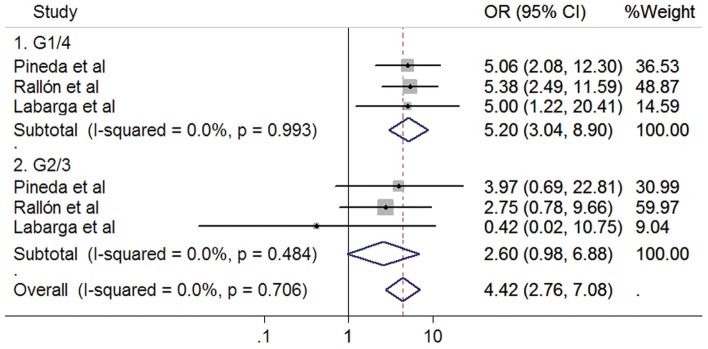
Studies conducted in HCV/HIV co-infected patients were classified into one group, without considering the treatment history due to the similar results observed in HCV mono-infected patients with and without treatment history and the limited number of studies. The pooled results indicated similar associations as those in HCV mono-infected patients. The rs12979860 CC genotype was associated with a better treatment response in HCV G1/4 patients receiving PegIFN/RBV therapy (OR 5.20, 95%CI 3.04–8.90, P<0.001; Figure 5), but not in HCV G2/3 patients (OR 2.60, 95%CI 0.98–6.88, P = 0.054). However, only one study was on rs8099917, and this study showed that re8099917 TT genotype favored the positive association in patients with HCV genotype 1 or 4, but not in genotype 2 or 3 [44].
Figure 5. Forrest plot for association between rs12979860 polymorphisms and response to PegIFN/RBV in HIV co-infected patients.
Publication bias
No obvious publication bias was observed in studies using Egger's test and Peters' test respectively, as shown in table 3.
Table 3. Results for publication bias test using Egger's test and Peters' test.
| Group | P for Egger | P for Peters |
| Rs12979860 | ||
| HCV G1/4 and treatment- naïve patient | 0.310 | 0.286 |
| HCV G2/3 and treatment- naïve patient | 0.889 | 0.946 |
| Patients with unsuccessful or unknown treatment history | 0.167 | 0.291 |
| HIV/HCV co-infected patient | 0.043 | 0.342 |
| Rs8099917 | ||
| HCV G1/4 and treatment- naïve patient | 0.331 | 0.879 |
| HCV G2/3 and treatment- naïve patient | 0.960 | 0.532 |
| Patients with unsuccessful or unknown treatment history | 0.189 | 0.143 |
Discussion
In the present study, we collected and summarized studies on associations between SNPs near IL28B gene (rs12979860 and rs8099917) and the treatment effect of PegIFN/RBV in chronic HCV patients. Roughly, a 3-fold significant increase of possibility to clear virus (SVR) was observed for rs12979860 CC genotype or rs8099917 TT genotype in patients infected with HCV genotype 1 or 4, irrespective of race, treatment history or the status of HIV co-infection. However, no relationship was found in patients infected with HCV genotype 2 or 3. A possible interpretation is that the effect of SNPs is attenuated by a better treatment response in patients with genotype 2 or 3 HCV as several studies have reported that rs12979860 CC or rs8099917 TT genotype was associated with faster early viral elimination in patients with genotype 2 or 3 HCV [17], [20], [21], [24] and the rate of SVR in treatment-naïve subjects infected with genotype 2 or 3 is 80.6%, much higher than 48.5% in patients with HCV genotype 1 or 4.
Rs12979860, located about 3 kb upstream to the IL28B coding region, resides in the putative promoter or the regulatory region of IL28B gene and may affect the gene expression as predicted by a bioinformatics tool, FastSNP (http://fastsnp.ibms.sinica.edu.tw/pages/input_SNPListAnalysis.jsp). The C allele has been reported to be linked to higher serum levels of IL28B, IL28A and IL29 levels, which are all involved in the induction of expression of IFN-responsive genes [47]. The CC genotype has also been reported to be associated with lower levels of intrahepatic interferon-stimulated gene (ISG) expression, which is linked to better IFN-based treatment response [48]. However, the exact mechanism underlying effects of the SNP on treatment response still remains to be clarified. Nonetheless, several facts are noteworthy. Frequencies of favorable CC genotype of rs12979860 vary in different races, but in the same direction as SVR rates to PegIFN/RBV treatment [49], [50]. And the frequencies of CC genotype increase from the lowest in patients failed to treatment, to intermediate in patients with treatment-induced clearance, and then to the highest in patients with spontaneous clearance [50], [51]. In our analysis, the SVR rate declines with the reduction of C allele (72.7% for CC, 41.6% for CT, 34.4% for TT, Table 2) when data from the Caucasian HCV G1/4 treatment-naïve patients were combined. Rs8099917 has been reported to be in strong linkage disequilibrium with rs12979860 [4], [14]–[18]. Similar associations with HCV treatment are observed and the predictive value could not be increased when determined simultaneously [38]. Therefore, rs8099917 may share similar mechanism as rs12979860 on the association with treatment response although the mechanism is still unclear and determination of the genotype of one SNP may be enough in clinical practice.
Limitations and cautions should be taken into account before making conclusions. Firstly, selection of patients could cause a bias. Several studies analyzed patients with compliance >80% in all patients [16], [17], [21], [24], [38] or >80% only in non-SVR patients [4], [19], [22]. Non-responders are more likely to discontinue therapy prematurely [3] and the frequencies of unfavorable genotypes tend to be higher in non-responders [50], [51]. Therefore, selection of patients according to the compliance may result in an underestimation of SNP effect on treatment response. Secondly, therapeutic effect could be influenced by multiple variables known or unknown. For example, baseline HCV RNA level has been recognized to be reversely correlated with treatment effect [4]. In this analysis, we could not adjust this factor because different studies applied different criteria to define high and low levels of HCV RNA [4], [15], [16], [23]. Therefore, the unified design should be considered in future studies to calculate the net contribution of IL28B to treatment response. Thirdly, a group of patients with unsuccessful or unknown treatment history in our review was meant to represent the actual complex clinical setting. The interpretation of results from this group should be cautious since the portions of patients with treatment history were different among different studies. However, the study carried out in patients all failed to previous treatment also supported the association concluded in our analysis [42], indicating the predictable values of SNP near the IL28B gene. Finally, several studies were excluded from our analysis due to no sufficient data to calculate the ORs stratified by races and HCV genotypes according to our criteria [50]–[54]. However, the same conclusion would be derived if these studies were included, only with the values of ORs slightly altered, since these studies also favor the results we get.
In summary, our findings suggest that SNPs of IL28B are useful baseline predictors for virological response in patients infected with genotype 1 or 4 HCV when treated with PegIFN/RBV, but not in patients with genotype 2 or 3 HCV. Therefore, determination of IL28B genotype may be necessary only in HCV genotype 1 or 4 patients. For those patients infected with difficult-to-treat genotype 1 or 4 HCV and also bearing rs12979860 non-CC genotype or rs8099917 non-TT genotype, it is urgent to develop more effective therapy strategies.
Supporting Information
Form for paper assessment and data collection.
(DOC)
Funding Statement
This work was supported by the National Natural Science Funds of China (Grant No: 81172726 and 81072369,) and the Natural Science Fund of Jilin Province (Grant No: 201215035). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lavanchy D (2009) The global burden of hepatitis C. Liver Int 29 Suppl 1: 74–81. [DOI] [PubMed] [Google Scholar]
- 2. Ghany MG, Strader DB, Thomas DL, Seeff LB (2009) Diagnosis, management, and treatment of hepatitis C:an update. Hepatology 49: 1335–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galle GW, et al. (2009) Peginterferon Alfa-2b or Alfa-2a with Ribavirin for Treatment of Hepatitis C Infection. N Engl J Med 361: 580–593. [DOI] [PubMed] [Google Scholar]
- 4. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, et al. (2009) Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461: 399–401. [DOI] [PubMed] [Google Scholar]
- 5. Shirakawa H, Matsumoto A, Joshita S, Komatsu M, Tanaka N, et al. (2008) Pretreatment prediction of virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients using viral and host factors. Hepatology 48: 1753–1760. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, et al. (2009) Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 41: 1105–1109. [DOI] [PubMed] [Google Scholar]
- 7. Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, et al. (2009) IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 41: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 8. Mangia A, Thompson AJ, Santoro R, Piazzolla V, Tillmann HL, et al. (2010) An IL28B polymorphism determines treatment response of hepatitis C virus genotype 2 or 3 patients who do not achieve a rapid virologic response. Gastroenterology 139: 821–827, 827 e821. [DOI] [PubMed] [Google Scholar]
- 9. Rallon NI, Naggie S, Benito JM, Medrano J, Restrepo C, et al. (2010) Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in HIV/hepatitis C virus-coinfected patients. AIDS 24: F23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, et al. (2009) Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461: 798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montes-Cano MA, Garcia-Lozano JR, Abad-Molina C, Romero-Gomez M, Barroso N, et al. (2010) Interleukin-28B genetic variants and hepatitis virus infection by different viral genotypes. Hepatology 52: 33–37. [DOI] [PubMed] [Google Scholar]
- 12. Lunge VR, da Rocha DB, Beria JU, Tietzmann DC, Stein AT, et al. (2012) IL28B polymorphism associated with spontaneous clearance of hepatitis C infection in a southern Brazilian HIV type 1 population. AIDS Res Hum Retroviruses 28: 215–219. [DOI] [PubMed] [Google Scholar]
- 13. Rumi MG, Aghemo A, Prati GM, D'Ambrosio R, Donato MF, et al. (2010) Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology 138: 108–115. [DOI] [PubMed] [Google Scholar]
- 14. Hayes CN, Kobayashi M, Akuta N, Suzuki F, Kumada H, et al. (2011) HCV substitutions and IL28B polymorphisms on outcome of peg-interferon plus ribavirin combination therapy. Gut 60: 261–267. [DOI] [PubMed] [Google Scholar]
- 15. Kawaoka T, Hayes CN, Ohishi W, Ochi H, Maekawa T, et al. (2011) Predictive value of the IL28B polymorphism on the effect of interferon therapy in chronic hepatitis C patients with genotypes 2a and 2b. J Hepatol 54: 408–414. [DOI] [PubMed] [Google Scholar]
- 16. Lin CY, Chen JY, Lin TN, Jeng WJ, Huang CH, et al. (2011) IL28B SNP rs12979860 is a critical predictor for on-treatment and sustained virologic response in patients with hepatitis C virus genotype-1 infection. PLoS One 6: e18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moghaddam A, Melum E, Reinton N, Ring-Larsen H, Verbaan H, et al. (2011) IL28B genetic variation and treatment response in patients with hepatitis C virus genotype 3 infection. Hepatology 53: 746–754. [DOI] [PubMed] [Google Scholar]
- 18. Lagging M, Askarieh G, Negro F, Bibert S, Soderholm J, et al. (2011) Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One 6: e17232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurosaki M, Tanaka Y, Nishida N, Sakamoto N, Enomoto N, et al. (2011) Pre-treatment prediction of response to pegylated-interferon plus ribavirin for chronic hepatitis C using genetic polymorphism in IL28B and viral factors. J Hepatol 54: 439–448. [DOI] [PubMed] [Google Scholar]
- 20. Scherzer TM, Hofer H, Staettermayer AF, Rutter K, Beinhardt S, et al. (2011) Early virologic response and IL28B polymorphisms in patients with chronic hepatitis C genotype 3 treated with peginterferon alfa-2a and ribavirin. J Hepatol 54: 866–871. [DOI] [PubMed] [Google Scholar]
- 21. Yu ML, Huang CF, Huang JF, Chang NC, Yang JF, et al. (2011) Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology 53: 7–13. [DOI] [PubMed] [Google Scholar]
- 22. Fattovich G, Covolo L, Bibert S, Askarieh G, Lagging M, et al. (2011) IL28B polymorphisms, IP-10 and viral load predict virological response to therapy in chronic hepatitis C. Aliment Pharmacol Ther 33: 1162–1172. [DOI] [PubMed] [Google Scholar]
- 23. Bitetto D, Fattovich G, Fabris C, Ceriani E, Falleti E, et al. (2011) Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology 53: 1118–1126. [DOI] [PubMed] [Google Scholar]
- 24. Lindh M, Lagging M, Farkkila M, Langeland N, Morch K, et al. (2011) Interleukin 28B gene variation at rs12979860 determines early viral kinetics during treatment in patients carrying genotypes 2 or 3 of hepatitis C virus. J Infect Dis 203: 1748–1752. [DOI] [PubMed] [Google Scholar]
- 25. Mangia A, Thompson AJ, Santoro R, Piazzolla V, Copetti M, et al. (2011) Limited use of interleukin 28B in the setting of response-guided treatment with detailed on-treatment virological monitoring. Hepatology 54: 772–780. [DOI] [PubMed] [Google Scholar]
- 26. de Rueda PM, Lopez-Nevot MA, Saenz-Lopez P, Casado J, Martin-Casares A, et al. (2011) Importance of host genetic factors HLA and IL28B as predictors of response to pegylated interferon and ribavirin. Am J Gastroenterol 106: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 27. Ladero JM, Martin EG, Fernandez C, Carballo M, Devesa MJ, et al. (2012) Predicting response to therapy in chronic hepatitis C: an approach combining IL28B gene polymorphisms and clinical data. J Gastroenterol Hepatol 27: 279–285. [DOI] [PubMed] [Google Scholar]
- 28. Sarrazin C, Schwendy S, Moller B, Dikopoulos N, Buggisch P, et al. (2011) Improved responses to pegylated interferon alfa-2b and ribavirin by individualizing treatment for 24–72 weeks. Gastroenterology 141: 1656–1664. [DOI] [PubMed] [Google Scholar]
- 29. Suppiah V, Gaudieri S, Armstrong NJ, O'Connor KS, Berg T, et al. (2011) IL28B, HLA-C, and KIR variants additively predict response to therapy in chronic hepatitis C virus infection in a European cohort: a cross-sectional study. PLoS Med 8: e1001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beinhardt S, Staettermayer AF, Rutter K, Maresch J, Scherzer TM, et al. (2012) Treatment of chronic hepatitis C genotype 1 patients at an academic center in Europe involved in prospective, controlled trials: is there a selection bias? Hepatology 55: 30–8. [DOI] [PubMed] [Google Scholar]
- 31. De Nicola S, Aghemo A, Rumi MG, Galmozzi E, Valenti L, et al. (2012) An IL28B polymorphism predicts pegylated interferon plus ribavirin treatment outcome in chronic hepatitis c genotype 4. Hepatology 55: 336–342. [DOI] [PubMed] [Google Scholar]
- 32. Howell CD, Gorden A, Ryan KA, Thompson AJ, Ibrahim C, et al. (2012) Single Nucleotide Polymorphism Upstream of Interleukin 28B Associated with Phase 1 and Phase 2 of Early Viral Kinetics in Patients Infected with HCV Genotype 1. J Hepatol 56: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Umemura T, Joshita S, Yoneda S, Katsuyama Y, Ichijo T, et al. (2011) Serum interleukin (IL)-10 and IL-12 levels and IL28B gene polymorphisms: pretreatment prediction of treatment failure in chronic hepatitis C. Antivir Ther 16: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 34. Scherzer TM, Stattermayer AF, Strasser M, Laferl H, Maieron A, et al. (2011) Impact of IL28B on treatment outcome in hepatitis C virus G1/4 patients receiving response-guided therapy with peginterferon alpha-2a (40KD)/ribavirin. Hepatology 54: 1518–1526. [DOI] [PubMed] [Google Scholar]
- 35. Miyaaki H, Ichikawa T, Yatsuhashi H, Taura N, Miuma S, et al. (2011) Suppressor of cytokine signal 3 and IL28 genetic variation predict the viral response to peginterferon and ribavirin. Hepatol Res 41: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 36. Lindh M, Lagging M, Arnholm B, Eilard A, Nilsson S, et al. (2011) IL28B polymorphisms determine early viral kinetics and treatment outcome in patients receiving peginterferon/ribavirin for chronic hepatitis C genotype 1. J Viral Hepat 18: e325–331. [DOI] [PubMed] [Google Scholar]
- 37. Hayashi K, Katano Y, Honda T, Ishigami M, Itoh A, et al. (2011) Association of interleukin 28B and mutations in the core and NS5A region of hepatitis C virus with response to peg-interferon and ribavirin therapy. Liver Int 31: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 38. Halfon P, Bourliere M, Ouzan D, Maor Y, Renou C, et al. (2011) A single IL28B genotype SNP rs12979860 determination predicts treatment response in patients with chronic hepatitis C Genotype 1 virus. Eur J Gastroenterol Hepatol 23: 931–935. [DOI] [PubMed] [Google Scholar]
- 39. Lyoo K, Song MJ, Hur W, Choi JE, Hong SW, et al. (2011) Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol 52: 363–366. [DOI] [PubMed] [Google Scholar]
- 40. Sakamoto N, Nakagawa M, Tanaka Y, Sekine-Osajima Y, Ueyama M, et al. (2011) Association of IL28B variants with response to pegylated-interferon alpha plus ribavirin combination therapy reveals intersubgenotypic differences between genotypes 2a and 2b. J Med Virol 83: 871–878. [DOI] [PubMed] [Google Scholar]
- 41. Sinn DH, Kim YJ, Lee ST, Gwak GY, Choi MS, et al. (2011) Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol 26: 1374–1379. [DOI] [PubMed] [Google Scholar]
- 42. O'Brien TR, Everhart JE, Morgan TR, Lok AS, Chung RT, et al. (2011) An IL28B genotype-based clinical prediction model for treatment of chronic hepatitis C. PLoS One 6: e20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pineda JA, Caruz A, Rivero A, Neukam K, Salas I, et al. (2010) Prediction of response to pegylated interferon plus ribavirin by IL28B gene variation in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis 51: 788–795. [DOI] [PubMed] [Google Scholar]
- 44. Aparicio E, Parera M, Franco S, Perez-Alvarez N, Tural C, et al. (2010) IL28B SNP rs8099917 is strongly associated with pegylated interferon-alpha and ribavirin therapy treatment failure in HCV/HIV-1 coinfected patients. PLoS One 5: e13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rallon NI, Soriano V, Naggie S, Restrepo C, Goldstein D, et al. (2011) IL28B gene polymorphisms and viral kinetics in HIV/hepatitis C virus-coinfected patients treated with pegylated interferon and ribavirin. AIDS 25: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Labarga P, Barreiro P, Mira JA, Vispo E, Rallon N, et al. (2011) Impact of IL28B polymorphisms on response to peginterferon and ribavirin in HIV-hepatitis C virus-coinfected patients with prior nonresponse or relapse. AIDS 25: 1131–1133. [DOI] [PubMed] [Google Scholar]
- 47. Langhans B, Kupfer B, Braunschweiger I, Arndt S, Schulte W, et al. (2011) Interferon-lambda serum levels in hepatitis C. Journal of Hepatology 54: 859–865. [DOI] [PubMed] [Google Scholar]
- 48. Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, et al. (2010) IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology 52: 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, et al. (2010) Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 139: 120–129 e118. [DOI] [PubMed] [Google Scholar]
- 50. McCarthy JJ, Li JH, Thompson A, Suchindran S, Lao XQ, et al. (2010) Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology 138: 2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, et al. (2010) Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138: 1338–1345, 1345 e1331–1337. [DOI] [PubMed] [Google Scholar]
- 52. Liao XW, Ling Y, Li XH, Han Y, Zhang SY, et al. (2011) Association of genetic variation in IL28B with hepatitis C treatment-induced viral clearance in the Chinese Han population. Antivir Ther 16: 141–147. [DOI] [PubMed] [Google Scholar]
- 53. Toyoda H, Kumada T, Tada T, Kawaguchi T, Murakami Y, et al. (2011) Impact of genetic polymorphisms near the IL28B gene and amino acid substitutions in the hepatitis C virus core region on interferon sensitivity/resistance in patients with chronic hepatitis C. J Med Virol 83: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 54. Ridruejo E, Solano A, Marciano S, Galdame O, Adrover R, et al. (2011) Genetic variation in Interleukin-28B predicts SVR in hepatitis C genotype 1 Argentine patients treated with PEG IFN and ribavirin. Ann Hepatol 10: 452–457. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Form for paper assessment and data collection.
(DOC)