Abstract
A patient in the thirties, currently undergoing chemotherapy for metastatic osteosarcoma diagnosed 3 years earlier, was admitted with in the emergency department with abdominal pain. Laparoscopic surgery revealed severe inflammation and an abscess. 18 cm of small intestine was removed because of intestinal necrosis. Histological examination showed several arterial tumour emboli, morphologically similar to the primary sarcoma. The patient died 1 year after successful surgery. Because of the improved survival of patients with osteosarcoma, acute mesenteric ischaemia should be considered in acute abdomen in these patients.
Background
Osteosarcoma (OS) is the second most frequent primary malignant bone tumour among children and adolescents, with an incidence of 1.8–4.5/1 000 000 under 15 years of age.1 Metastases are present at the time of diagnosis in 20% of the cases. In total, 40% develop metastases and 90% of these occur in the lungs.2 Only 2% of the metastases are extraskeletal or pulmonal.2
When excluding atrial myxoma as the most frequent origin of arterial tumour emboli (ATE), their origin is primary pulmonary tumours (41 %) and sarcomas (28 %). There are only reports of 60 cases of ATE up until 1993.3 Intestinal ischaemia due to occlusion of the superior mesenteric artery (SMA) occurred in only five of these cases.
We describe a patient with a primary OS in one of the femora and no previous embolic episodes, presenting with intestinal ischaemia due to ATE occluding a peripheral branch of the SMA.
Case presentation
A patient in the thirties, diagnosed 3 years before this event with a primary OS-tumour in one of the femora, contacted the oncology ward. Pulmonary metastases were discovered 1 year after initial diagnosis, for which the patient was receiving treatment with iphosphamide and etoposide. The patient presented with constant abdominal pain lasting 3 days. There had been vomiting and flatus, but no defecation. There were signs of peritonitis with rebound tenderness in the lower right fossa when palpated. The temperature was 37.9°C, C reactive protein 126 and white blood cell of 10.3×109/l.
On suspicion of acute appendicitis, explorative laparoscopy was performed, and severe inflammation and a small abscess was found 25–40 cm oral of the ileocoecal junction. After conversion to laparotomy, 18 cm of small intestine was removed and ileoileal anastomosis was performed. There were no postoperative complications and the patient was discharged 3 days after surgery.
Investigations
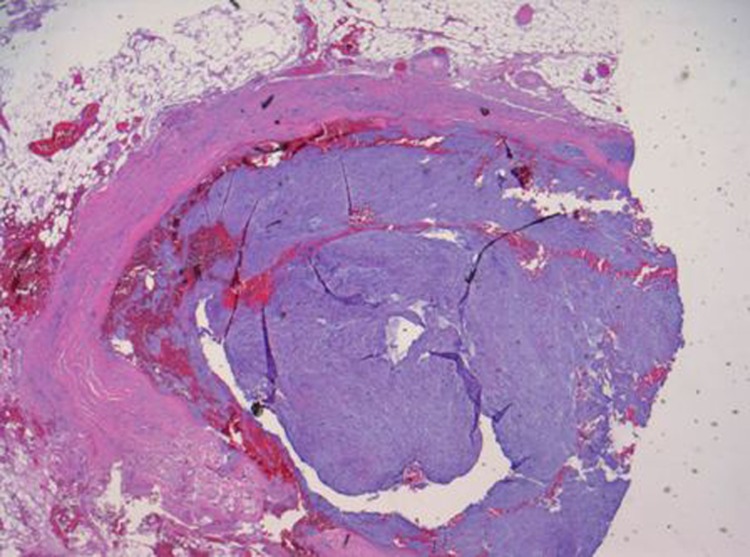
Histological examination revealed ischaemic changes and intravascular sarcoma metastases in several vessels of the SMA, morphologically similar to the previously diagnosed femoral sarcoma (figure 1). The line of resection was not free of tumour tissue. There was ischaemic necrosis in the intestinal segment evident of acute ischaemia.
Figure 1.
Dense myxoid tissue filling the vessel lumen. Adherent to the endothelium without penetrating the basal lamina.
A routine positron emission tomography-CT was performed 5 days before the acute admission, showing slight increase in tumour masses in both lungs, one of the femora, the left scapula and several vertebrae, but none in the abdomen. No arterial phase scan was performed. It was therefore not possible to diagnose tumour in the arteries, but the patient had no abdominal symptoms at the time.
Outcome and follow-up
Later the patient developed sarcoma metastases in both scapulae and the opposite femur, and pulmonary metastases were complicated with invasion into the left pulmonary artery. The patient died 4 years after initial diagnosis due to dissemination of the cancer.
Discussion
A literature search in PubMed using MeSH-terms, for example, ‘OS’ and ‘ATE’, revealed no reports later than a review by Chandler et al in 1993.3
The five cases in this review describe male patients, 58–64 years old, none of them diagnosed with cancer before admission. Symptoms at admission were heterogeneous, comprising abdominal pain, nausea and vomiting, or haemoptysis and weight loss. The patients were later diagnosed with carcinoma, sarcoma or lymphoma. Two of the six patients had pulmonary surgery performed before ATE in their SMA, and two of the six patients had no pulmonary involvement. None of these patients received chemotherapy. Postoperative survival in four of the five patients ranged from a few days to 4 months.
Acute mesenterial ischaemia (AMI) is a challenging diagnosis, because it is a rare cause of acute abdomen. Only 1–2% of these patients suffer from AMI,4 with extensive atherosclerosis being by far the most common cause. The mortality is 60–80% when AMI is caused by atherosclerosis, and even higher in other cases.4 This could reflect a delay in diagnosis, since the intestine is vital in >90 % of patients with AMI diagnosed the first 12 h of symptoms debut. The patients usually present with abdominal pain disproportionate to the clinical findings, with abdominal angina or signs of sepsis.4
The golden standard for diagnosing AMI is digital subtraction angiography (DSA) or selective mesenteric angiography, because they can also identify non-occlusive mesenteric ischaemia.4 DSA is not routinely used due to availability and practicalities like unstable patients and transport.5 Plain x-ray and CT-scans can only be used for excluding differential diagnoses. Serological markers are not routinely used in clinical practice, though several have been proposed.5
Most ATE are associated with primary or secondary pulmonary cancer invading the veins or the left atrium. Surgical manipulation is the major cause of ATE (41%), and other important factors are size and growth rate of the tumour. The pattern of localisation of ATE is similar to that of atherosclerotic emboli. In the review by Chandler et al, only five of 80 ATE were located in the SMA.3 Recommended intervention is heparinisation to prevent further aggregation and embolectomy using a Fogarthy catheter, or laparotomy and embolectomy with resection of the infarcted intestine and primary anastomosis.3 4 Intestinal perfusion should be tested intraoperatively using fluorescein or Doppler ultrasound, and if the perfusion is uncertain, a second-look procedure within 12–24 h or creation of a mucous fistula is recommended.
Because the ATE in this case did not penetrate the vessels basal lamina, they are by definition not metastases. Since the tumour cells lodged in the SMA probably are not the only ones which embolised from the pulmonary metastases, it seems reasonable to consider ATE as a sign of further dissemination. This is however, not a contra indication to perform embolectomy, due to the high success rate of the procedure.3
In the period of 1982–2002, highly malignant OS with axial primary tumour and/or pulmonary metastases at the time of diagnosis have shown an increase in survival from 9% to 32%, mainly due to improvements in chemotherapy and more frequent use of surgery.6 Because of this increase in survival for patients with OS and pulmonary metastases, the incidence of ATE could probably increase. Therefore one should consider AMI as a differential diagnosis when encountering acute abdomen in these patients.
Learning points.
-
▶
In patients with OS, ATE often arise from pulmonic metastases and can present as an embolism in the mesenteric circulation.
-
▶
ATE distribute and lodge similar to atherosclerotic emboli.
-
▶
Mortality of AMI due to non-atherosclerotic embolisms is >60 %.
-
▶
Increase in survival for patients with OS and pulmonary metastases, makes AMI an important differential diagnosis.
Footnotes
Competing interests None.
Patient consent Not obtained.
References
- 1.Ta HT, Dass CR, Choong PF, et al. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev 2009;28:247–63 [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Longhi A, Fagioli F, et al. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer 2005;41:2836–45 [DOI] [PubMed] [Google Scholar]
- 3.Chandler C. Malignant arterial tumor embolization. J Surg Oncol 1993;52:197–202 [DOI] [PubMed] [Google Scholar]
- 4.Yasuhara H. Acute mesenteric ischemia: the challenge of gastroenterology. Surg Today 2005;35:185–95 [DOI] [PubMed] [Google Scholar]
- 5.Evennett NJ, Petrov MS, Mittal A, et al. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg 2009;33:1374–83 [DOI] [PubMed] [Google Scholar]
- 6.Picci P, Mercuri M, Ferrari S, et al. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol 2010;21:1366–73 [DOI] [PubMed] [Google Scholar]