Abstract
Objective:
Cognitive impairment and neurocirculatory abnormalities such as orthostatic hypotension (OH), supine hypertension (SH), and failure to decrease blood pressure at night (nondipping) occur relatively commonly in Parkinson disease (PD); however, whether cognitive dysfunction in early PD is related to neurocirculatory abnormalities has not been established. Cognitive dysfunction in PD is associated with white matter hyperintensities on MRI. We report results of an analysis of neuropsychological and hemodynamic parameters in patients with early PD.
Methods:
Among 87 patients, 25 had normal cognition, 48 had mild cognitive impairment, and 14 had dementia, based on comprehensive neuropsychological tests. Orthostatic vital signs and ambulatory 24-hour blood pressure monitoring were recorded, and brain magnetic resonance scans were obtained for all patients.
Results:
Cognitive impairment was associated with OH, SH, and white matter hyperintensities but not with nondipping. Dementia and white matter hyperintensities were common in SH. Of 13 patients with OH + SH, every one had mild cognitive impairment or dementia.
Conclusions:
Cognitive dysfunction is related to neurocirculatory abnormalities, especially OH + SH, in early PD, raising the possibility that early detection and effective treatment of those abnormalities might slow the rate of cognitive decline.
It is increasingly being recognized that Parkinson disease (PD) involves prominent nonmotor manifestations such as dementia and autonomic failure.
A variety of neurocirculatory abnormalities have been noted in PD, including orthostatic hypotension (OH),1–4 supine hypertension (SH),5–7 labile blood pressure, and the absence of a decrease in pressure during the night, a phenomenon called nondipping.8 These abnormalities are related to each other. SH is found in a substantial proportion of patients with PD with OH.5,9
Patients with PD are susceptible to cognitive dysfunction, even early in the disease.10 Eventually both cognitive and autonomic failure commonly occur. Although studies have suggested interdependence of these manifestations, the results have been inconsistent.11–14 Among elderly individuals, cognitive decline and other neurobehavioral signs are associated with low blood pressure, pressure fluctuations, and nondipping.15–19
The hypothesis tested in this study was that neurocirculatory abnormalities may contribute to cognitive abnormalities accompanying early PD. We assessed whether cognitive abnormalities or white matter intensities on MRI were related to OH, SH, or nondipping by investigating associations among cognitive data from a variety of rating scales, neurocirculatory data from head-up tilt testing and 24-hour ambulatory blood pressure monitoring, and neuroimaging data from a quantitative measure of white matter hyperintensities seen on MRI in Korean patients with early PD.
METHODS
Patients.
The study was approved by the ethics committee of the Seoul St. Mary's Hospital. Each patient gave written informed consent before participating.
Early PD was diagnosed in 87 individuals at the Department of Neurology, Seoul St. Mary's Hospital, according to UK Brain Bank criteria.20 None of the patients had ever taken antiparkinsonian medication.
Clinical information included age, gender, education, disease duration, current medications, and history of arterial hypertension, diabetes mellitus, and cigarette smoking. Twenty-seven patients (31%) had a history of systemic hypertension (mean duration 8.7 years). Of these, 24 were taking ≥1 antihypertensive medication.
Excluded were patients with atypical PD or secondary parkinsonism or those taking medications known to influence cognitive or autonomic functions. PET using [18F]N-(3-fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl)nortropane and myocardial [123I]metaiodobenzylguanidine scintigraphy was done in selected patients to exclude secondary parkinsonism or multiple system atrophy.21 All patients were evaluated by the Unified Parkinson's Disease Rating Scale (UPDRS) Part I to Part III and classified by modified Hoehn and Yahr stage. Blood pressure monitoring was performed after any antihypertensive medications were discontinued for ≥7 days.
Tilt-table testing.
Each patient was studied in a testing room (23 ± 1°C) after having fasted overnight except for water. Electrocardiographic and noninvasive blood pressure monitoring devices were connected (YM6000; Mediana Technologies, Miami, FL). After 30 minutes of supine rest, head-up tilt-table testing at 60° was done for up to 20 minutes, using a Manumed Special Tilt 1 Section (Enraf Nonius, Rotterdam, the Netherlands). Blood pressure was obtained every 5 minutes before and 1, 3, 5, 10, 15, and 20 minutes during tilt, 1 minute posttilt, and as indicated for patient safety. Mean supine baseline and the lowest tilt values for blood pressure were recorded. For statistics, the lower values were chosen from 3 and 5 minutes.
OH was defined as a fall in blood pressure of ≥20 mm Hg systolic and ≥10 mm Hg diastolic after at least 3 minutes of tilt.22 SH was defined by systolic pressure ≥150 mm Hg or diastolic pressure ≥90 mm Hg.5,6
Ambulatory blood pressure monitoring.
Automated 24-hour blood pressure recording (Mobil-O-Graph NG; I.E.M., Stolberg, Germany) tracked blood pressure and heart rate every 15 minutes during the day (8:00 am–10:00 pm) and every 30 minutes during the night (10:00 pm–8.00 am). Mean daytime and nighttime systolic, diastolic, and pulse pressures were recorded. Pulse pressure provided an indirect index of overall arterial stiffness.23 Measures of blood pressure variability were the SD and the coefficient of variation of systolic pressure.24 Percentage nocturnal decrease of mean arterial blood pressure (MBP) was calculated as (MBPday − MBPnight)/MBPday × 100. Subjects with <10% nocturnal decrease of MBP compared with daytime were considered nondippers.25
MRI and CHIPS scale.
All patients underwent 3.0-T MRI (Magnetom Verio 3T; Siemens). White matter hyperintensities were quantified on axial sections of fluid-attenuated inversion recovery sequence images, using the recently developed visual rating scale, the Cholinergic Pathways Hyperintensities Scale (CHIPS),26 which quantifies the extent of white matter hyperintensities in the periventricular and subcortical white matter. CHIPS scores are closely associated with cognitive status in PD.27
Neuropsychological tests.
Information about memory problems and other subjective cognitive deficits was obtained from caregiver interviews. General cognitive status and dementia severity were evaluated using the Korean version of the Mini-Mental State Examination, Clinical Dementia Rating scale, and the sum of the box of the Clinical Dementia Rating scale.
To assess cognitive domains we used the Seoul Neuropsychological Screening Battery,28 which covers attention, language, praxis, 4 elements of Gerstmann syndrome, visuospatial function, verbal and visual memory, and frontal/executive functions. Quantifiable indices were Digit Span Forward and Backward; the Korean version of the Boston Naming Test; the Rey-Osterrieth Complex Figure Copy; the Seoul Verbal Learning Test for verbal memory; the Rey-Osterrieth Complex Figure Test for nonverbal, visuospatial memory; the Controlled Oral Word Association Test (semantic: animals and grocery items and phonemic: Korean letters) for word fluency; and the Stroop Color/Word Conflict Test. Frontal motor functions were assessed by motor impersistence, contrasting program, go-no go test, fist-edge-palm, alternating hand movement, alternating square and triangle, and Luria loop tests. Test scores were classified as abnormal when they were <16% of scores of age-, sex-, and education-matched normal subjects.28
Mild cognitive impairment was diagnosed if at least 1 of 5 cognitive domains was abnormal.29 Patients with mild cognitive impairment had no history or symptoms of memory problems or other cognitive disorders, according to the dementia screening questionnaire. Dementia was diagnosed according to clinical diagnostic criteria for probable PD dementia.30
Data analysis and statistics.
Data analysis consisted of neurocirculatory parameters by cognitive status and cognitive domains by neurocirculatory parameters (OH, SH, OH + SH, and nondipping). Additional evaluations assessed blood pressure fluctuations (SD and the coefficient of variation of systolic pressure).
Independent means t tests or 1-way analyses of variance (with Bonferroni post hoc testing) were used to compare groups, and χ2 tests were used to compare frequencies for categorical variables. Analyses of covariance were done to identify a priori confounding covariates. Relationships among blood pressure profiles and cognitive domains and CHIPS scores were tested by Spearman correlation coefficients. Linear regression analyses were performed on Mini-Mental State Examination and CHIPS scores, with several associated factors as covariates. Statistical tests used SPSS 15.0 software. Statistical significance was defined by p < 0.05.
RESULTS
Among the patients, 35 were men and 52 were women. Mean age (±SD) was 67.5 ± 9.2 years and motor symptoms duration was 1.8 ± 0.8 years. UPDRS scores averaged 22.4 ± 16.6 and Hoehn and Yahr stage scores averaged 1.7 ± 0.7. The patients therefore had relatively mild PD of short duration.
Twenty-five patients had normal cognition, 48 had mild cognitive impairment, and 14 had dementia. The groups had similar gender makeup, disease duration, level of education, presence of hypertension and diabetes mellitus, cigarette smoking, and serum homocysteine (table 1). Mean age was higher in the mild cognitive impairment group than in the other groups. There was more severe motor impairment in the dementia group by both UPDRS and Hoehn and Yahr stages.
Table 1.
Clinical, laboratory, and blood pressure profiles in patient groups according to cognitive statusa
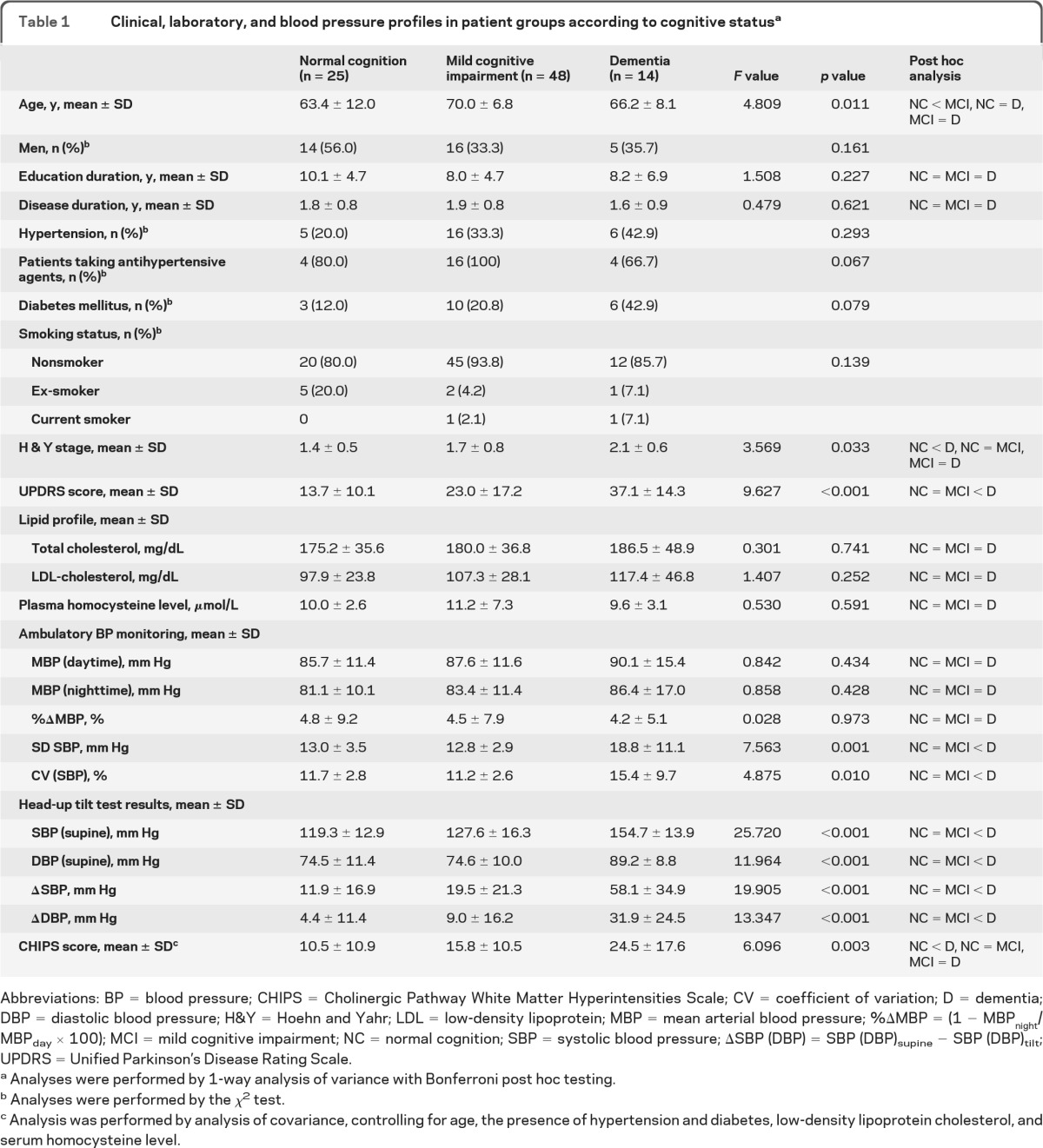
Abbreviations: BP = blood pressure; CHIPS = Cholinergic Pathway White Matter Hyperintensities Scale; CV = coefficient of variation; D = dementia; DBP = diastolic blood pressure; H&Y = Hoehn and Yahr; LDL = low-density lipoprotein; MBP = mean arterial blood pressure; %ΔMBP = (1 − MBPnight/MBPday × 100); MCI = mild cognitive impairment; NC = normal cognition; SBP = systolic blood pressure; ΔSBP (DBP) = SBP (DBP)supine − SBP (DBP)tilt; UPDRS = Unified Parkinson's Disease Rating Scale.
Analyses were performed by 1-way analysis of variance with Bonferroni post hoc testing.
Analyses were performed by the χ2 test.
Analysis was performed by analysis of covariance, controlling for age, the presence of hypertension and diabetes, low-density lipoprotein cholesterol, and serum homocysteine level.
Thirty-two patients (36.8%) had OH. Groups with vs without OH were similar with respect to age, gender, level of education, duration of illness, severity of parkinsonism, and percentage of patients with a history of hypertension. The proportion of patients receiving antihypertensive treatment was lower in the OH group (table e-1 on the Neurology® Web site at www.neurology.org). Supine mean pressure was higher and the nadir pressure during tilt was lower in the OH group. Among patients with OH, the mean tilting time before orthostatic symptoms was 3.4 ± 1.1 minutes. Fourteen patients developed OH within 3 minutes and 18 between 3 and 10 minutes. All patients without OH were tested for 20 minutes. The frequencies of nondipping and circadian blood pressure profiles were similar in the groups with vs without OH (tables e-1 and e-2).
Cognition and OH.
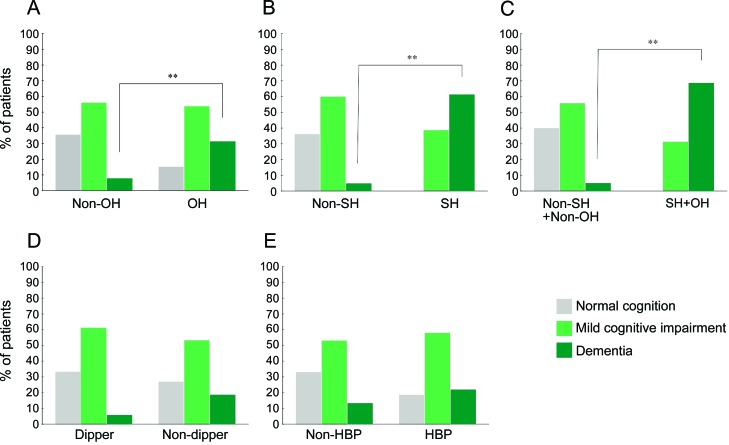
The OH and non-OH groups differed in prevalences of cognitive impairment and dementia (figure 1A). The OH group had more severe impairment in verbal immediate/delayed memory (table e-3). There was a trend toward higher CHIPS scores in the OH group (figure 2A). Individual values for orthostatic changes in blood pressure were correlated with values for verbal immediate/delayed memory and with CHIPS scores (tables 2 and 3).
Figure 1. Cognitive status as a function of neurocirculatory abnormalities in Parkinson disease.
(A) Orthostatic hypotension (OH) (p = 0.003). (B) Supine hypertension (SH) (p < 0.001). (C) OH + SH (p < 0.001). (D) Nondipping phenomenon (p = 0.172). (E) Arterial hypertension (HBP) (p = 0.297).
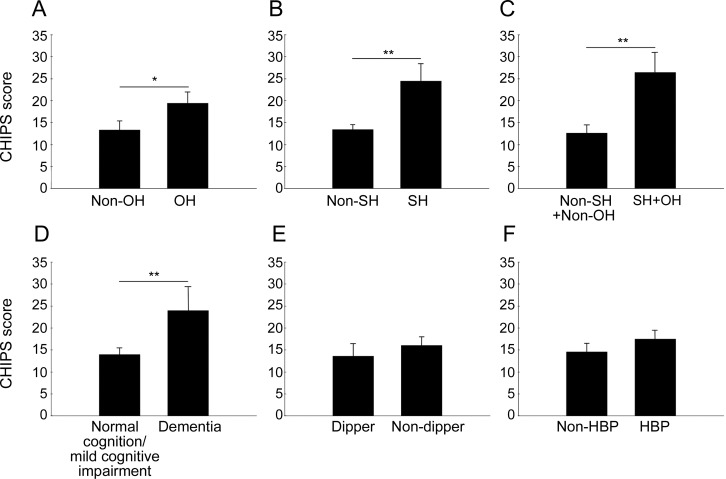
Figure 2. Cholinergic Pathways Hyperintensities Scale (CHIPS) mean (±SEM) scores as a function of neurocirculatory abnormalities in Parkinson disease.
(A) Orthostatic hypotension (OH). (B) Supine hypertension (SH). (C) OH + SH. (D) Dementia. (E) Nondipping. (F) Arterial hypertension (HBP). Statistical tests were analyses of covariance, controlling for age, the presence of hypertension or diabetes, low-density lipoprotein cholesterol, and serum homocysteine level. *p < 0.05; **p < 0.001.
Table 2.
Correlations of blood pressure profiles in head-up tilt test with each cognition domaina
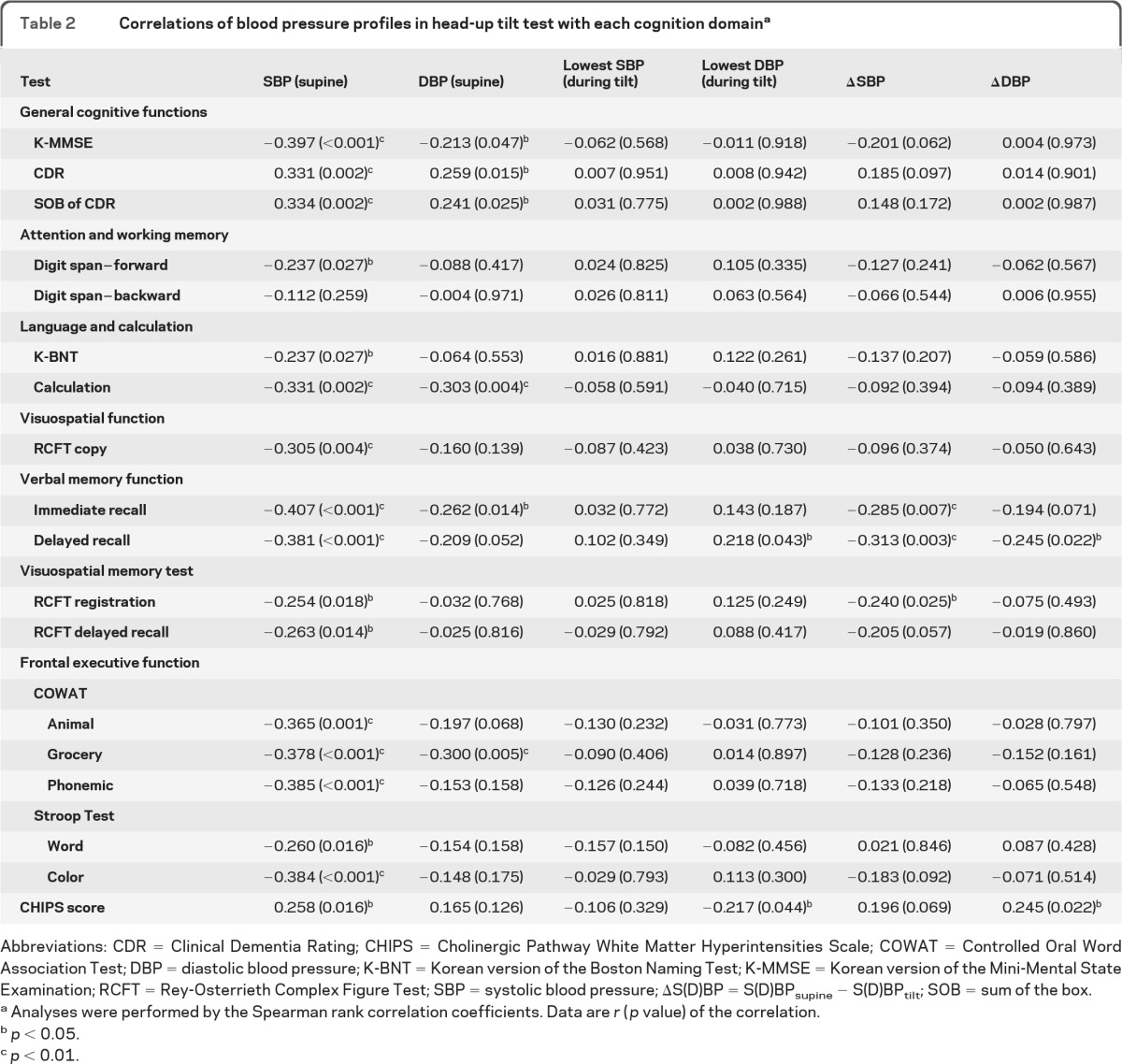
Abbreviations: CDR = Clinical Dementia Rating; CHIPS = Cholinergic Pathway White Matter Hyperintensities Scale; COWAT = Controlled Oral Word Association Test; DBP = diastolic blood pressure; K-BNT = Korean version of the Boston Naming Test; K-MMSE = Korean version of the Mini-Mental State Examination; RCFT = Rey-Osterrieth Complex Figure Test; SBP = systolic blood pressure; ΔS(D)BP = S(D)BPsupine − S(D)BPtilt; SOB = sum of the box.
Analyses were performed by the Spearman rank correlation coefficients. Data are r (p value) of the correlation.
p < 0.05.
p < 0.01.
Table 3.
Linear regression analysis for contributors of Mini-Mental State Examination score and CHIPS score
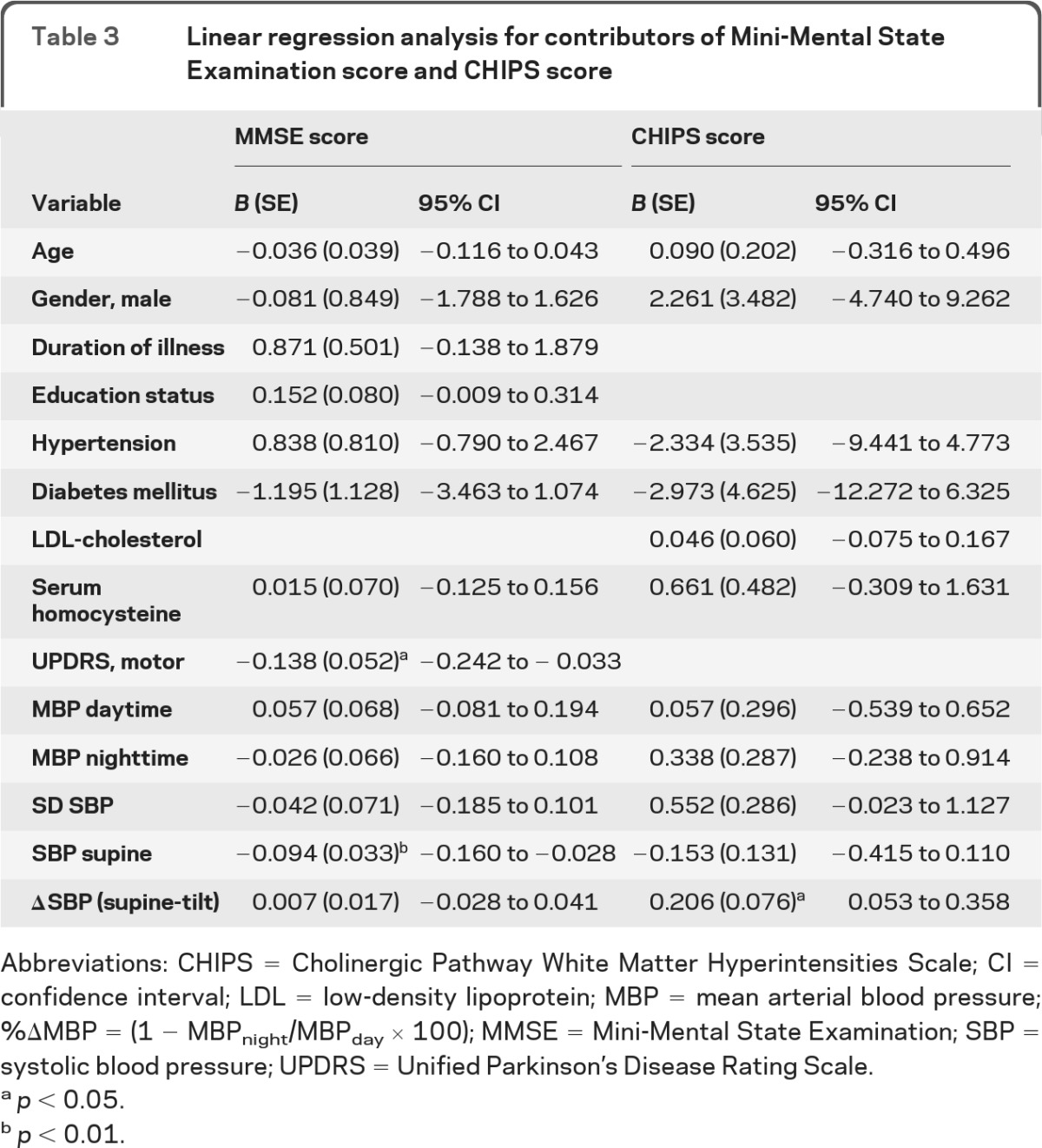
Abbreviations: CHIPS = Cholinergic Pathway White Matter Hyperintensities Scale; CI = confidence interval; LDL = low-density lipoprotein; MBP = mean arterial blood pressure; %ΔMBP = (1 − MBPnight/MBPday × 100); MMSE = Mini-Mental State Examination; SBP = systolic blood pressure; UPDRS = Unified Parkinson's Disease Rating Scale.
p < 0.05.
p < 0.01.
Patients with dementia had marked, progressive reductions in blood pressure during orthostasis compared with the groups with normal cognition or mild cognitive impairment. Among 14 patients with dementia, 10 (71.4%) had OH, whereas among 25 patients with normal cognitive function, only 5 had OH (χ2 = 10.029, p = 0.002).
Cognition and SH.
Eighteen patients (20.7%) had SH, with supine systolic pressure >150 mm Hg. Groups with vs without SH had similar mean ages at onset of parkinsonism and similar gender makeup, education level, and cigarette smoking. The magnitude of the fall in pressure during orthostasis was greater in the SH than in the non-SH group (table e-1). The daytime/nighttime systolic pressure was also higher in the SH group.
All patients with SH were nondippers. The proportions of patients with a history of hypertension or diabetes mellitus were higher in the SH group, the duration of illness was shorter, and parkinsonian motor symptoms were more severe.
Systolic pressure during supine rest was correlated with general cognitive functions and most neuropsychological domains and CHIPS scores (table 2). The mean supine blood pressure and SD and coefficient of variation of systolic pressure were higher in the dementia group than in the other groups (figure e-1, table 1). Supine systolic pressure was also associated with decreased Mini-Mental State Examination scores (table 3).
All 18 patients with SH had at least some cognitive dysfunction (p < 0.001 by sign test) (table e-3, figure 1B). In most neuropsychological test domains, the SH group had more severe defects than did the group without SH. Conversely, among 14 patients with dementia, 11 (78.6%) had SH, whereas among patients with normal cognitive function, none had SH (χ2 = 27.360, p < 0.001). Mean CHIPS scores were higher in patients with SH than in patients without SH (figure 2B).
Cognition and OH + SH.
Patients with OH + SH (n = 13) had more severe cognitive impairment and higher CHIPS scores than did those with neither OH nor SH (n = 50) (figures 1C and 2C). None of the OH + SH patients had normal cognition; most (9 of 13 [69%]) had dementia. The mean CHIPS score in the OH + SH patients (26.5 ± 16.4) was twice that of the remaining patients (13.7 ± 11.0, p = 0.001).
Cognition and blood pressure variability.
Frontal executive functions were negatively related to the SD of systolic pressure, and nocturnal blood pressure changes were related to visuospatial memory registration (table e-4). Many aspects of the neuropsychological tests, especially in memory/frontal executive functions, were also correlated with the pulse pressure.
Cognition and CHIPS scores.
The mean CHIPS score was higher in patients with dementia (24.5 ± 17.6) than in those with normal cognition or mild cognitive impairment (14.0 ± 10.9, p = 0.004) (figure 2D). CHIPS scores did not differ between the groups with normal cognition vs mild cognitive impairment, although there was a trend toward higher scores in the latter.
Cognition and nondipping.
A total of 79.3% of the patients were nondippers. Dipper and nondipper groups differed in percent changes of blood pressures between day and night, with higher overnight pressures in the nondipper group (table e-1). Dipper and nondipper groups were similar in terms of gender, patient education, duration of illness, and severity of parkinsonism (table e-1). Mean age and the proportion of patients with hypertension were higher in the nondipper group. All patients with OH + SH were nondippers, whereas among 50 patients with neither OH nor SH, 39 were nondippers (χ2 = 3.465, p = 0.063).
The dipper and nondipper groups did not differ in cognitive status, although many patients with dementia were nondippers (figure 1D). There were subtle differences in neuropsychological test results and CHIPS scores (table e-3, figure 2E), with most test scores being nonsignificantly lower in the nondipper group.
Cognition and history of hypertension.
Patient groups with and without a history of hypertension had similar clinical characteristics except for the proportion with diabetes mellitus (table e-1). Cognitive status and CHIPS scores were unrelated to hypertension (figures 1E and 2F). Results from most neuropsychological domains were unrelated to the presence of hypertension, except for minor differences in visuospatial function and word fluency (table e-3).
DISCUSSION
In this study of patients with early PD, cognitive impairment was clearly associated with neurocirculatory abnormalities, especially OH and SH. Both OH and SH were more prevalent in patients with dementia than in patients with normal cognitive function; conversely, not a single patient with OH + SH had normal cognitive function.
Both cognitive dysfunction and neurocirculatory abnormalities were associated with elevated white matter hyperintensities (CHIPS) scores on MRI. The results therefore indicate close links among cognitive impairment, MRI evidence for cumulative vascular brain injury, and neurocirculatory abnormalities in PD.
Our data from patients with relatively recent onset of PD, who had never been treated with antiparkinsonian medication, provide support for the view that OH, although relatively uncommon in PD, can be an early finding related to the disease process itself.3,4 In PD, OH reflects cardiac and extracardiac sympathetic noradrenergic denervation and baroreflex failure.31
Patients with neurogenic OH typically also have SH.5,6 Our study confirms this association; however, this finding made it impossible to analyze the relative contributions of OH and SH to the cognitive abnormalities separately. All patients with OH + SH had cognitive impairment, and approximately 50% of patients with cognitive impairment had OH, SH, or both.
The findings fit with the proposition that neurocirculatory abnormalities might contribute to cognitive decline in PD. If this were correct, then treatment targeting those abnormalities could constitute a novel means to prevent development of dementia in PD. Association does not imply causation; however, it is possible that the cognitive and neurocirculatory abnormalities reflect shared central pathogenetic mechanisms. Brain abnormalities related to dementia might contribute to neurovascular instability and blood pressure dysregulation.32 There is a U-shaped association between blood pressure and cognitive performance among elderly subjects,33,34 but in the present study cognitive dysfunction was not associated with the occurrence of hypertension per se.
In line with the Braak schema for the pathogenetic sequence in synucleinopathies,35 PD may feature early involvement of medullary centers mediating baroreflex regulation of sympathetic and parasympathetic outflows,31 coupled with early deposition of α-synuclein in sympathetic noradrenergic nerves that results in cardiac and (at least in PD + OH) extracardiac noradrenergic denervation. Whereas baroreflex failure by itself does not cause OH, baroreflex failure in the setting of sympathetic noradrenergic denervation might do so. Arteriolar wall/lumen ratios could reflect compensatory architectural changes in blood vessel walls, because high wall/lumen ratios augment vasoconstriction for a given amount of released transmitter. The ability to continue to modulate blood pressure at least partly could come at the cost of progressively increasing arterial stiffness and SH.
Our results also show that in early PD, cognitive dysfunction is related to measures of blood pressure lability such as the SD of systolic pressure. Correlations between pulse pressure and cognitive dysfunction and CHIPS scores suggested a role of arterial stiffness.
Strengths and limitations.
A strength of this study is that we enrolled only patients with newly diagnosed PD that was relatively mild who had never taken any dopaminergic medication. We could therefore infer that the association of cognitive impairment with neurocirculatory abnormalities does not result from antiparkinsonian treatment.
A weakness of the study is that early autonomic or cognitive involvement might reflect a particular population within a spectrum of disorders between PD and diffuse Lewy body disease.36 We attempted to reduce selection bias by including patients who fulfilled the UK Brain Bank clinical diagnostic criteria for PD and excluding patients with suspected cases of diffuse Lewy body disease.20,36 It can be difficult, however, to differentiate PD from diffuse Lewy body disease at early stages. There is considerable debate and controversy in the field about distinguishing mild cognitive impairment, PD with dementia, and diffuse Lewy body disease. Indeed, relatively recent recognition of several nonmotor manifestations of PD is forcing reconsideration of what PD is from a nosologic point of view. More severe autonomic symptoms and clinical laboratory findings might be expected in patients with diffuse Lewy body disease.37
Most of the patients with hypertension were taking antihypertensive medications. Although the results showed no correlations between indices of cognitive decline and history of hypertension, and patients with OH + SH were taking antihypertensive medication infrequently, care should be taken in the conclusion that arterial hypertension and the use of antihypertensive medication do not contribute to cognitive impairment.
We did not assess other mechanisms that might play a pathophysiologic role in SH, such as renal impairment.38 To our knowledge, hypertension from renal dysfunction is not associated with OH. Conversely, in PD, SH is clearly associated with OH, as confirmed here.
The study was performed in a confined geographical region with a relatively homogeneous ethnic background. Further studies are needed in patients of different ethnic origins to be able to generalize to the overall population of patients with PD worldwide.
Individual values for neurocirculatory parameters (SH, OH, nondipper status, and blood pressure lability) were closely associated with each other. Because of this, it was difficult to identify their contributions to cognitive dysfunction separately. In PD, as in other forms of primary chronic autonomic failure, OH is often associated with SH.5,6 Unlike OH, SH alone often occurs late in the day or at night and may not elicit symptoms.7 Although a link between SH and increased cardiovascular risk, including stroke, has been established,39,40 a chronic adverse effect of SH has not been demonstrated in PD.
In general, our results fit with the view that myriad episodes of cerebral hypo- and hyperperfusion, related to SH, OH, and blood pressure lability, contribute to vascular brain injuries and thereby to dementia in PD. The findings and their interpretation lead us to propose that efforts to minimize blood pressure fluctuations in PD might be beneficial in terms of maintaining cognitive function.
Supplementary Material
GLOSSARY
- CHIPS
Cholinergic Pathways Hyperintensities Scale
- MBP
mean arterial blood pressure
- OH
orthostatic hypotension
- PD
Parkinson disease
- SH
supine hypertension
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
J.-S. Kim: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, study supervision, obtaining funding. Y.-S. Oh: analysis or interpretation of data, acquisition of data, statistical analysis, study supervision. K.-S. Lee: analysis or interpretation of data, acquisition of data, study supervision. Y.-I. Kim: analysis or interpretation of data, acquisition of data, study supervision. D.-W. Yang: analysis or interpretation of data, acquisition of data, study supervision. D.S. Goldstein: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Senard JM, Rai S, Lapeyre-Mestre M, et al. Prevalence of orthostatic hypotension in Parkinson's disease. J Neurol Neurosurg Psychiatry 1997; 63: 584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wenning GK, Scherfler C, Granata R, et al. Time course of symptomatic orthostatic hypotension and urinary incontinence in patients with postmortem confirmed parkinsonian syndromes: a clinicopathological study. J Neurol Neurosurg Psychiatry 1999; 67: 620–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein DS, Holmes CS, Dendi R, Bruce SR, Li ST. Orthostatic hypotension from sympathetic denervation in Parkinson's disease. Neurology 2002; 58: 1247–1255 [DOI] [PubMed] [Google Scholar]
- 4. Goldstein DS. Orthostatic hypotension as an early finding in Parkinson's disease. Clin Auton Res 2006; 16: 46–54 [DOI] [PubMed] [Google Scholar]
- 5. Pathak A, Senard JM. Blood pressure disorders during Parkinson's disease: epidemiology, pathophysiology and management. Expert Rev Neurother 2006; 6: 1173–1180 [DOI] [PubMed] [Google Scholar]
- 6. Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 2003; 42: 136–142 [DOI] [PubMed] [Google Scholar]
- 7. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci 2011; 310: 123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt C, Berg D, Herting, et al. Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Mov Disord 2009; 24: 2136–2142 [DOI] [PubMed] [Google Scholar]
- 9. Lagi A, Spini S. Clinostatic hypertension and orthostatic hypotension. Clin Cardiol 2010; 33: E10–E15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010; 9: 1200–1213 [DOI] [PubMed] [Google Scholar]
- 11. Allcock LM, Kenny RA, Mosimann UP, et al. Orthostatic hypotension in Parkinson's disease: association with cognitive decline? Int J Geriatr Psychiatry 2006; 21: 778–783 [DOI] [PubMed] [Google Scholar]
- 12. Idiaquez J, Benarroch EE, Rosales H, Milla P, Ríos L. Autonomic and cognitive dysfunction in Parkinson's disease. Clin Auton Res 2007; 17: 93–98 [DOI] [PubMed] [Google Scholar]
- 13. Peralta C, Stampfer-Kountchev M, Karner E, et al. Orthostatic hypotension and attention in Parkinson's disease with and without dementia. J Neural Transm 2007; 114: 585–588 [DOI] [PubMed] [Google Scholar]
- 14. Oh ES, Lee JH, Seo JG, Sohn EH, Lee AY. Autonomic and cognitive functions in Parkinson's disease (PD). Arch Gerontol Geriatr 2011; 52: 84–88 [DOI] [PubMed] [Google Scholar]
- 15. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens 2008; 26: 1636–1641 [DOI] [PubMed] [Google Scholar]
- 16. Guo H, Tabara Y, Igase M, et al. Abnormal nocturnal blood pressure profile is associated with mild cognitive impairment in the elderly: the J-SHIPP study. Hypertens Res 2010; 33: 32–36 [DOI] [PubMed] [Google Scholar]
- 17. Yap PL, Niti M, Yap KB, Ng TP. Orthostatic hypotension, hypotension and cognitive status: early comorbid markers of primary dementia? Dement Geriatr Cogn Disord 2008; 26: 239–246 [DOI] [PubMed] [Google Scholar]
- 18. Mehrabian S, Duron E, Labouree F, et al. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci 2010; 299: 45–48 [DOI] [PubMed] [Google Scholar]
- 19. Rose KM, Couper D, Eigenbrodt ML, Mosley TH, Sharrett AR, Gottesman RF. Orthostatic hypotension and cognitive function: the Atherosclerosis Risk in Communities Study. Neuroepidemiology 2010; 34: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988; 51: 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King AE, Mintz J, Royall DR. Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord 2011; 26: 1218–1224 [DOI] [PubMed] [Google Scholar]
- 22. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996; 6: 125–126 [DOI] [PubMed] [Google Scholar]
- 23. Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci 2007; 113: 157–170 [DOI] [PubMed] [Google Scholar]
- 24. Tohgi H, Chiba K, Kimura M. Twenty-four-hour variation of blood pressure in vascular dementia of the Binswanger type. Stroke 1991; 22: 603–608 [DOI] [PubMed] [Google Scholar]
- 25. Manabe Y, Fujii D, Kono S, et al. Systemic blood pressure profile correlates with cardiac 123I-MIBG uptake in patients with Parkinson's disease. J Neurol Sci 2011; 307: 153–156 [DOI] [PubMed] [Google Scholar]
- 26. Bocti C, Swartz RH, Gao FQ, Sahlas DJ, Behl P, Black SE. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke 2005; 36: 2126–2131 [DOI] [PubMed] [Google Scholar]
- 27. Shin J, Choi S, Lee JE, Lee HS, Sohn YH, Lee PH. Subcortical white matter hyperintensities within the cholinergic pathways of Parkinson's disease patients according to cognitive status. J Neurol Neurosurg Psychiatry 2012; 83: 315–321 [DOI] [PubMed] [Google Scholar]
- 28. Kang YW, Na DL. Seoul Neuropsychological Screening Battery. Incheon, Korea: Human Consulting Co; 2003 [Google Scholar]
- 29. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56: 303–308 [DOI] [PubMed] [Google Scholar]
- 30. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007; 22: 1689–1707 [DOI] [PubMed] [Google Scholar]
- 31. Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 2012; 46: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skoog I, Andreasson LA, Landahl S, Lernfelt B. A population-based study on blood pressure and brain atrophy in 85-year-olds. Hypertension 1998; 32: 404–409 [DOI] [PubMed] [Google Scholar]
- 33. Guo Z, Viitanen M, Winblad B, Fratiglioni L. Low blood pressure and incidence of dementia in a very old sample: dependent on initial cognition. J Am Geriatr Soc 1999; 47: 723–726 [DOI] [PubMed] [Google Scholar]
- 34. Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. JAMA 1999; 281: 438–445 [DOI] [PubMed] [Google Scholar]
- 35. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003; 24: 197–211 [DOI] [PubMed] [Google Scholar]
- 36. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65: 1863–1872 [DOI] [PubMed] [Google Scholar]
- 37. Walter BL. Cardiovascular autonomic dysfunction in patients with movement disorders. Cleve Clin J Med 2008; 75: S54–S58 [DOI] [PubMed] [Google Scholar]
- 38. Garland EM, Gamboa A, Okamoto L, et al. Renal impairment of pure autonomic failure. Hypertension 2009; 54: 1057–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coleman CT, Stowasser M, Jenkins C, Marwick TH, Sharman JE. Central hemodynamics and cardiovascular risk in nondippers. J Clin Hypertens 2011; 13: 557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pringle E, Phillips C, Thijs L, et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens 2003; 21: 2251–2257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.