Abstract
Objective:
To investigate the vascular contribution to longitudinal changes in Alzheimer disease (AD) biomarkers.
Methods:
The Alzheimer's Disease Neuroimaging Initiative is a clinic based, longitudinal study with CSF, PET, and MRI biomarkers repeatedly measured in participants with normal cognition (NC), mild cognitive impairment (MCI), and mild AD. Participants with severe cerebrovascular risks were excluded. Cardiovascular risk scores and MRI white matter hyperintensities (WMHs) were treated as surrogate markers for vascular burden. Generalized estimating equations were applied, and both vascular burden and its interaction with time (vascular burden × time) or time-varying WMHs were entered into regression models to assess whether biomarker rates of change were modified by vascular burden.
Results:
Cardiovascular risk profiles were not predictive of progression in CSF β42-amyloid, [18F]fluorodeoxyglucose (FDG) PET uptake, and MRI hippocampal atrophy. Greater baseline cardiovascular risks or WMHs were generally associated with cognitive impairment, particularly poor executive function. WMHs increased over time with a faster rate in MCI and AD than in NC. Increased time-varying WMH was associated with faster decline in executive function and lower FDG uptake in NC. Otherwise, WMH was not associated with CSF and MRI biomarkers in the 3 groups. These findings remained unchanged after accounting for APOE4.
Conclusion:
Increased WMHs are associated with aging, decreased glucose metabolism, and decline in executive function but do not affect AD-specific pathologic progression, suggesting that the vascular contribution to dementia is probably additive although not necessarily independent of the amyloid pathway.
Both Alzheimer disease (AD) and vascular pathology are common in the elderly population, and multiple brain pathologic conditions account for most patients with dementia.1 Many cardiovascular risk factors including midlife hypertension, diabetes, dyslipidemia, and smoking seem to increase the risk of AD, suggesting a vascular contribution to the etiology of AD.2,3 Within the framework of the neurovascular unit, vascular dysfunction may reduce the clearance of β-amyloid (Aβ) via the blood-brain barrier or indirectly increase Aβ deposition.4 Amyloid deposition is considered the pivotal event in the AD pathologic cascade,5 but whether the accumulation is accelerated by vascular risks remains unclear.
White matter hyperintensities (WMHs) on brain MRI reflect cardiovascular risk profiles, and greater WMH volume is associated with cerebral hypometabolism and cognitive decline.6–8 White matter injury in AD may result from arteriosclerosis, cerebral amyloid angiopathy, venous collagenosis, secondary degeneration related to neuronal loss, and toxic effects of soluble Aβ.9 Brain microinfarcts may cause cognitive impairment via reduction of brain reserve, but the mechanism appears to be independent of typical AD lesions.10 A recent study from the Alzheimer's Disease Neuroimaging Initiative (ADNI) has shown that the longitudinal changes in CSF Aβ42, [18F]fluorodeoxyglucose (FDG) PET uptake, and MRI hippocampal volume are reflective of AD progression after cerebral amyloid deposition.11 Vascular effects on brain reserve may lower the clinical threshold for cognitive impairment without influencing the rate of AD pathologic progression. Because participants with severe cerebrovascular risks were excluded from ADNI, we aimed to assess mild vascular effects on the longitudinal change of AD biomarkers using the cardiovascular risk profile and WMHs.
METHODS
Study population.
This is a cohort study with a total of 819 research participants (normal cognition [NC], 229; mild cognitive impairment [MCI], 397; and Alzheimer disease, [AD] 193) enrolled in ADNI from 59 centers in the United States and Canada during 2005–2007. ADNI is supported by the NIH, private pharmaceutical companies, and nonprofit organizations with the primary goal of examining the utility of serial biomarker measurement in AD and pre-AD stages. Full inclusion and exclusion criteria are detailed at http://www.adni-info.org. In brief, screening criteria for entry into the study included the Mini-Mental State Examination (MMSE) score, Clinical Dementia Rating scale score, and an education-adjusted cutoff score on delayed recall of one paragraph from the Logical Memory subtest of the Wechsler Memory Scale–Revised.12 All participants were recruited between the ages of 55 and 90 years and had at least 6 years of education and a study partner able to provide an independent evaluation of functioning. Use of specific psychoactive medications and a Hachinski Ischemic Scale score of 4 or greater were exclusion criteria. We used the data from ADNI up to the date November 1, 2011.
Standard protocol approvals, registrations, and patient consents.
The study procedures were approved by institutional review boards of all participating institutions. Written informed consent was obtained from all research participants or their representatives.
Follow-up timeline.
Detailed schedules of assessment for NC, MCI, and AD are posted in the general procedure manual on the ADNI Web site http://www.adni-info.org/Scientists/pdfs/ADNI_Protocol_extension_A2_091908.pdf. In brief, after the baseline visit, subsequent visits took place in person at 6- or 12-month intervals.
Cognitive function assessment.
In addition to MMSE score, the Alzheimer's Disease Assessment Scale−Cognitive Subscale (ADAS-cog) and an executive function composite score were used as dependent measures to examine relationships between vascular burden and cognitive change. The ADAS-cog contains 11 items covering language, memory, praxis, and comprehension function. Higher scores indicate poorer cognitive function. An executive function composite score was calculated by summing the total correct in digit span (forward and backward), category fluency (animals and vegetables), and digit symbol substitution tests. Baseline and multiple follow-up MMSE, ADAS-cog, and executive function assessments were available for all participants.
Biomarkers of AD pathology.
CSF proteins.
CSF samples were collected in the morning after an overnight fast, shipped to the University of Pennsylvania Alzheimer's Disease Biomarker Laboratory and analyzed using a standardized protocol.13 Aβ42, total tau, phosphorylated tau were measured (pg/mL) in each of the CSF aliquots using the multiplex xMAP Luminex (Luminex Corp, Austin, TX) platform with immunoassay kit–based reagents (for research only–based reagents; INNO-BIA AlzBio3; Innogenics, Ghent, Belgium).
FDG-PET.
The protocol to acquire ADNI PET data at sites nationwide is detailed at http://www.loni.ucla.edu/ADNI/Data/ADNI_Data.shtml, and methods for FDG-PET analysis have been described previously.14 In brief, PET images were acquired 30–60 minutes postinjection. Images were averaged, spatially aligned, interpolated to a standard voxel size, intensity normalized, and smoothed to a common resolution of 8-mm full-width at half-maximum. PET volumes were intensity normalized to a single region comprising the cerebellar vermis and the pons defined by the Montreal Neurological Institute template. We used predefined regions of interest (FDG ROIs) associated with brain regions typically affected by AD to reflect glucose metabolism. Mean FDG uptake was extracted and averaged from 5 ROIs (right/left temporal gyrus, right/left angular gyrus, and bilateral posterior cingulate gyrus) for each participant.
MRI hippocampal volume.
The 1.5-T MRI protocol was described elsewhere15 and was standardized across all sites: 2 T1-weighted MRI scans, using a sagittal volumetric magnetization-prepared rapid gradient echo sequence, with an echo of 4 msec, repetition time of 9 msec, flip angle of 8°, and acquisition matrix size of 256 × 256 × 166 in the x-, y-, and z-dimensions with a nominal voxel size of 0.94 × 0.94 × 1.2 mm. The images were aligned, skull-stripped, and segmented and passed rigorous quality control checks. FreeSurfer software (http://surfer.nmr.mgh.harvard.edu) was applied to obtain bilateral hippocampal volumes in mm3 from this segmentation.
APOE4 allele.
Blood samples at baseline were collected, and APOE genotyping was performed at the National Cell Repository for AD. APOE4 gene carriers were participants who had at least one APOE4 allele.
Surrogate markers of vascular burden.
Cardiovascular risk profile.
Cardiovascular risk score was calculated using the office-based cardiovascular risk profile prediction function from the Framingham Heart Study, which took age, gender, body mass index, blood pressure, smoking, and diabetes into account16; higher scores indicated higher risks of cardiovascular events. The cardiovascular risk score was normally distributed and treated as a continuous but time-fixed variable in the analysis.
WMH volume.
The automated imaging procedure to estimate WMH volume was detailed in an earlier ADNI publication.7 WMH volume was not normally distributed and therefore was log-transformed for analysis. All participants had at least one MRI WMH measurement at baseline, and 38% (310/819) had repeated measures for 3 years.
Statistical analyses.
AD biomarker trajectories.
Participants with repeated measures were entered into analyses. We used repeated measures linear regression (an exchangeable working within-subject correlation model via a generalized estimating equation [GEE])17 to estimate average rates of change in cognitive function and AD biomarkers. The primary GEE model of biomarker trajectory treated time-varying biomarkers as the outcome with covariates of time and baseline age in the regression.
Longitudinal effect of vascular burden.
Cardiovascular risk score and WMHs were proxy measures of vascular burden. We first examined the interrelationship among WMH, age, APOE4, and cardiovascular risk score at baseline in multivariable linear regression models. For participants with repeated WMHs, we delineated WMH changes over time in NC, MCI, and AD groups. Each vascular burden proxy as well as its interaction with time (vascular burden proxy × time) was then entered into the GEE models of biomarkers in NC, MCI, and AD (model 1: cardiovascular risk score as vascular proxy; model 2: baseline WMHs as vascular proxy). Coefficients of the interaction terms reflected the direction and magnitude of how vascular risks modified biomarker rates of change at different stages. For a subgroup of participants with repeated measures of WMHs, time-varying WMH was taken into the GEE models to evaluate how AD biomarkers varied with WMHs over time (model 3).
Secondary analyses.
Although our focus was the association between vascular burden and AD biomarkers, we used similar GEE models to evaluate vascular effects on cognitive decline indexed by time-varying MMSE, ADAS-cog, and executive function scores. In addition, we tested whether conversion from NC to MCI or from MCI to AD was affected by baseline vascular risks in logistic models with age adjustment.
Sensitivity analyses.
APOE4 carriers are predisposed to develop AD and a previous study from ADNI also demonstrated that APOE4 accelerated hippocampal atrophy in MCI and AD.11 Therefore, we included APOE4 carrier status in GEE models to test the robustness of any vascular effect.
All statistical analyses and graphics were performed in R (version 2.11.1). All tests of statistical significance were conducted at the 2-tailed α level of 0.05.
RESULTS
Baseline cognitive function, AD biomarkers, cardiovascular risk score, WMH volume, length of follow-up, and other demographic features of the NC, MCI, and AD groups are shown in table 1. The effect of age seemed stronger than that of cardiovascular risk on baseline WMH, whereas APOE4 did not appear to affect WMH (table 2). WMH volume significantly increased over time, and the average rate of change (rate: 10–3 log-transformed volume/month) was faster in the MCI (7.6) and AD (7.4) groups than in the NC (4.9) group after adjustment for age.
Table 1.
Baseline characteristics of 819 participants in ADNI
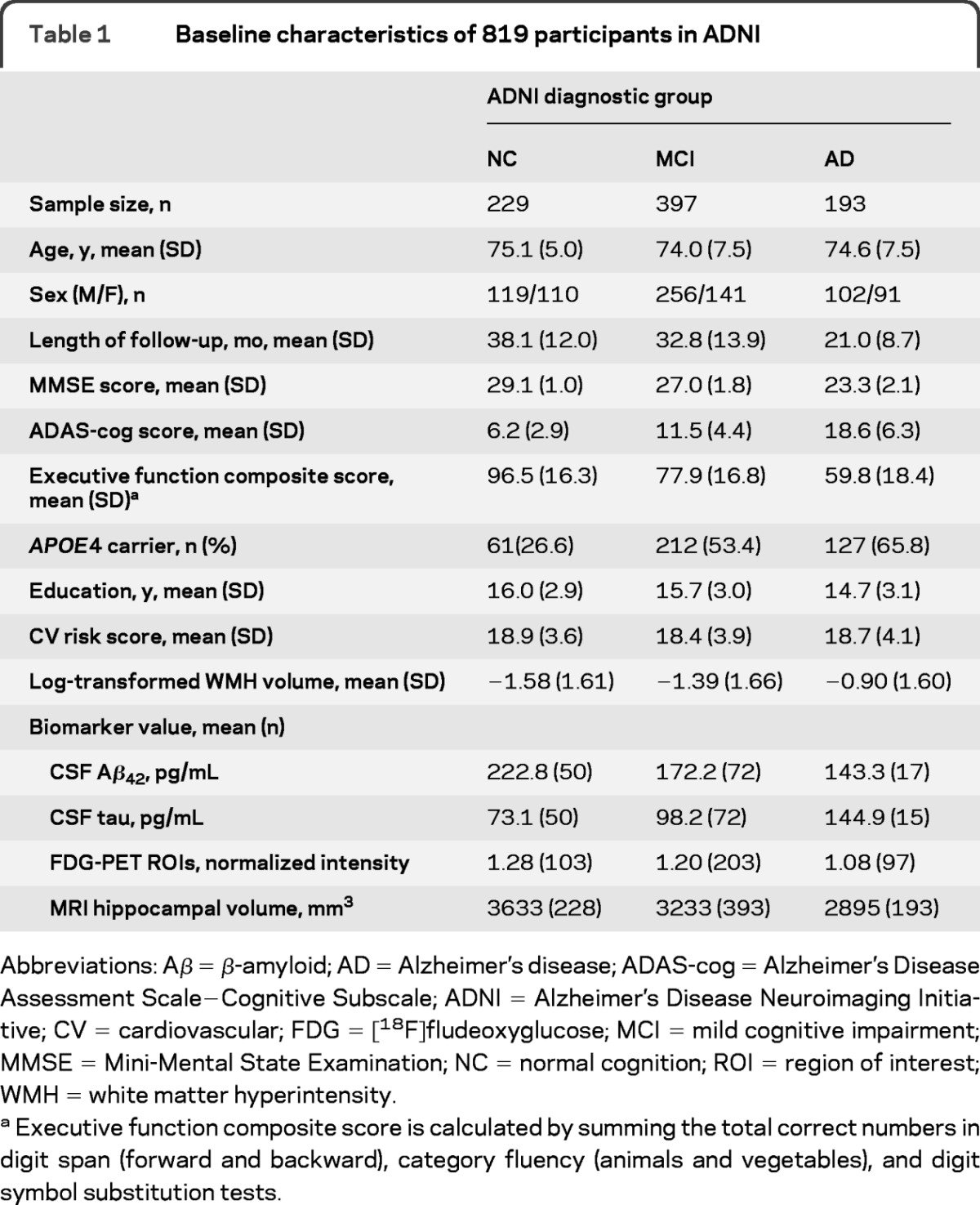
Abbreviations: Aβ = β-amyloid; AD = Alzheimer's disease; ADAS-cog = Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADNI = Alzheimer's Disease Neuroimaging Initiative; CV = cardiovascular; FDG = [18F]fludeoxyglucose; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; NC = normal cognition; ROI = region of interest; WMH = white matter hyperintensity.
Executive function composite score is calculated by summing the total correct numbers in digit span (forward and backward), category fluency (animals and vegetables), and digit symbol substitution tests.
Table 2.
Association of baseline WMH with age, cardiovascular risk score, and APOE4a
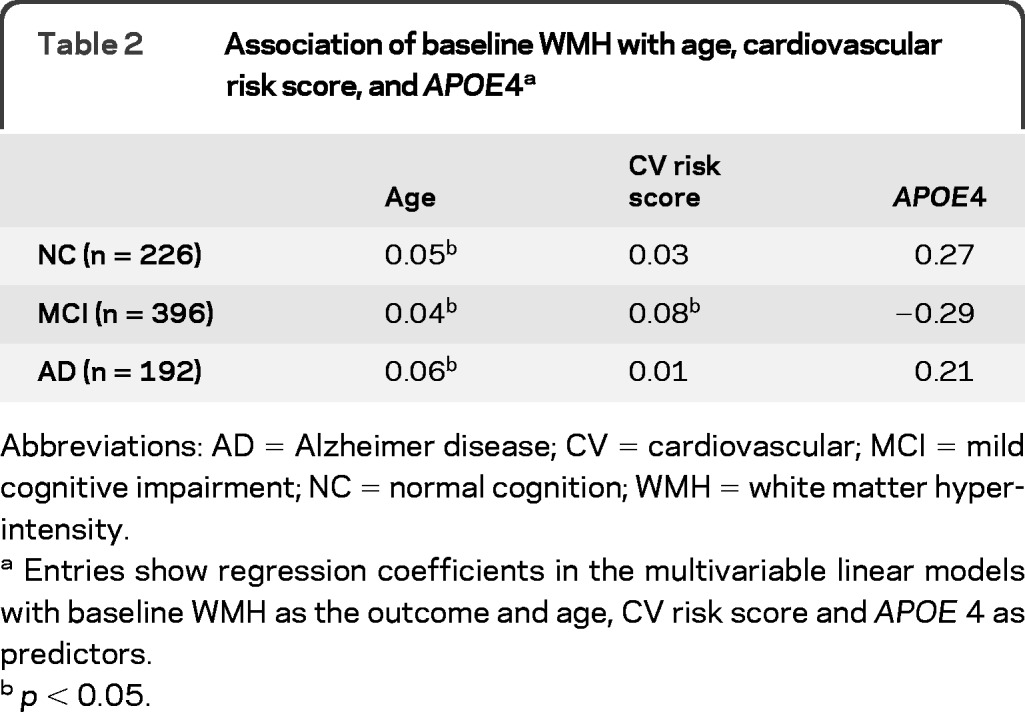
Abbreviations: AD = Alzheimer disease; CV = cardiovascular; MCI = mild cognitive impairment; NC = normal cognition; WMH = white matter hyperintensity.
Entries show regression coefficients in the multivariable linear models with baseline WMH as the outcome and age, CV risk score and APOE 4 as predictors.
p < 0.05.
Vascular contribution to AD biomarker changes are summarized in tables 3–5. CSF Aβ42 declined over time in the NC and MCI groups, but there was no association between vascular burden and CSF Aβ42 cross-sectionally and longitudinally. An increase in WMHs was associated with greater reduction of FDG uptake in cognitively normal participants. In addition, increased baseline WMH volume was associated with faster MRI hippocampal atrophy also in cognitively normal participants, but this finding was not replicated when time-varying WMH was used for analysis. These results remained unchanged after accounting for APOE4.
Table 3.
Regression coefficients in GEE models for CSF Aβ42 biomarkera
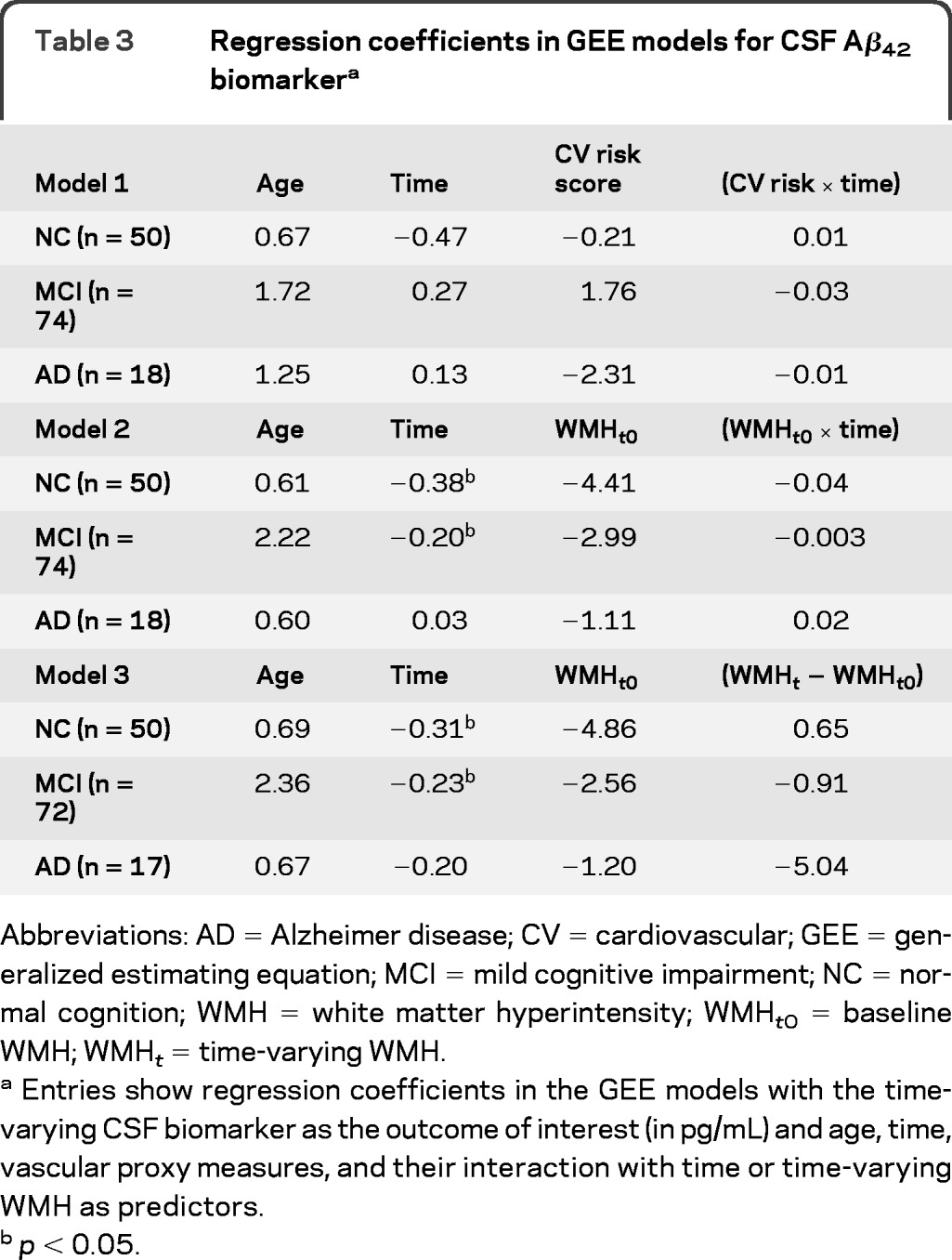
Abbreviations: AD = Alzheimer disease; CV = cardiovascular; GEE = generalized estimating equation; MCI = mild cognitive impairment; NC = normal cognition; WMH = white matter hyperintensity; WMHt0 = baseline WMH; WMHt = time-varying WMH.
Entries show regression coefficients in the GEE models with the time-varying CSF biomarker as the outcome of interest (in pg/mL) and age, time, vascular proxy measures, and their interaction with time or time-varying WMH as predictors.
p < 0.05.
Table 4.
Regression coefficients in GEE models for FDG-PET biomarkera

Abbreviations: AD = Alzheimer disease; CV = cardiovascular; FDG = [18F]fludeoxyglucose; GEE = generalized estimating equation; MCI = mild cognitive impairment; NC = normal cognition; WMH = white matter hyperintensity; WMHt0 = baseline WMH; WMHt = time-varying WMH.
Entries show regression coefficients in the GEE models with time-varying PET biomarker as the outcome of interest (unit: 10–3 normalized intensity); and age, time, vascular proxy measures and their interaction with time or time-varying WMH as predictors.
p < 0.05.
Statistical significance disappears after accounting for APOE 4.
Table 5.
Regression coefficients in GEE models for MRI hippocampal atrophya
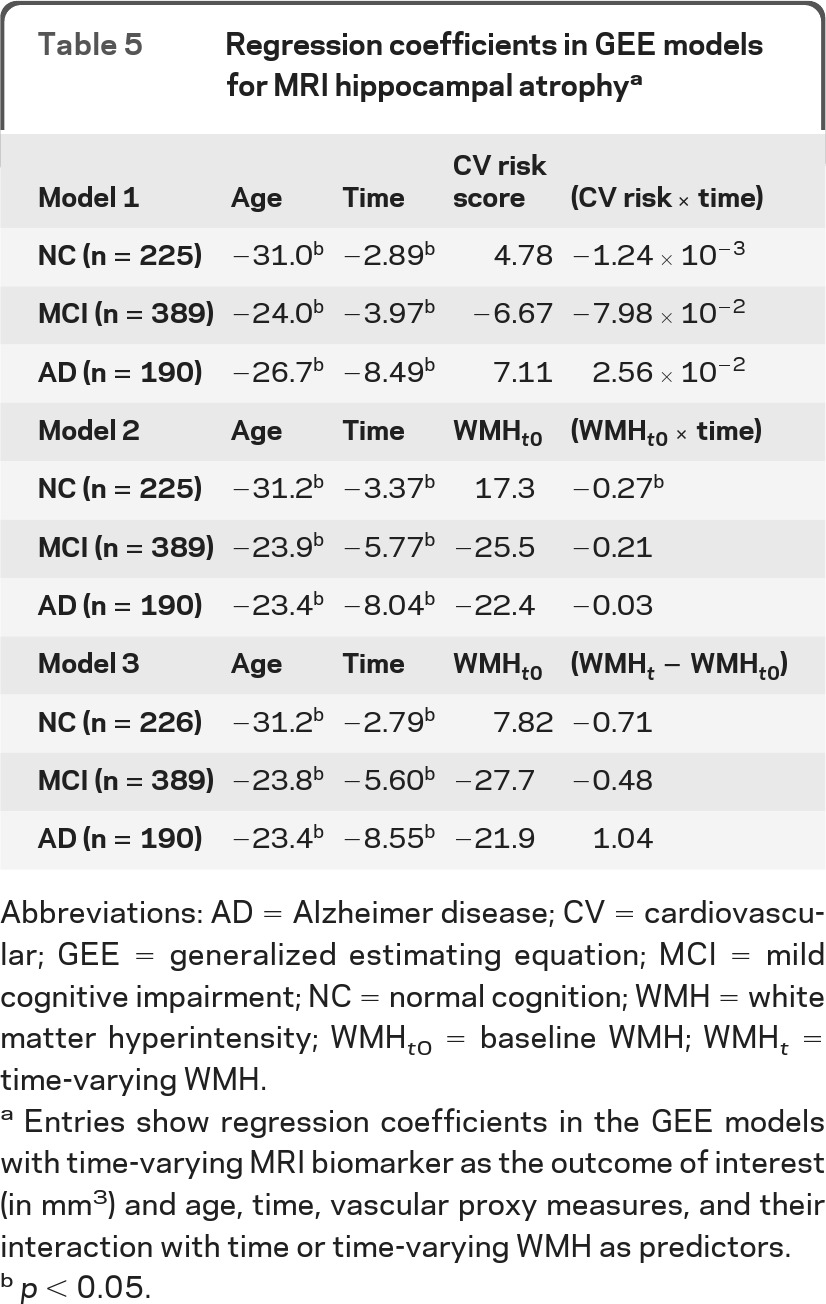
Abbreviations: AD = Alzheimer disease; CV = cardiovascular; GEE = generalized estimating equation; MCI = mild cognitive impairment; NC = normal cognition; WMH = white matter hyperintensity; WMHt0 = baseline WMH; WMHt = time-varying WMH.
Entries show regression coefficients in the GEE models with time-varying MRI biomarker as the outcome of interest (in mm3) and age, time, vascular proxy measures, and their interaction with time or time-varying WMH as predictors.
p < 0.05.
For the relationship between cognitive function and vascular risks, higher cardiovascular risk scores were not predictive of MMSE or ADAS-cog scores in any group but were associated with poor executive function in the MCI group. Greater baseline WMH volume was associated with lower MMSE score in the AD group, higher ADAS-cog score in the NC and AD groups, and poorer executive function in the MCI and AD groups. Neither cardiovascular risk score nor WMH volume was associated with rates of cognitive decline indexed by MMSE and ADAS-cog scores (tables e-1 and e-2 on the Neurology® Web site at www.neurology.org). However, a greater increase in WMH volume was associated with faster decline in executive function in the NC group (table e-3). Approximately 9% (20 of 229) of cognitively normal participants converted to MCI and 43% (169 of 397) of participants with MCI converted to AD, but baseline cardiovascular risk scores and WMHs were not different between converters and nonconverters.
DISCUSSION
Within a limited range of vascular burden, we found no evidence of an effect of vascular risk on the longitudinal change of CSF Aβ42, FDG uptake in typical AD-related ROIs, and MRI hippocampal atrophy during cognitive decline. CSF Aβ42 is an amyloid-specific marker for AD, and its decline appears to be faster early in the disease course11; however, none of these vascular proxy measures was associated with CSF Aβ42 either cross-sectionally or longitudinally. Likewise, MRI hippocampal volume serves as a sensitive surrogate marker for AD pathology,18 but neither cardiovascular risk profile nor WMH was clearly associated with hippocampal volume or its rate of atrophy. Although there was a suggestion of a relationship between WMH and longitudinal hippocampal atrophy in the NC group, the lack of a relationship between WMH change and hippocampal change argues against its significance. Our findings are consistent with previous studies that cerebrovascular burden, at least of mild degree, and AD pathology may be 2 independent factors contributing to dementia.10,19–21
Vascular factors not only increase the risk of AD but also predict dementia progression in AD.22 Their contribution is considered additive in augmenting the expression of AD.23 Animal models have shown increased amyloid deposition after stroke, and white matter injury may predispose to the neurodegenerative effects of Aβ.9
Although the neurovascular hypotheses concerning Aβ clearance seem plausible,24 little evidence from human studies suggests that vascular risks are also amyloidogenic.25 Even though amyloid deposition can be enhanced by circulatory defects, vascular effects may be easily overwhelmed once AD pathology becomes advanced.20 However, it is possible that vascular risks play an initiating role, and, therefore, observation of their influence on amyloid accumulation may require longer study periods.
The significant association between higher baseline WMH volume and lower FDG uptake in the AD group may reflect the fact that vascular burden contributes to reduced synaptic activity in patients with AD. However, the rate of glucose hypometabolism was not influenced by vascular risks in MCI or AD, arguing against the interaction between vascular risks and AD pathologic progression. We used the average normalized intensity from 5 FDG ROIs including bilateral temporal and angular gyri and posterior cingulate gyrus, which were commonly affected in AD. Although an earlier study demonstrated that WMH was associated with frontoparietal metabolism in FDG-PET rather than our ROIs,26 the underlying neuropathology for these regions is still undetermined. FDG-PET generally represents synaptic activity,27 and ROIs may be sensitive to AD pathologic changes. However, FDG ROIs may be not specific enough to exclude vascular or other pathologic contributions. Our finding in the NC group that a greater amount of WMH was associated with faster decline of FDG ROI uptake is in line with this notion. These cognitively normal participants are not necessarily going to develop AD, and they may be considerably different from participants with MCI and AD in ADNI. Therefore, the significant associations between FDG uptake in PET and WMH volume in our study may reflect the general effect of vascular burden on synaptic activity rather than AD-specific pathology.
Age has long been considered a prominent predictor of WMH severity,28 and here with repeated measures we have further shown that after adjustment for age, WMH volume increased significantly over time and the rate of increase appeared to be faster for participants with cognitive impairment. Although vascular risks such as hypertension are important factors for WMH severity,29 we cannot exclude the contribution from other pathologic conditions. A voxel-based morphometric MRI study has shown that gray matter reduction is correlated with the increase in WMH volume30; however, the temporal relationship between these findings is unknown. People with MCI or AD may have accelerated gray matter or cortical atrophy and thus develop faster WMH progression. Without further investigation of the underlying neuropathology of WMH, we cannot be sure that WMH change simply represents the progression of vascular burden.
APOE4 is a strong genetic risk factor for AD and predictive of cognitive decline.31 As a key component in the transport of cholesterol and lipid, APOE4 plays a role in both coronary risk and cerebral amyloid deposition.32,33 We did not find any association between APOE4 and cardiovascular risk score or WMH. The relationship between AD biomarkers and vascular burden was not affected by the presence of APOE4 either. Conversely, APOE4 has been shown to be associated with lower baseline CSF Aβ42, FDG uptake, and accelerated hippocampal atrophy in an earlier ADNI study.11 Therefore, APOE4 seems to contribute to AD via the amyloid pathway more than through increasing vascular burden.
Greater cardiovascular risks and WMH volume were, as expected, associated with worse cognitive performance, particularly executive function in the MCI group, consistent with previous studies, which showed that WMH was more associated with psychomotor speed or executive function.26,34 We also found that although cognitive performance on MMSE and ADAS-cog, typical measures for AD, was not influenced by changes in WMH volume, the decline in executive function significantly correlated with the increase of WMH volume in the NC group. This finding suggested that vascular burden and AD-type pathology target different cognitive domains in aging and dementia.
There are several limitations in our study. First, vascular burden in the study was generally low because enrollment criteria excluded people with Hachinski Ischemic Scale scores of 4 or greater. As a result, the range of vascular burden in ADNI was narrow, and our findings cannot be generalized to populations with more than mild vascular risks. Furthermore, our length of observation ranged from 2 to 5 years. The influence of mild vascular burden may not be detectable within the period of observation. Nevertheless, this limitation provides us a unique opportunity to evaluate mild vascular effects that are uncomplicated by comorbidities, motor and sensory alterations, and other signs and symptoms that might increase measurement error, especially for cognition. Another limitation is that we do not have pathologic data showing the concordance between these AD-type biomarker changes and the severity of amyloid deposition. How specific these AD biomarkers are to detect the amyloid pathologic cascade at each cognitive stage in contrast with vascular progression is not known. Cardiovascular risk profile was derived from hypertension, diabetes, and other factors measured only once at baseline, and many of them may not have been quantified with adequate precision. Although the cardiovascular risk score was normally distributed, it predicts future cardiovascular events but is not necessarily reflective of the underlying vascular pathology. Outcomes could also have been affected by treatment of hypertension or other vascular risk factors, and the degree of medical control was not considered in our analyses. These treatments presumably change over time, depending on the previous outcome and also affecting the follow-up predictor as well as outcome. Handling of these time-varying confounders is beyond the scope of ADNI data, and we are not sure how the lack of handling of these confounders would affect our results.
The unique strength of the study is its longitudinal setting and repeated measurement to capture the dynamics of AD biomarkers and WMHs. Time-varying predictors and outcome are rarely available in population studies; therefore, the assumption that an age effect is uniform across different participants is not as necessary as in cross-sectional studies. In addition, the majority of participants had 3 or more repeated measures, allowing us to evaluate not only the single difference between 2 time points but also the variance of change. With more than 3 or 4 repeated measures, the regression toward the mean effect can be further minimized.
The longitudinal changes in AD biomarkers were not modified by mild vascular risks during cognitive decline in ADNI. There is no evidence that cerebral amyloid deposition is affected by mild vascular burden. Vascular contribution to AD dementia is probably additive although not necessarily independent of the amyloid pathway.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ASAD-cog
Alzheimer's Disease Assessment Scale–Cognitive Subscale
- FDG
[18F]fluorodeoxyglucose
- GEE
generalized estimating equation
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NC
normal cognition
- WMH
white matter hyperintensity
Footnotes
Supplemental data at www.neurology.org
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (http%3A//adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found on the Neurology® Web site at www.neurology.org.
AUTHOR CONTRIBUTIONS
Study concept and design: Raymond Y. Lo and William J. Jagust. Data interpretation: Raymond Y. Lo and William J. Jagust. Drafting of the manuscript: Raymond Y. Lo. Statistical analysis: Raymond Y. Lo. Critical revision of the manuscript: William J. Jagust.
Study Funding
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: Abbott, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Amorfix Life Sciences Ltd., AstraZeneca, Bayer HealthCare, BioClinica, Inc., Biogen Idec Inc., Bristol-Myers Squibb Company, Eisai Inc., Elan Pharmaceuticals Inc., Eli Lilly and Company, F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc., GE Healthcare, Innogenetics, N.V., Janssen Alzheimer Immunotherapy Research & Development, LLC, Johnson & Johnson Pharmaceutical Research & Development LLC, Medpace, Inc., Merck & Co., Inc., Meso Scale Diagnostics, LLC, Novartis Pharmaceuticals Corporation, Pfizer Inc., Servier, Synarc Inc., and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514 and the Dana Foundation.
DISCLOSURE
W. Jagust has served on a scientific advisory board for Genentech, Inc.; has served as a consultant for Bayer Healthcare, GE Healthcare, Synarc, Janssen Alzheimer Immunotherapy, Genentech, Inc., TauRx, and Merck & Co; and receives research support from the NIH. R. Lo reports no disclosure. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197– 2204 [DOI] [PubMed] [Google Scholar]
- 2. Breteler MM. Vascular risk factors for Alzheimer's disease: an epidemiologic perspective. Neurobiol Aging 2000; 21: 153– 160 [DOI] [PubMed] [Google Scholar]
- 3. Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord 2009; 23: 1– 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci 2005; 28: 202– 208 [DOI] [PubMed] [Google Scholar]
- 5. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010; 9: 119– 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995; 45: 2077– 2084 [DOI] [PubMed] [Google Scholar]
- 7. Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol 2010; 67: 1370– 1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 2004; 35: 1857– 1861 [DOI] [PubMed] [Google Scholar]
- 9. Desai MK, Mastrangelo MA, Ryan DA, Sudol KL, Narrow WC, Bowers WJ. Early oligodendrocyte/myelin pathology in Alzheimer's disease mice constitutes a novel therapeutic target. Am J Pathol 2010; 177: 1422– 1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 2011; 70: 774– 780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lo RY, Hubbard AE, Shaw LM, et al. Longitudinal change of biomarkers in cognitive decline. Arch Neurol 2011; 68: 1257– 1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010; 74: 201– 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaw LM. PENN biomarker core of the Alzheimer's Disease Neuroimaging Initiative. Neurosignals 2008; 16: 19– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011; 32: 1207– 1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008; 27: 685– 691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743– 753 [DOI] [PubMed] [Google Scholar]
- 17. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44: 1049– 1060 [PubMed] [Google Scholar]
- 18. Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 2002; 58: 1476– 1482 [DOI] [PubMed] [Google Scholar]
- 19. Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004; 62: 1148– 1155 [DOI] [PubMed] [Google Scholar]
- 20. Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol 2006; 60: 677– 687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marchant NL, Reed BR, Decarli CS, et al. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging 2012; 33: 1006.e25– 1006.e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology 2007; 69: 1850– 1858 [DOI] [PubMed] [Google Scholar]
- 23. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA 277: 813– 817, 1997. [PubMed] [Google Scholar]
- 24. Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol 2009; 118: 103– 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reed BR, Marchant NL, Jagust WJ, Decarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging 2012; 33: 1979– 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuczynski B, Reed B, Mungas D, Weiner M, Chui HC, Jagust W. Cognitive and anatomic contributions of metabolic decline in Alzheimer disease and cerebrovascular disease. Arch Neurol 2008; 65: 650– 655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage 2003; 20: 1894– 1898 [DOI] [PubMed] [Google Scholar]
- 28. Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry 1991; 29: 55– 67 [DOI] [PubMed] [Google Scholar]
- 29. Dufouil C, de Kersaint-Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology 2001; 56: 921– 926 [DOI] [PubMed] [Google Scholar]
- 30. Wen W, Sachdev PS, Chen X, Anstey K. Gray matter reduction is correlated with white matter hyperintensity volume: a voxel-based morphometric study in a large epidemiological sample. Neuroimage 2006; 29: 1031– 1039 [DOI] [PubMed] [Google Scholar]
- 31. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOEϵ4 effect. N Engl J Med 2009; 361: 255– 263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 2007; 298: 1300– 1311 [DOI] [PubMed] [Google Scholar]
- 33. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 6820– 6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000; 47: 145– 151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


