Abstract
Objectives:
Increasing evidence suggests that uncontrolled seizures have deleterious effects on cognition and behavior, particularly in the developing brain.
Methods:
In a community-based cohort, 198 children, aged <8 years with new-onset epilepsy were followed prospectively and reassessed with the Wechsler Intelligence Scales for Children, Third Edition (WISC-III) 8–9 years later. Linear regression analyses with interactions between age at onset (age) and pharmacoresistance (PR) were used to test whether earlier onset conveyed greater vulnerability to the effects of uncontrolled seizures. Full-scale IQ (FSIQ) and the 4 subdomain scores were examined. Adjustment for adaptive behavior scores in a subset was performed. A dichotomous indicator for IQ <80 or ≥80 was used to permit inclusion of children who were not tested, particularly those who were untestable.
Results:
FSIQ was not correlated with age. PR was associated with an 11.4 point lower FSIQ (p = 0.002) and similar decrements in each WISC-III domain. There were substantial age-PR interactions for FSIQ (p = 0.003) and 3 domain scores, indicating a lessening impact of PR with increasing age. The dichotomous IQ indicator was strongly correlated with age at onset in the pharmacoresistant group (p < 0.0001) and not in the non-pharmacoresistant group (p = 0.61). Adjustment for adaptive behavior measured near onset did not alter the conclusions.
Conclusions:
Uncontrolled seizures impair cognitive function with effects being most severe in infancy and lessening with increasing age at onset. These findings further emphasize the need for early aggressive treatment and seizure control in infants and young children.
Seizures and epilepsy are increasingly being recognized to have moderate to profound effects on cognitive function. There is an increasing body of literature demonstrating that, in children with refractory epilepsy who are evaluated for epilepsy surgery, earlier age at onset is associated with lower IQ or developmental scores,1–5 and longer duration of epilepsy before surgery is negatively correlated with IQ and ability to rebound after surgery2,4,6. A drug treatment trial for infantile spasms reported a striking correlation between both age at onset and delay to treatment with developmental scores assessed later at age 4 years.7 These studies focused on severe epilepsy (spasms) or pharmacoresistant surgical epilepsy and by design do not allow comparisons with cognitive outcomes in well-controlled epilepsy. Consequently, they cannot directly address the separate roles of onset age vs seizures in the developing brain.
In a prospective community-based study of children with epilepsy, we examined the association of cognitive scores assessed 8–9 years after initial diagnosis and age at onset of epilepsy, pharmacoresistance, and the interaction between the two. We specifically hypothesized that the impact of pharmacoresistance would be greatest in children with the youngest age at onset and decrease with increasing age at onset consistent with the literature cited above.
METHODS
Children were recruited in 1993–1997 when epilepsy was first diagnosed by a pediatric neurologist in the State of Connecticut.8 All parents completed a baseline questionnaire (see e-Methods on the Neurology® Web site at www.neurology.org). In the subset of children who were not yet in kindergarten at the time of the baseline questionnaire, the Vineland Adaptive Behavior Scales (VABS) was also administered.9 We used the VABS composite score, which is standardized to a mean of 100 and SD of 15.
Neurologic examination results were taken from the medical records and coded as definitely abnormal vs normal. Brain imaging data came from the cumulated records and were used regardless of timing on the grounds that the structural abnormalities were such that they could have been ascertained at the initial diagnosis.10 A composite variable was created to reflect the presence of either an imaging or examination abnormality (negative if examination and imaging results were normal; positive if either or both were abnormal). Children with no imaging were classified based on the examination results only.
Children were prospectively followed by phone calls with parents every 3–4 months and periodic review of medical records. Pharmacoresistant epilepsy was determined based on the failure of 2 appropriate drugs to control seizures fully, with evidence that the doses had been increased in an effort to maximize drug effectiveness. This is comparable to the recently proposed definition of pharmacoresistance.11 At about 8–9 years after initial study entry, study subjects and their parents were invited to participate in an assessment protocol. This consisted of a parent interview and several assessments of the child including neuropsychological testing with the Wechsler Intelligence Scales for Children, Third Edition (WISC-III) or Wechsler Adult Intelligence Scale−Revised (WAIS-R) and a behavioral measure, the Child Behavior Checklist (CBCL) for children <17 years when tested.12 Because of specific hypotheses regarding changes with age and to ensure consistency of measures across all children, we limited analyses to children who were young enough to be tested with the WISC-III.13 This approach focused the analytic sample on children with onset at <8 years of age. We focused analyses on the full-scale IQ (FSIQ) and subdomain scores for verbal comprehension, perceptual organization, freedom from distractibility, and processing speed.
Because severely impaired children could not meaningfully take part in our neurocognitive battery, we used, as a secondary outcome, a composite measure of cognitive function, developed previously,14 to categorize children's performance as consistent with FSIQ ≥80 or <80. The determination was made based on all available information including our own standardized testing, school reports, and cognitive-developmental testing and assessments done for clinical purposes. Use of this indicator allowed us to reflect a broader range of children than in our targeted age sample without excluding those too impaired to be assessed with the WISC-III questionnaires, and other procedures are further described in the e-Methods.
Analyses were performed in SAS (version9.2). For standard bivariate comparisons, t tests, χ2 tests, and Pearson correlations were used. We used multivariable linear regression to assess the contributions of clinical variables to cognitive function scores and specifically to test the interaction between age at onset and pharmacoresistance.
Standard protocol approvals, registrations, and patient consents.
All procedures used in this study were approved by the institutional review boards of the participating institutions. Written informed consent of parents and young adults and assent of children were obtained.
RESULTS
Of the original 613 children in the cohort, 431 were <8 years old at the time of their first unprovoked seizure, of whom 355 participated at some level in the later assessment protocol. Of those who did not participate at all in the assessment, 11 were deceased, 46 were lost to follow-up, 13 refused participation, and 6 others were not included for other reasons. Of the 355 who participated, 128 did not have neuropsychological testing, and 227 participated in the testing protocol, 29 with the WAIS-R and 198 with the WISC-III (figure e-1). The latter 198 formed the primary analytic sample. A subset of 149 was also tested with the VABS at initial study entry and formed a subset for some analyses. Clinical-only MRI scans were available for 17, and 176 had a research MRI with or without additional clinical scans. Of the remaining 5, all had normal neurologic examination results; one had a normal CT scan, and the other 4 had no imaging performed.
In the primary analytic sample (N = 198), there were 88 (44.4%) girls. The average age at the first unprovoked seizure (onset) was 3.7 years (SD 2.2 years) and average age at diagnosis (study entry) was 4.3 years (SD 2.1 years). Thirty-eight (19.2%) met the study criteria for pharmacoresistance, and 26 had either abnormal results for the neurologic examination or imaging; 6 had abnormal results for both. The range of epilepsy syndromes reflected those seen in this age group although the inclusion of children with the most severe forms of epilepsy was limited by their ability to participate in neurocognitive testing. Sixty-five children met the criteria for specific epilepsy syndromes including 3 with West syndrome, 18 with benign epilepsy with central temporal spikes (BECTS), 30 with childhood absence epilepsy (CAE), 5 with myoclonic-atonic epilepsy, and 9, each with a different other syndrome. The majority, 133 children, most of whom had nonsyndromic epilepsy with focal features (n = 110), did not meet the criteria for specific syndromes. Of those remaining, 12 had generalized or mixed features but no specific syndromic diagnosis and 11 had epilepsy that could not be clearly characterized (i.e., undetermined). Identified underlying causes in this group included intrauterine insults and intraventricular hemorrhages (n = 13), malformations (n = 3), tuberous sclerosis (n = 4), and 1–2 each for trauma, infection, postneonatal stroke, tumor, and cyst. The mean FSIQ was 94.2 (SD 20.5), which is lower than the standardization mean of 100. This pattern was seen for each of the 4 domain scores. Age at onset was not correlated with any of the WISC-III scores (largest correlation = 0.13, p = 0.08 for perceptual organization). Pharmacoresistance and imaging/examination abnormalities were each associated with all 5 cognitive scores with typical differences for positive vs negative imaging/examination findings on the order of 5–10 points (table 1).
Table 1.
Univariate statistics and bivariate associations for main clinical predictor variables and cognitive outcome scores
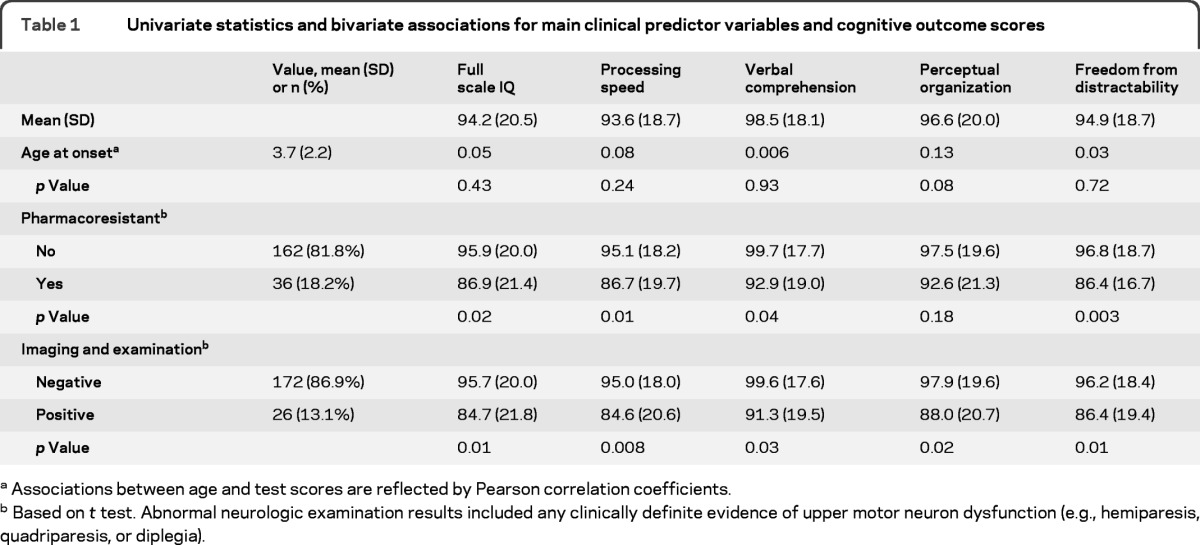
Associations between age and test scores are reflected by Pearson correlation coefficients.
Based on t test. Abnormal neurologic examination results included any clinically definite evidence of upper motor neuron dysfunction (e.g., hemiparesis, quadriparesis, or diplegia).
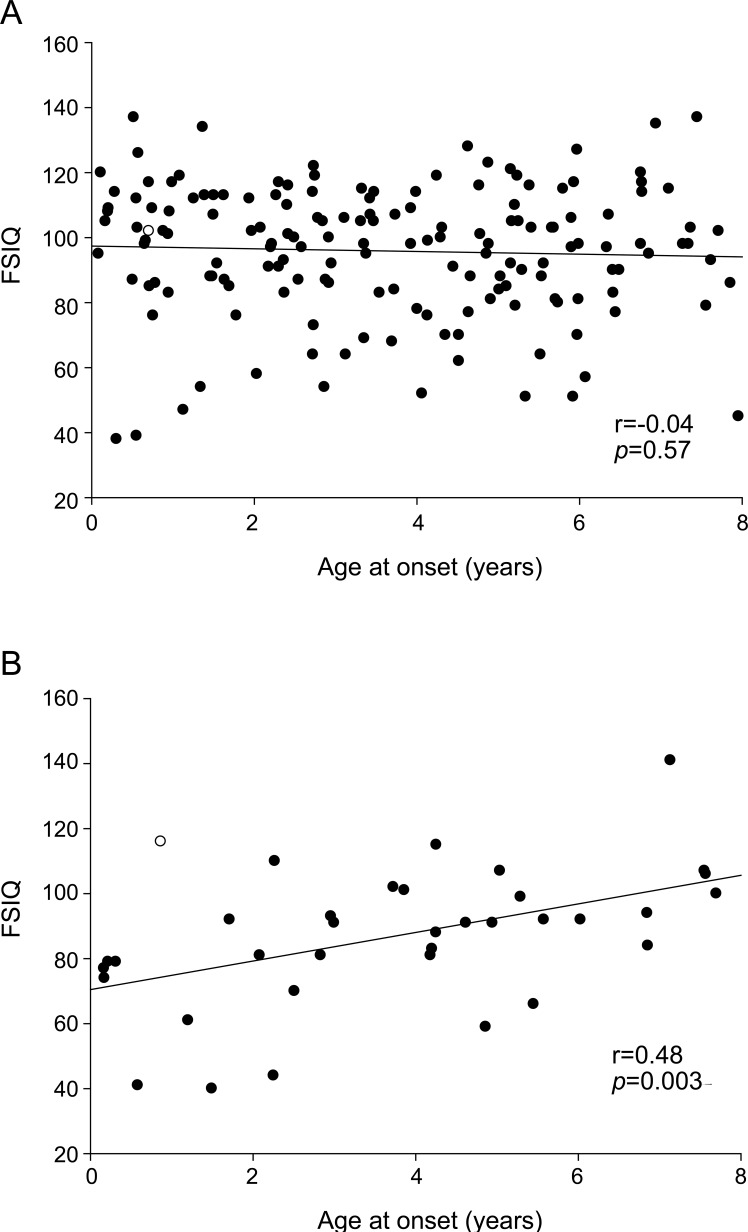
In a series of linear regression models, each of the 5 cognitive scores was treated as the independent variable. Each was modeled as a function of positive imaging/examination, age at onset, pharmacoresistance, and an interaction term between onset and pharmacoresistance to model the change in impact pharmacoresistance with increasing age. In each model, the presence of imaging or examination abnormalities was associated with a decrement of approximately 7–9 points relative to those with negative imaging and examination findings. Consistent with the unadjusted bivariate findings for age, age at onset was not associated with any of the cognitive scores. With the interaction term in the model, this estimate reflects the age effect within the nonpharmacoresistant group. Pharmacoresistance was strongly associated with each of the test scores. With inclusion of the interaction term, the estimate reflects the effect of pharmacoresistance at the youngest end of the age range. The term for the interaction between age and pharmacoresistance was statistically significant for 4 of 5 cognitive scores (table 2) and reflects the lessening impact of pharmacoresistant epilepsy with increasing age at onset of epilepsy. Scatterplots of FSIQ (figure 1) and the 4 domain scores (figure e-2) by age at onset also indicated no association with age in those without pharmacoresistance but a noticeable trend for lower scores with younger age at onset in the pharmacoresistant group. Adjustment for the depressed-withdrawn scale of the CBCL did not alter these basic findings.
Table 2.
Multiple linear regression results for the primary analytic samplea
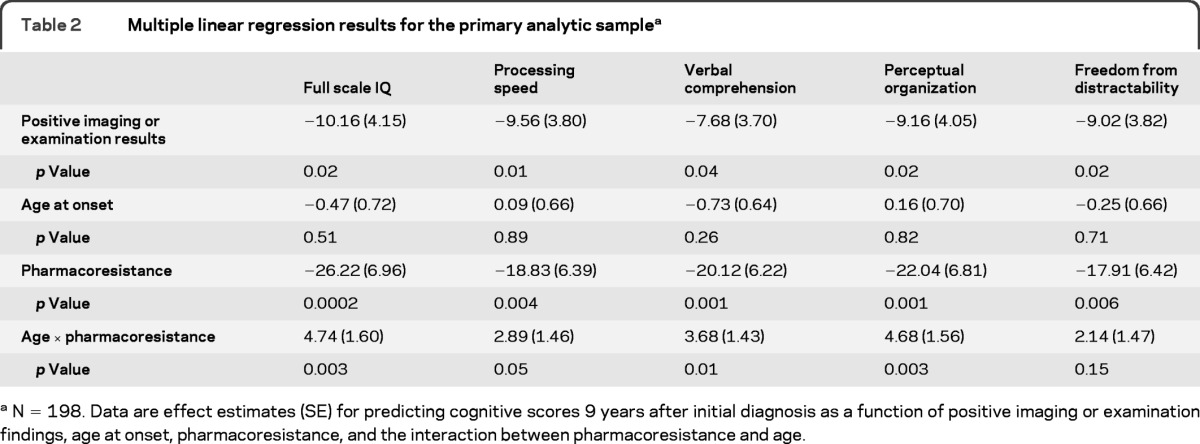
N = 198. Data are effect estimates (SE) for predicting cognitive scores 9 years after initial diagnosis as a function of positive imaging or examination findings, age at onset, pharmacoresistance, and the interaction between pharmacoresistance and age.
Figure 1. Full-scale IQ (FSIQ) and age at onset by pharmacoresistance.
Scatterplot and regression line for the association between age at onset and FSIQ in (A) children whose epilepsy was not pharmacoresistant and (B) children with pharmacoresistant epilepsy.
In the reduced subset of children who had a VABS at study entry (n = 149), we reran the regression models with adjustment for the composite score. The results for age at onset, pharmacoresistance, and their interaction did not change appreciably (table 3). The impact of having abnormal results for an imaging study or neurologic examination, however, was largely accounted for by the VABS composite score. We also present the impact of pharmacoresistance without the interaction to emphasize the independent effect of uncontrolled seizures above and beyond early measures of function.
Table 3.
Multiple linear regression results in children who were young enough to have been assessed with the VABS when first recruited into the studya
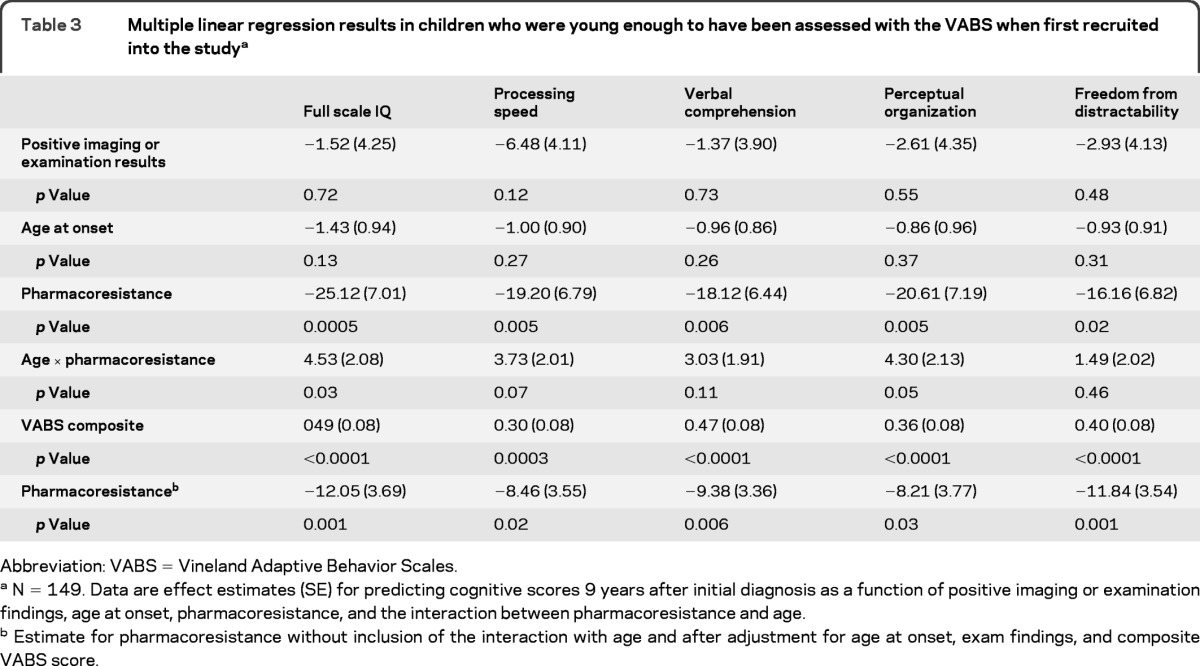
Abbreviation: VABS = Vineland Adaptive Behavior Scales.
N = 149. Data are effect estimates (SE) for predicting cognitive scores 9 years after initial diagnosis as a function of positive imaging or examination findings, age at onset, pharmacoresistance, and the interaction between pharmacoresistance and age.
Estimate for pharmacoresistance without inclusion of the interaction with age and after adjustment for age at onset, exam findings, and composite VABS score.
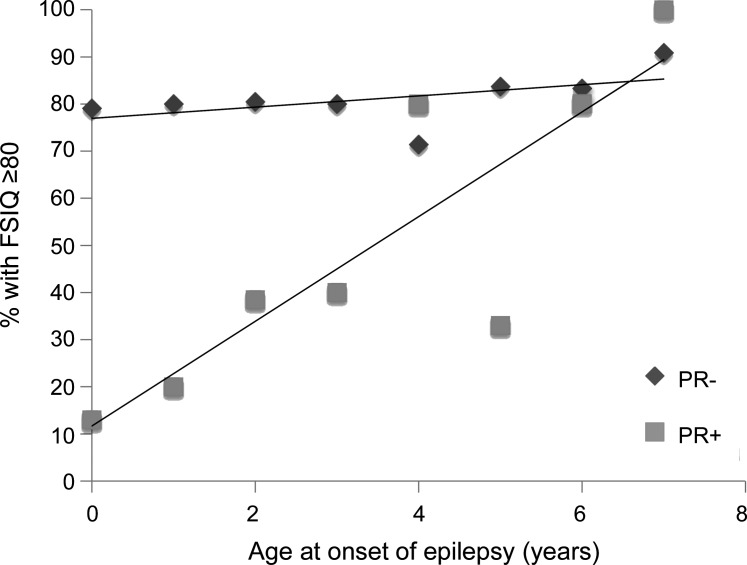
To assess the impact of the excluded patients (not tested and those who received the WAIS), a disproportionate number of whom had some level of cognitive impairment, we looked at the proportion with measured or estimated FSIQ ≥80 as a function of age at onset and pharmacoresistance (n = 355) (figure 2). Eighty-two (23%) were in the IQ <80 category. There was no association between age and proportion with FSIQ ≥80 in the nonpharmacoresistant group (p = 0.37), but a striking correlation in the pharmacoresistant group (p < 0.0001).
Figure 2. Full-scale IQ (FSIQ) ≥80 by age at onset and pharmacoresistance.
Proportion of children with a FSIQ ≥80 by year of age at onset of epilepsy and by whether seizures were pharmacoresistant (PR+) or not pharmacoresistant (PR−). Children who did not participate in the cognitive testing battery were included in the data for this plot (N = 326 total).
Because we used the full sample of available patients, we included children with electroclinical syndromes that have a relatively self-limited course, are not generally associated with intellectual disability, and are usually fairly easily treated, in particular, CAE and BECTS. These syndromes also do not present in the first 1−2 years of life. To make our sample more comparable to the types of epilepsies reported in refractory surgical and tertiary center samples, we repeated the analyses after exclusion of 53 children with specific diagnoses for one of these or other self-limited syndromes. In this reduced sample (n = 145), despite the loss in statistical power, the results were very similar to those for the original sample (table e-1). In addition, the correlation between age and FSIQ in the pharmacoresistant group (n = 28) was 0.35 (p = 0.06) which, in magnitude, is highly comparable to what others have reported in surgical series.1–5 Finally, the associations between FSIQ 80< vs ≥80 and age at onset in those with and without pharmacoresistance were highly comparable to those in full group, with no evidence of a trend in the nonpharmacoresistant group (p = 0.61) and a striking trend ranging from 87% having FSIQ ≤80 for age <1 year to 0% for age 7 years (p = 0.001) (figure e-3).
DISCUSSION
Mounting clinical evidence1,3,7,15 as well as laboratory models16 point to a deleterious impact of seizures themselves on cognition, particularly when they occur in the context of a developing brain. This phenomenon has been dubbed epileptic encephalopathy.17,18 The fundamental concept is that seizures interfere with the mechanisms of learning and memory and, in a developing brain, actually impede acquisition of mature function during critical periods of development. In a previous analysis from this cohort,19 we demonstrated that, in very young children and infants (onset 0–3 years), VABS scores declined over the first 3 years in children with pharmacoresistant seizures but stayed relatively constant in those with well-controlled seizures. That analysis did not extend into later childhood or address cognitive function per se. By design, it could not consider an interaction with age. However, ample supportive evidence for this phenomenon exists elsewhere in the literature. Several studies have demonstrated substantial correlations between FSIQ and age at onset in a variety of refractory childhood onset epilepsies treated surgically1,3–5 or pharmacologically.7 Investigators have demonstrated that earlier intervention, especially for seizures beginning in infancy, results in better developmental outcomes and the ability to rebound after surgery.2,6,20 These studies, as has ours, mostly focused on pharmacoresistance. Factors such as seizure frequency, as seen in one study,1 and severity would provide more tightly controlled assessments of the impact of seizures in the young brain. Given the diversity of epilepsy and seizure types and the number with pharmacoresistant epilepsy, we were not able to provide a detailed analysis of this.
Our findings add to these data in 3 ways. 1) The effect is not simply due to age at onset because, in the absence of pharmacoresistance, age was not associated with cognitive scores. This fact could not be demonstrated in the surgical series, which did not include patients with well-controlled seizures. Our findings are foreshadowed by earlier results in our cohort regarding the stability of the VABS within the normal range over time in the group with well-controlled seizures at least during the first few years after the initial diagnosis of epilepsy.19 2) Another finding not evident in other studies is that, although the initial level of adaptive function (VABS) is strongly correlated with later cognitive function, it does not account for the impact of pharmacoresistance on later function. These results provide further support for the concerns regarding uncontrolled seizures in the developing brain and the importance of rapid early seizure control.7,21 3) Previous studies examined FSIQ only. Our data suggest a more pervasive effect across the cognitive subdomains of the WISC, in particular verbal comprehension and perceptual organization. In contrast, in adults, the impact of seizures seems be largely on measures of fluid intelligence (attention and processing speed) and other functions not sampled by the WISC (visual and verbal memory and fine motor function).15,22,23 The traumatic brain injury literature provides similar patterns of findings. Insults during early brain developmental may be partially correctable through mechanisms of plasticity; however, the burden of deficit is then spread across a large range of cognitive functions.24–26 Our study is also representative of children in the community and is not limited to those seen in tertiary referral centers.
To avoid any potential confound between age and test version, we focused on children tested with the WISC. Consequently, we do not know nor can we test whether the impact of age stops at approximately 8 years at onset. Many aspects of mature brain function are present by this age, and we suspect that the developmental interferences probably play a lesser role after this point. Interestingly, one study compared adults with earlier (onset <∼8 years, median age in the sample) vs later onset of epilepsy and found that, relative to control subjects, those with early onset had substantially lower IQ scores whereas IQ scores of those with later onset were not greatly different from those of control subjects.27
Analysis of the dichotomous secondary outcome measure, FSIQ <80 vs ≥80, allowed inclusion of children excluded from analysis of WISC-III IQ scores and further strengthens our main findings. The surgical series reported previously included a wide range of epilepsies; however, certain syndromes would automatically have been excluded, most noticeably BECTS and CAE. The findings were comparable after exclusion of children with self-limited epilepsies.
Notably, the early VABS score was more important in predicting later cognitive scores than were the imaging and neurologic examination findings. Given that epilepsy in most individuals does not have a clear underlying cause, it may be that measures such as adaptive function in very young children are a more sensitive marker for underlying brain disorders that affect later cognitive function. These disorders would include those specifically identified (e.g., brain malformation), those inferred based on examination (e.g., a hemiparesis), and also those for which there is no specific indication of underlying cause.
Although we initially recruited children with degenerative disorders into the study, they had died by the time of the 9-year analyses. The children participating in the 9-year assessment with identified underlying causes had static insults or conditions. Most (176 children) of the 198 children in the primary sample had research MRIs. Of those with clinical scans only, we had obtained and had been able to reinterpret most scans for research purposes.10 Of children who were pharmacoresistant, only one did not have any imaging beyond the initial evaluation period. Thus, for primary analyses, we probably identified most if not all major structural abnormalities. We note that, although the VABS explained the effects of imaging and examination abnormalities, it did not account for the effects of pharmacoresistance when tested alone or when an interaction with age was included. In fact, in the group tested with the WISC-III, pharmacoresistance per se was not associated with the VABS composite at onset (mean VABS scores of 99.0 and 95.7 in the controlled vs pharmacoresistant groups, p = 0.39).
Some children were still taking antiepileptic drugs (AEDs) when tested. AED status was correlated with pharmacoresistance. Thus, we were unable to study any additional effects of AEDs. AEDs can have an impact on cognitive function with certain ones having an especially high risk.28,29 Phenobarbital has residual effects even after discontinuation in young children treated for febrile seizures.30 In utero exposure to certain AEDs, most notably sodium valproate, is associated with substantial decrements in cognitive function in early childhood.31 There are no data that specifically address whether AEDs differentially affect acquisition of cognitive skills as a function of age of postnatal exposure. If AEDS had a more detrimental effect in the early developing brain than later on, however, we would expect to see an effect of age at onset in the nonpharmacoresistant group also because most children were treated for at least a few years, and we did not see such an effect. Further, surgical series also report some improvement in cognitive scores after surgery, suggesting that the seizures themselves negatively affect cognition and that the earlier the surgery is done, the better the postsurgical ability to acquire developmental and cognitive skills.2,20,28,32
The hazards associated with seizures—injuries, mortality, and loss of autonomy—are all extremely important. Our data add to a building body of literature that highlights the cognitive consequences of poorly controlled seizures in the developing brain and provides more support for emerging recommendations and guidelines regarding early identification of pharmacoresistance11 and referral to specialty care, particularly for children,21 although similar considerations also occur in adults.33
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- BECTS
benign epilepsy with central temporal spikes
- CAE
childhood absence epilepsy
- CBCL
Child Behavior Checklist
- FSIQ
full-scale IQ
- VABS
Vineland Adaptive Behavior Scales
- WAIS-R
Wechsler Adult Intelligence Scale−Revised
- WISC-III
Wechsler Intelligence Scales for Children, Third Edition
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Berg is the PI of the study, designed the original study, conducted the statistical analyses described herein and drafted the original manuscript. Dr. Zelko contributed to interpretation and presentation of the analyses, and to drafting and revising the manuscript. Dr. Levy and Dr. Testa participated in the original cohort recruitment and contributed to drafting and revising the manuscript.
DISCLOSURE
A. Berg receives support from grant NINDS-R37-NS31146. She has received travel funding and honoraria from Eisai, the British Pediatric Neurological Association, and the Epilepsy Research Center (Melbourne); travel funding from UCB the American Epilepsy Society and the International League Against Epilepsy; BIAL, awards from the American Epilepsy Society and British Pediatric Neurological Association; and consulting fees from Dow Agro Science. She serves on the Editorial Boards of Epileptic Disorders, Epilepsy&Behavior and Neurology. She is past Chair of the ILAE's Commission on Classification and Terminology, Current Chair of the ILAE's Task Force on Classification-Diagnostic Manual, member of the AES Psychiatric Task Force, Steward for the NINDS Benchmarks in Epilepsy Research. F. Zeko receives funding from NINDS-R37-NS31146. S. Levy receives consulting payments for her participation in the study funded by NINDS-R37-NS31146. F. Testa receives consulting payments for her participation in the study funded by NINDS-R37-NS31146. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Vasconcellos E, Wyllie E, Sullivan S, et al. Mental retardation in pediatric candidates for epilepsy surgery: the role of early seizure onset. Epilepsia 2001; 42: 268– 274 [DOI] [PubMed] [Google Scholar]
- 2. Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia 2005; 46: 561– 567 [DOI] [PubMed] [Google Scholar]
- 3. Cormack F, Cross JH, Isaacs E, et al. The development of intellectual abilities in pediatric temporal lobe epilepsy. Epilepsia 2007; 48: 201– 224 [DOI] [PubMed] [Google Scholar]
- 4. D'Argenzio L, Colonnelli MC, Harrison S, et al. Cognitive outcome after extratemporal epilepsy surgery in childhood. Epilepsia 2011; 52: 1966– 1972 [DOI] [PubMed] [Google Scholar]
- 5. Vendrame M, Alexopoulos AV, Boyer K, et al. Longer duration of epilepsy and earlier age at epilepsy onset correlate with impaired cognitive developmental in infancy. Epilepsy Behav 2009; 16: 431– 435 [DOI] [PubMed] [Google Scholar]
- 6. Jonas R, Nguyen S, Hu B, et al. Cerebral hemispherectomy: Hospital course, seizure, developmental, language, and motor outcomes. Neurology 2004; 62: 1712– 21 [DOI] [PubMed] [Google Scholar]
- 7. O'Callaghan FJK, Lux AL, Darke K, et al. The effect of lead time to treatment and of age at onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia 2011; 52: 1359– 1364 [DOI] [PubMed] [Google Scholar]
- 8. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia 1999; 40: 445– 452 [DOI] [PubMed] [Google Scholar]
- 9. Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition Survey Forms Manual. Circle Pines, MN: American Guidance Service; 1984 [Google Scholar]
- 10. Berg AT, Mathern GW, Bronen RA, et al. Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain 2009; 132: 2785– 2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51: 1069– 1077 [DOI] [PubMed] [Google Scholar]
- 12. Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001 [Google Scholar]
- 13. Wechsler D. Wechsler Intelligence Scale for Children, 3rd ed. San Antonio, TX: Psychological Corp; 1991 [Google Scholar]
- 14. Berg AT, Langfitt JT, Testa FM, et al. Global Cognitive Function in Children with Epilepsy: a community-based study. Epilepsia 2008; 49: 608– 614 [DOI] [PubMed] [Google Scholar]
- 15. Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 2006; 60: 80– 87 [DOI] [PubMed] [Google Scholar]
- 16. Lin H, Holmes GL, Kubie JL, Muller RU. Recurrent seizures induce a reversible impairment in a spatial hidden goal task. Hippocampus 2009; 19: 817– 827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dulac O. Epileptic encephalopathy. Epilepsia 2001; 42 (suppl 3): 23– 26 [DOI] [PubMed] [Google Scholar]
- 18. Berg AT, Cross JH. Towards a modern classification of the epilepsies? Lancet Neurol 2010; 9: 459– 461 [DOI] [PubMed] [Google Scholar]
- 19. Berg AT, Smith SN, Frobish D, et al. Longitudinal assessment of adaptive behavior in infants and young children with newly diagnosed epilepsy: influences of etiology, syndrome, and seizure control. Pediatrics 2004; 114: 645– 650 [DOI] [PubMed] [Google Scholar]
- 20. Loddenkemper T, Holland KD, Stanford LD, Kotagal P, Bingaman W, Wyllie E. Developmental outcome after epilepsy surgery in infancy. Pediatrics 2007; 119: 930– 935 [DOI] [PubMed] [Google Scholar]
- 21. Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia 2006; 47: 952– 959 [DOI] [PubMed] [Google Scholar]
- 22. Taylor J, Kolamunnage-Dona R, Marson AG, Smith PEM, Aldenkamp AP, Baker GA. Patients with epilepsy: cognitively compromised before start of antiepileptic drug treatment? Epilepsia 2010; 51: 48– 56 [DOI] [PubMed] [Google Scholar]
- 23. Taylor J, Baker GA. Newly diagnosed epilepsy: cognitive outcomes at 5 years. Epilepsy Behav 2010; 18: 387– 403 [DOI] [PubMed] [Google Scholar]
- 24. Anderson V, Catroppa C, Morse S, Haritou F. Recovery of intellectual ability following traumatic brain injury in childhood: impact of injury severity and age at injury. Pediatr Neurosurg 2000; 32: 282– 290 [DOI] [PubMed] [Google Scholar]
- 25. Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics 2005; 116: 1374– 1382 [DOI] [PubMed] [Google Scholar]
- 26. Anderson V, Spencer-Smith M, Leventer R, et al. Childhood brain insult: can age at insult help us predict outcome? Brain 2009; 132: 45– 56 [DOI] [PubMed] [Google Scholar]
- 27. Hermann B, Seidenberg M, Bell B, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia 2002; 43: 1062– 1071 [DOI] [PubMed] [Google Scholar]
- 28. Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology 2011; 76: 1330– 1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meador KJ. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy Res 2006; 68: 63– 67 [DOI] [PubMed] [Google Scholar]
- 30. Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures: effects on intelligence and on seizure recurrence. N Engl J Med 1990; 322: 364– 369 [DOI] [PubMed] [Google Scholar]
- 31. Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009; 360: 1597– 1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee YJ, Kang H-C, Lee JS, et al. Resective pediatric epilepsy surgery in Lennox-Gastaut syndrome. Pediatrics 2010; 125: e58– e66 [DOI] [PubMed] [Google Scholar]
- 33. Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ. Essential services, personnel, and facilities in specialized epilepsy centers: revised 2010 guidelines. Epilepsia 2010; 51: 2322– 2333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.