Abstract
Objective:
To examine the association between cognitive function and dementia with vitamin D concentration in adults.
Methods:
Five databases were searched for English-language studies up to August 2010, and included all study designs with a comparative group. Cognitive function or impairment was defined by tests of global or domain-specific cognitive performance and dementia was diagnosed according to recognized criteria. A vitamin D measurement was required. Two authors independently extracted data and assessed study quality using predefined criteria. The Q statistic and I2 methods were used to test for heterogeneity. We conducted meta-analyses using random effects models for the weighted mean difference (WMD) and Hedge's g.
Results:
Thirty-seven studies were included; 8 contained data allowing mean Mini-Mental State Examination (MMSE) scores to be compared between participants with vitamin D <50 nmol/L to those with values ≥50 nmol/L. There was significant heterogeneity among the studies that compared the WMD for MMSE but an overall positive effect for the higher vitamin D group (1.2, 95% confidence interval [CI] 0.5 to 1.9; I2 = 0.65; p = 0.002). The small positive effect persisted despite several sensitivity analyses. Six studies presented data comparing Alzheimer disease (AD) to controls but 2 utilized a method withdrawn from commercial use. For the remaining 4 studies the AD group had a lower vitamin D concentration compared to the control group (WMD = −6.2 nmol/L, 95% CI −10.6 to −1.8) with no heterogeneity (I2 < 0.01; p = 0.53).
Conclusion:
These results suggest that lower vitamin D concentrations are associated with poorer cognitive function and a higher risk of AD. Further studies are required to determine the significance and potential public health benefit of this association.
Vitamin D insufficiency may be a modifiable risk factor for dementia as the role of vitamin D in brain function is becoming clearer.1,2 At the molecular level, the brain has the ability to synthesize the active form of vitamin D (1,25-dihydroxyvitamin D) within many cell types and regions with predominance in the hypothalamus and the large neurons within the substantia nigra.3 Many genes are regulated by vitamin D allowing cells to synthesize relevant products in response to routine signals and stimuli, demonstrating that vitamin D acts as an autocrine and paracrine agent.4 Functionally, vitamin D contributes to neuroprotection by modulating the production of nerve growth factor (NGF),5 neurotrophin 3,5 glial cell derived neurotrophic factor (GDNF),6 nitric oxide synthase (iNOS),7 and choline acetyl transferase.8
Low vitamin D concentrations are prevalent worldwide for all age groups.9 Two recent systematic reviews examined the association between vitamin D and cognitive performance,10,11 but found insufficient evidence to make a conclusion. Both studies were limited in their scope of study inclusion, and provided no meta-analysis. Therefore, to understand better the association among vitamin D concentration, cognitive function, and dementia, we examined the evidence explicitly, by conducting a comprehensive systematic literature review and meta-analysis.
METHODS
The systematic review was conducted with a prospective protocol and data and managed using the Web-based systematic review software SRS 4.0 (Mobius Analytics, Ottawa, Canada).
Literature search strategy.
To identify all relevant primary studies we developed a comprehensive literature search strategy in collaboration with a professional research librarian. To increase the comprehensiveness of our review12,13 we searched MEDLINE, EMBASE, AMED, PsychINFO, and the Cochrane Central database, restricting only the end date to August 31, 2010. The search strategy used subject headings and text words for “vitamin D” and “cognition,” using both common and chemical names for vitamin D. For example, cognition search terms included subject headings of “dementia,” “cognitive disorder,” “cognition,” “delirium,” and key words such as “memory,” “executive function,” “global impairment,” “Alzheimer,” and “neuropsychological test” (see appendix e-1 on the Neurology® Web site at www.neurology.org for the detailed search strategy). We reviewed reference lists of included articles and previous systematic reviews for additional relevant citations.
Study selection and data abstraction.
Eligible studies were selected in 2 stages, first by screening title and abstracts for relevance followed by full text review. Any published English-language study examining the relationship between vitamin D and cognition in human adults (>18 years) was considered for inclusion. A vitamin D measurement was necessary to assess accurately vitamin D status. We accepted all validated neuropsychological tests (e.g., global function, executive function, psychomotor speed, attention, memory, or intelligence) as measures of cognitive function. Any recognized diagnostic criterion for dementia was accepted. All studies with a comparative group (randomized controlled trials [RCTs], cohort, case-control, and cross-sectional studies) were eligible. Data abstraction included information on study design, study setting, population characteristics, vitamin D type and method of analysis, cognitive measures, and statistical methods. At both stages, 2 of the authors reviewed each citation. Conflicts were resolved between reviewers or by group consensus.
Assessment of methodologic quality.
Five domains were used to assess study quality: population, outcome, exposure, statistical analysis, and the specific domain of randomization for RCT studies. Key elements for these domains were adapted for assessing quality of diagnostic tests, from the Newcastle-Ottawa quality assessment scales for case-control and cohort studies,14 the Jadad scale15 for RCT studies, and the Quality Assessment of Diagnostic Accuracy Studies for diagnostic accuracy studies.16 Each quality item was rated as met, unmet, or unclear. For the item “missing data reported,” the rating of “not applicable” was given if there were no missing data. An overall quality score was not calculated.
Statistical methods.
From each primary study we extracted effect estimates of the relationship between vitamin D and cognition. Vitamin D values were expressed in SI units. For 25-hydroxyvitamin D [25(OH)D] the conversion factor used was 1 ng/mL = 2.496 nmol/L and for 1,25-dihydroxyvitamin D [1,25(OH)D] it was 1 pg/mL = 2.4 pmol/L. Unless otherwise specified, the term vitamin D in this manuscript refers to 25(OH)D.
Heterogeneity was assessed using a test based on the deviations of the individual study estimates from the summary estimate of effect17 and quantified using the I2, which describes the proportion of the variance due to heterogeneity among studies rather than sampling error.18 An I2 >50% is considered to represent substantial heterogeneity.19 Random effects meta-analyses20 for the weighted mean difference and Hedge's g were conducted using MetaAnalyst 3.0.21 All p values are 2-sided and confidence intervals (CIs) represent 95% CIs. Funnel plots were used to assess potential publication bias.
There were sufficient data to conduct 2 meta-analyses. The first compared the mean 25(OH)D concentration between Alzheimer disease (AD) and control groups. We included only studies using the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) or the Diagnostic and Statistical Manual of Mental Disorders (DSM-III, III-R, or IV) with a cognitive score (e.g., Mini-Mental State Examination [MMSE]).
The second meta-analysis compared mean MMSE scores between individuals with vitamin D <50 and ≥50 nmol/L. The vitamin D cutpoint of 50 nmol/L was selected, as it was the most common cutpoint reported in these studies, and is commonly used to define vitamin D deficiency.22 If an article presented data using 2 cutpoints (e.g., <25, ≥25–50, and ≥50 nmol), a weighted mean MMSE and SD were calculated collapsing the groups into <50 and ≥50 nmol/L. An additional analysis was conducted to compare the extreme groups (<25 and ≥50 nmol/L) where data permitted. Authors were contacted when complete data were not available. Of the 11 authors contacted,23–33 8 provided additional data.23–30,33 One author25 also provided data for additional participants (n = 23) and for a comparison group (n = 63) not included in the original publication.
Subgroup and sensitivity analyses.
To explain heterogeneity, we determined a priori the following subgroups: study design (cross-sectional, case-control), type of vitamin D assay (radioimmunoassay [RIA], competitive protein binding assay [CPBA], ELISA), percent female (<50%, 50%–99%, 100%), and adjusted for potential confounders (effect estimates adjusted for at least age and sex). For AD vs control analysis, we based additional subgroups on the diagnostic criterion (NINCDS-ADRDA, DSM, or other) and the type of control group (community-based, clinic-based, and other). Subgroup effect estimates were compared using Wald-type tests.17
When heterogeneity was not explained by the a priori subgroups, other a posteriori subgroups were considered as a sensitivity analysis to determine the impact of exclusion of specific studies on the overall estimate of effect. Sensitivity analyses were conducted to examine the effect of including “any dementia” instead of a diagnosis of AD, and using any cognitive screening measure (Short Portable Mental Status Questionnaire and Abbreviated Mental Test) instead of the MMSE. Because the cognitive screening instruments have different scales, the sensitivity analysis was performed using Hedge's g.
RESULTS
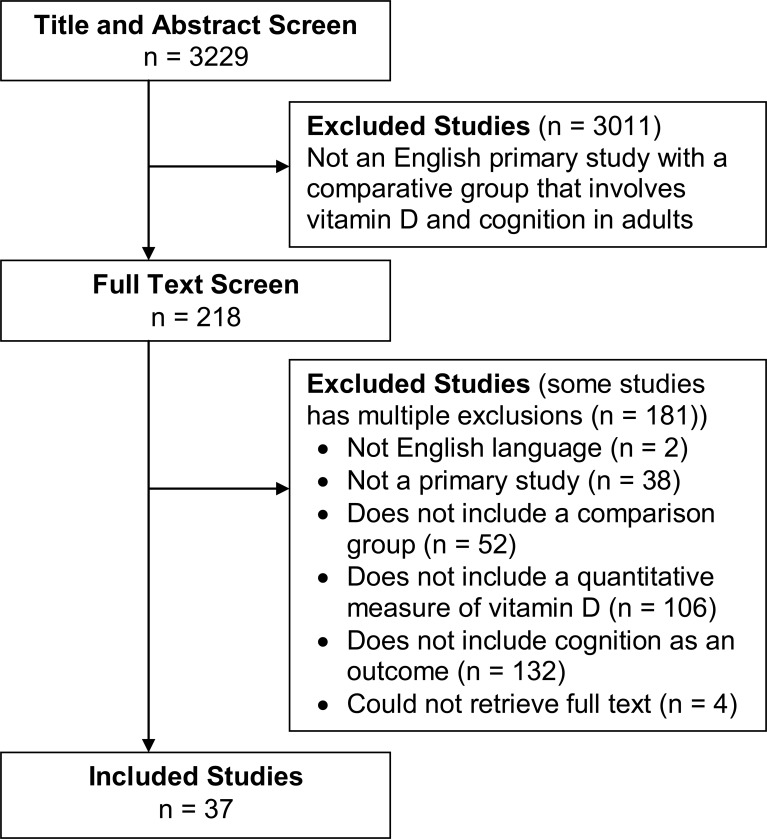
Figure 1 describes the flow of the selection of studies. The 37 studies included in this review (21 cross-sectional,23–31,34–45 10 case-control,46–55 1 before-after with comparison group,56 2 prospective cohort,32,57 and 3 randomized control trials33,58,59) are summarized in tables e-1 and e-2 for 25(OH)D studies, and in table e-3 for 1,25(OH)D studies. Table e-4 contains details of the inclusion/exclusion criteria and statistical data.
Figure 1. Flow diagram showing the study selection process for this systematic review.
The study sample sizes varied widely from 27 to 17,099. Thirty studies included only older participants, generally 65 years or older. Nine studies included only women and 2 studies included only men. Exclusion criteria varied across studies. Individuals taking nutritional supplements such as calcium or vitamin D, drugs known to affect calcium metabolism, or hormonal treatments such as corticosteroids or estrogens were excluded from some studies. A history of liver or kidney disease, stroke, osteoporosis or nontraumatic fractures, and positive risk factors for osteoporosis (e.g., hyperparathyroidism), were also used as exclusion criteria. Most studies involved community-dwelling adults, while 728,30,32,38–40,57 were random population-based samples. Of these, 238,57 used the same data source (InCHIANTI study), 2 included only male subjects,32,39 and 8 included only female subjects.30,41,44,49,51,53–55 Five studies26,27,33,44,56 examined the association between vitamin D and cognition solely in patients from long-term care institutions.
All studies measured 25(OH)D concentration except 145 where 1,25(OH)D was measured (table e-3). Four studies41,47, 54,55 measured both 25(OH)D and 1,25(OH)D, and of these, 3 compared AD to control groups, while 1 examined the relationship between vitamin D and several cognitive measures.55 Similar results were found for both types of vitamin D.
The most frequently used method for measurement of vitamin D was the DiaSorin RIA method (n = 15). Various vitamin D cutpoints were used and classified as deficient, insufficient (<25 nmol/L, ≥25–50 nmol/L, <50 nmol/L) or sufficient (≥25 nmol/L, ≥50 mmol/L, ≥50 to <75 nmol/L, >75 nmol/L). Quartiles or quintiles of vitamin D concentration were also used to investigate the relationship between cognition and vitamin D.
In 14 studies23,36,41,42,45–47,49–54,57 the cognition outcome included the diagnosis of dementia (mild dementia, AD, Alzheimer-type dementia, senile dementia of the Alzheimer type, and vascular type dementia). Dementia was most commonly defined according to DSM or NINCDS-ADRDA criteria (n = 10). Other definitions included patient history,51 criteria developed by the Japanese Ministry of Health, Labor, and Welfare,42 Dementia Screening Scale of Hasegawa score and Ischemic score,41 or Clinical Dementia Rating score.43
Of the 24 studies that included a test of cognitive function, the most commonly used test was the MMSE (n = 12). Domain-specific measures included memory/learning, attention/processing speed, language/verbal fluency, executive function, and intelligence. Of the 15 studies that used domain-specific tests, there were in total 40 diffferent types. Few studies included more than 1 to 3 domain-specific tests.
In most cases, the relationship between vitamin D and cognition was assessed by comparing either mean vitamin D concentrations between patients diagnosed with dementia and controls, or mean neuropsychological test scores between vitamin D groups (cutpoints or percentile). Of the 10 studies that used a control group, 6 were compared to an AD group and 4 to another dementia group. Sixteen studies evaluated 1 or more cognitive measures compared to 1 or more vitamin D groups. Twenty-four studies provided effect estimates adjusted for at least age or sex (either by study design or analytically). Twelve studies included adjustment for additional factors which included season, sunlight exposure, site/center, alcohol, smoking, body mass index, cognitive score (baseline), diabetes, hypertension, chronic diseases/morbidity, physical activity, physical performance, education, energy intake (total), multivitamin use, vitamin E intake, self-reported health, instrumental activities of daily living, impaired mobility, race/ethnicity, depression, psychoactive drugs, kidney function, and biochemical measures of albumin, apoE, B1, B6, B12, calcium, homocysteine, and PTH. Five of the 12 studies adjusted for seasonal variation of vitamin D.
Quality assessment.
Figure e-1 provides a summary of commonly related sources of bias according to study design, except for the 1 before-after and 2 cohort studies. The most common unmet or unclear items concerned blinding, as it was unclear whether the laboratory which measured vitamin D was aware of the cognitive status associated with the samples, or if assessors of cognitive function were aware of the vitamin D status of the participant. In 12 of the 20 cross-sectional studies,24,26–29,35,36,40–44 it was unclear whether the participants were representative of the population from which they were recruited. In 4 of these 12 studies,26,29,37,44 there was no adjustment for age and gender as confounders. Most studies reported no missing data, or explained how those with missing data differed from those with complete data. For the 3 RCTs the major quality issue was failure to report how vitamin D was measured (n = 2). One cohort study32 included some participants who had the outcome.
Cross-sectional and case-control studies.
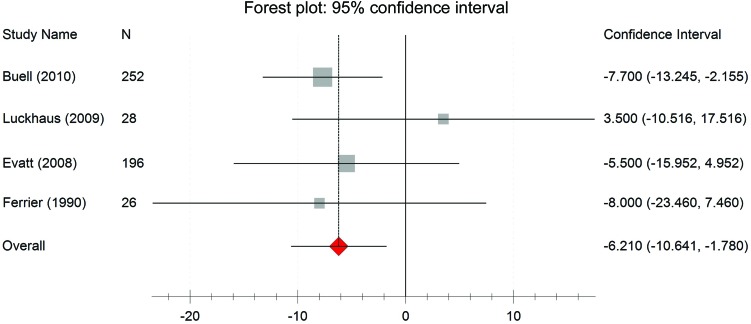
Six studies,35,46, 47,50,53,54 comprising data from 888 participants, demonstrated a lower mean 25(OH)D concentration in patients with AD than in controls (figure e-2). The mean difference was −15.0 nmol/L 25(OH)D (−26.2, −3.9); however, there was statistically significant heterogeneity (I2 = 0.96; p < 0.001). Despite this heterogeneity, all studies except 1 indicated a lower vitamin D concentration in patients with AD compared to controls. The heterogeneity among these study results is explained by the type of vitamin D assay. The difference in vitamin D concentration between AD and control groups in the studies using the CPBA method was −32.6 nmol/L (−39.6, −25.5), significantly greater than the difference in studies using an ELISA method (−2.3 nmol/L [−10.7, 6.2]) or RIA method (−7.7 nmol/L [−13.0, −2.5]). When the analysis was restricted to studies using methods other than the CPBA method the overall difference was −6.2 nmol/L (95% confidence interval [CI] −10.6 to −1.8), with results consistent across studies (I2 < 0.01; p = 0.53) (figure 2). Similar results were found when studies comparing any dementia against control groups employed methods other than CPBA to measure vitamin D (−6.3 nmol/L [−10.6, −2.0]; I2 < 0.01; p = 0.70).
Figure 2. Vitamin D concentration in Alzheimer disease and control groups.
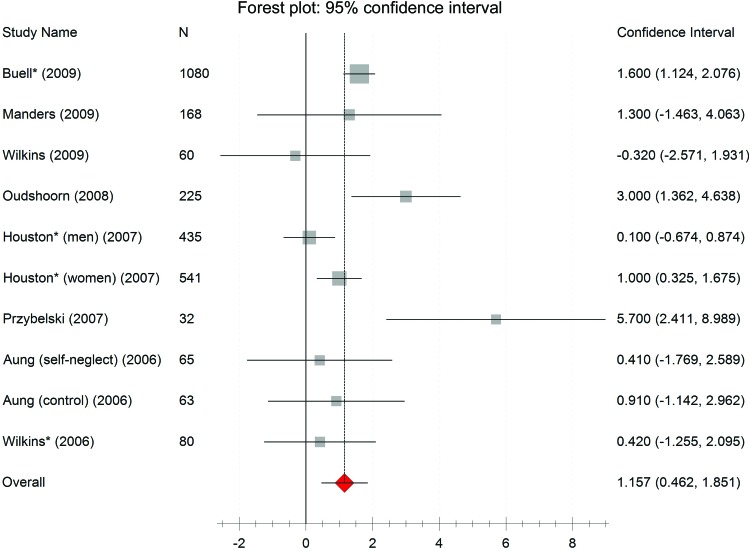
Eight studies,24,25,29,35,38,43,60,61 including data from 2,749 participants, contributed to the analysis comparing mean MMSE scores between participants with 25(OH)D concentration <50 nmol/L to those with concentrations ≥50 nmol/L (figure 3). Taken together, these studies showed a higher average MMSE score in those participants with higher vitamin D concentrations. The average difference in MMSE score was 1.2 (0.5, 1.9), although there was statistically significant heterogeneity (I2 = 0.65; p = 0.002). Most studies indicated small positive differences between groups. None of the a priori subgroup analyses were able to explain the heterogeneity among studies. The 2 studies which had the greatest group difference24,29 also had the lowest MMSE scores in the <50 nmol/L group compared to the other studies. Including studies using any general cognitive screening measure along with MMSE did not change the results substantially (data not shown). There were 2 studies containing the same cohort of data (InCHIANTI): 1 was cross-sectional38 and the other longitudinal.57 Slightly fewer participants were included in the longitudinal study since at least 1 follow-up visit was required. Substituting this subgroup in the analysis increased the average difference slightly to 1.4 (0.8, 2.1).
Figure 3. Mini-Mental State Examination and vitamin D group (<50 nmol/L and ≥50 nmol/L).
Seven of 8 studies24,25,29,35,38,43,60 provided data which allowed comparison of the 2 extreme groups of vitamin D (<25 nmol/L and ≥50 nmol/L). The mean difference in MMSE between these groups was 1.4 (95% CI 0.6, 2.1), with some heterogeneity (I2 = 0.50; p = 0.04). These studies also revealed a mean difference in MMSE score of 1.3 (0.5, 2.0) when comparing the <50 nmol/L to ≥50 nmol/L groups (I2 = 0.67; p = 0.02). There was no evidence of publication bias for meta-analyses shown in figure e-3.
Cohort studies.
The 2 cohort studies32,57 had very similar characteristics, but the results were conflicting. Both included individuals over 65 years, assessed cognition using the MMSE (or 3MS) and Trails part B (1 study also included Trails part A),32 and performed multivariate regression with 25(OH)D quartiles adjusting for many factors (6 were common to both). One study included only males,32 and reported no significant association between vitamin D quartile and baseline cognitive impairment or incident cognitive decline. In the other study,57 the mean follow-up period was shorter (2 years vs 4.6 years), and the lowest 25(OH)D quartile was different by one-half. In this study, participants deficient in 25(OH)D (<25 nmol/L) experienced an increased risk of substantial cognitive decline over 6 years, compared to those with sufficient concentrations (>75 nmol/L) (multivariate adjusted RR = 1.60, 95% CI 1.19 to 2.00). Individuals with 25(OH)D concentrations <25 nmol/L declined by an additional 0.3 points per year compared to those sufficient in 25(OH)D (>75 nmol/L) (p = 0.03), even after restricting the sample to individuals without dementia (p = 0.04).
RCT studies.
The intervention in 2 of the RCTs was a supplement containing various nutrients in addition to vitamin D33,59 and only 1 study treated with vitamin D alone.58 In this study, long-stay patients with baseline 25(OH)D <40 nmol/L were treated with 9,000 IU vitamin D2 for 8–40 weeks or placebo (lactose tablets). No significant difference for the single cognitive measure (mental assessment score) was found between groups, but the number of participants was extremely small (n = 82).
DISCUSSION
This systematic review summarized studies that contained measurements of 25(OH)D and related these to measures of cognition or dementia. The meta-analyses showed individuals with AD had lower 25(OH)D concentrations compared to those without AD, and MMSE scores were lower in individuals with lower 25(OH)D concentrations. The studies included various populations, study numbers, study designs, cognitive tests, confounders, statistical tests, vitamin D methods, and groupings.
Our results differ from 2 other published systematic reviews of vitamin D and cognitive performance.10,11 Barnard and Colon-Emeric11 suggested that cognitive function measured by MMSE was not associated with 25(OH)D concentration although their conclusions were based on whether the relationship between vitamin D and cognitive test scores in the original studies was statistically significant. Since statistical significance depends on sample size, solely focusing on this criterion could mask a small consistent effect in underpowered studies. As well, several factors limited the comprehensiveness of the prior reviews. Annweiler et al.10 searched Medline, PsychINFO, and the Cochrane Library, including all adults, but restricted studies to those which used regression models to investigate the relationship between vitamin D and cognition. The review by Barnard and Colon-Emeric11 was 6 months longer, limited databases to PubMed and Web of Science, and included only adults 65 years of age or older. We were more comprehensive in our search strategy (including 5 databases) and in our inclusion criteria which resulted in a larger number of articles screened (3,229 compared to 99 for Annweiler et al.) and studies included (37 compared to 5 for both Annweiler et al. and Barnard and Colon-Emeric). Including a broader range of studies allowed us to perform meta-analyses to clearly identify research gaps and to explore and empirically test potential sources of heterogeneity.
Our meta-analysis comparing cognition (using MMSE) to 25(OH)D provided suggestive evidence of an association; the nature of the relationship is unclear. Most studies did not perform regression analysis to provide an answer to this question. Of the 5 studies that did use regression models, 328,57,62 found the relationship to be linear, but 2 found no relationship.32,40 This discrepancy may be a function of the type of cognitive measure assessed. Little is known about the function of vitamin D in relationship to the different cognitive domains. The potential for a nonlinear relationship is also a possibility. The relationship between vitamin D and its effect on parathyroid hormone (PTH) is hyperbolic and data from the European Male Ageing Study39 found the relationship between 4 cognition test scores and 25(OH)D to exhibit a threshold at 35 nmol/L.
We also found that the method of 25(OH)D measurement was an important determinant of heterogeneity. The CPBA method explained the heterogeneity in the meta-analysis comparing AD to control groups (figure e-2). This method has since been withdrawn from the market due to accuracy issues. The analytical measurement of 25(OH)D is difficult and both between method discrepancies63 and within the same method over time64 have been described. Standardization and harmonization of 25(OH)D methods is currently being addressed,65 but in the interim, it is important to consider the type of analytical method being used when comparing results from different studies.
In addition to the analytical difficulties, assessment of vitamin D status is also a problem as measured vitamin D reflects exposure which may vary throughout the year. Most vitamin D is obtained endogenously when the skin is exposed to UVB. People living above the latitude of 33 ° north will receive sufficient radiation only between 10 am and 3 pm from April to September.66 Exogenous sources of vitamin D come from natural (e.g., salmon, sardines, and tuna) and fortified foods (e.g., milk products) as well as supplements. Other factors affecting vitamin D concentration include higher skin pigmentation, older age, and female gender. Genetic factors are also likely to contribute to the vitamin D status.9 There is no time-integrated measure of vitamin D concentration or stable biologic response gauge to more accurately assess how much vitamin D a person is exposed to. Many of the studies in this systematic review did not consider factors which can vary vitamin D status. Furthermore, individuals who have cognitive decline are more likely to have poor nutrition and spend less time outdoors. These limitations contribute to the uncertainty in the primary study results and our meta-analyses. It is therefore impossible to rule out reverse causality as an alternative explanation.
This systematic review provides sufficient evidence to warrant further investigation to determine if a cause and effect relationship exists between vitamin D and cognitive impairment. To date, no treatment study has examined this question where both vitamin D and cognition were measured over a sufficient period in a large at-risk population.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- CPBA
competitive protein binding assay
- GDNF
glial cell derived neurotrophic factor
- iNOS
nitric oxide synthase
- MMSE
Mini-Mental State Examination
- NGF
nerve growth factor
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association
- PTH
parathyroid hormone
- RCT
randomized controlled trial
- RIA
radioimmunoassay
- WMD
weighted mean difference
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Balion: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Dr. Griffith: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Ms. Strifler: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Henderson: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Patterson: drafting/revising the manuscript. Dr. Heckman: drafting/revising the manuscript. Dr. Llewellyn: drafting/revising the manuscript, interpretation of the data. Dr. Raina: drafting/revising the manuscript, study supervision.
DISCLOSURE
C. Balion receives research support from the Canadian Institutes of Health Research, the Agency for Healthcare Research and Quality, the Canada Foundation for Innovation (CFI), and the Ontario Ministry of Health and Long-Term Care. L. Griffith receives research support from the Canadian Institutes of Health Research, Agency for Healthcare Research and Quality, and Ontario Ministry of Long-Term Care. L. Strifler has received funding from the Ontario Ministry of Long-Term Care. M. Henderson has received funding from the Ontario Ministry of Long-Term Care. C. Patterson has received funding from the Ontario Ministry of Long-Term Care. G. Heckman has received consultant fees from Novartis and Janssen-Ortho for participation in advisory boards related to cholinesterase inhibitors. He is also funded through the Schlegel Research Chair in Geriatric Medicine at the University of Waterloo and has received funding from the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, and Ontario Ministry of Health and Long-Term Care. D. Llewellyn has received funding from the Nutrition Society and American Society of Nutrition for conference travel and has grants funded by the James Tudor Foundation, the Sir Halley Stewart Trust, the Norman Family Charitable Trust, the Peninsula Medical School Foundation, and the Age Related Diseases and Health Trust. P. Raina receives research support from the Canadian Institutes of Health Research, Agency for Healthcare Research and Quality, Ontario Ministry of Health and Long-Term Care, and Canada Foundation for Innovation. Go to Neurology.org for full disclosures.
REFERENCES
- 1. McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J 2008; 22: 982– 1001 [DOI] [PubMed] [Google Scholar]
- 2. Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med 2008; 29: 415– 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005; 29: 21– 30 [DOI] [PubMed] [Google Scholar]
- 4. Morris HA, Anderson PH. Autocrine and paracrine actions of vitamin D. Clin Biochem Rev 2010; 31: 129– 138 [PMC free article] [PubMed] [Google Scholar]
- 5. Neveu I, Naveilhan P, Jehan F, et al. 1,25-Dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res 1994; 24: 70– 76 [DOI] [PubMed] [Google Scholar]
- 6. Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 1996; 7: 2171– 2175 [DOI] [PubMed] [Google Scholar]
- 7. Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia 1998; 22: 282– 294 [PubMed] [Google Scholar]
- 8. Sonnenberg J, Luine VN, Krey LC, Christakos S. 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology 1986; 118: 1433– 1439 [DOI] [PubMed] [Google Scholar]
- 9. Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009; 20: 1807– 1820 [DOI] [PubMed] [Google Scholar]
- 10. Annweiler C, Schott AM, Berrut G, et al. Vitamin D and ageing: neurological issues. Neuropsychobiology 2010; 62: 139– 150 [DOI] [PubMed] [Google Scholar]
- 11. Barnard K, Colon-Emeric C. Extraskeletal effects of vitamin D in older adults: cardiovascular disease, mortality, mood, and cognition. Am J Geriatr Pharmacol 2010; 8: 4– 33 [DOI] [PubMed] [Google Scholar]
- 12. Stevinson C, Lawlor DA. Searching multiple databases for systematic reviews: added value or diminishing returns? Complement Ther Med 2004; 12: 228– 232 [DOI] [PubMed] [Google Scholar]
- 13. Suarez-Almazor ME, Belseck E, Homik J, Dorgan M, Ramos-Remus C. Identifying clinical trials in the medical literature with electronic databases: MEDLINE alone is not enough. Control Clin Trials 2000; 21: 476– 487 [DOI] [PubMed] [Google Scholar]
- 14. Wells GA, Shea B, O'Connel D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm ed. Ottawa, Canada: Department of Epidemiology and Community Medicine, University of Ottawa, Canada; 2009 [Google Scholar]
- 15. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1– 12 [DOI] [PubMed] [Google Scholar]
- 16. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 10, 3: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993; 2: 121– 145 [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003; 327: 557– 560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deeks J, Higgings J, Altman D. Analysing and presenting results. In: Higgings JPT, Green S. eds. Cochrane Handbook for Systematic Review of Interventions, 2011 version 5.1.0. Available at: www.cochrane-handbook.org. Accessed September 1, 2010
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177– 188 [DOI] [PubMed] [Google Scholar]
- 21. Wallace B, Schmid C, Lau J, Trikalinos T. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol 2009; 9: 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96: 53– 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilkins CH, Birge SJ, Sheline YI, Morris JC. Vitamin D deficiency is associated with worse cognitive performance and lower bone density in older African Americans. J Natl Med Assoc 2009; 101: 349– 354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oudshoorn C, Mattace-Raso FU, van d V, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer's disease. Dement Geriatr Cogn Disord 2008; 25: 539– 543 [DOI] [PubMed] [Google Scholar]
- 25. Aung K, Burnett J, Smith SM, Dyer CB. Vitamin D deficiency associated with self-neglect in the elderly. J Elder Abuse Neglect 2006; 18: 63– 78 [DOI] [PubMed] [Google Scholar]
- 26. Perez-Llamas F, Lopez-Contreras MJ, Blanco MJ, Lopez-Azorin F, Zamora S, Moreiras O. Seemingly paradoxical seasonal influences on vitamin D status in nursing-home elderly people from a Mediterranean area. Nutrition 2008; 24: 414– 420 [DOI] [PubMed] [Google Scholar]
- 27. Liu BA, Gordon M, Labranche JM, Murray TM, Vieth R, Shear NH. Seasonal prevalence of vitamin D deficiency in institutionalized older adults. J Am Geriatr Soc 1997; 45: 598– 603 [DOI] [PubMed] [Google Scholar]
- 28. Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol 2009; 22: 188– 195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function Arch Biochem Biophys 2007; 460: 202– 205 [DOI] [PubMed] [Google Scholar]
- 30. Annweiler C, Schott AM, Allali G, et al. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology 2010; 74: 27– 32 [DOI] [PubMed] [Google Scholar]
- 31. Hii S, Scherer S. Vitamin D deficiency and secondary hyperparathyroidism in older people with low trauma fractures. Australas J Ageing 2004; 23: 45– 47 [Google Scholar]
- 32. Slinin Y, Paudel ML, Taylor BC, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology 2010; 74: 33– 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manders M, De Groot LCPG, Hoefnagels WHL, et al. The effect of a nutrient dense drink on mental and physical function in institutionalized elderly people. J Nutr Health Aging 2009; 13: 760– 767 [DOI] [PubMed] [Google Scholar]
- 34. Benge JF, Perrier ND, Massman PJ, Meyers CA, Kayl AE, Wefel JS. Cognitive and affective sequelae of primary hyperparathyroidism and early response to parathyroidectomy. J Int Neuropsychol Soc 2009; 15: 1002– 1011 [DOI] [PubMed] [Google Scholar]
- 35. Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci 2009; 64: 888– 895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010; 74: 18– 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El Ghoneimy AT, Gad AH, Samir H, et al. Impact of vitamin D deficiency on cognition in patients with multiple sclerosis. Egypt J Neurol Psychiatry Neurosurg 2009; 46: 223– 234 [Google Scholar]
- 38. Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: The inCHIANTI Study. J Gerontol A Biol Sci Med Sci 2007; 62: 440– 446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Psychiatry 2009; 80: 722– 729 [DOI] [PubMed] [Google Scholar]
- 40. McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology 2007; 29: 49– 54 [DOI] [PubMed] [Google Scholar]
- 41. Ogihara T, Miya K, Morimoto S. Possible participation of calcium-regulating factors in senile dementia in elderly female subjects. Gerontology 1990; 36 (suppl 30): 25– 30 [DOI] [PubMed] [Google Scholar]
- 42. Sakuma M, Endo N, Oinuma T, et al. Vitamin D and intact PTH status in patients with hip fracture. Osteoporosis Int 2006; 17: 1608– 1614 [DOI] [PubMed] [Google Scholar]
- 43. Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 2006; 14: 1032– 1040 [DOI] [PubMed] [Google Scholar]
- 44. Woo J, Lau EM, Chan E, Leung PC. Calcium absorption, cognitive function, and osteoporosis. J Clin Exp Gerontol 1991; 13: 263– 272 [Google Scholar]
- 45. Ravaglia G, De Ronchi D, Forti P, et al. Nutritional status and dementia in oldest-old women. Arch Gerontol Geriatr 1998; 427– 430 [Google Scholar]
- 46. Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol 2008; 65: 1348– 1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferrier IN, Leake A, Taylor GA, et al. Reduced gastrointestinal absorption of calcium in dementia. Age Ageing 1990; 19: 368– 375 [DOI] [PubMed] [Google Scholar]
- 48. Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: The Tromso study. J Neurol 2006; 253: 464– 470 [DOI] [PubMed] [Google Scholar]
- 49. Kipen E, Helme RD, Wark JD, Flicker L. Bone density, vitamin D nutrition, and parathyroid hormone levels in women with dementia. J Am Geriatr Soc 1995; 43: 1088– 1091 [DOI] [PubMed] [Google Scholar]
- 50. Luckhaus C, Mahabadi B, Grass-Kapanke B, et al. Blood biomarkers of osteoporosis in mild cognitive impairment and Alzheimer's disease. J Neural Transm 2009; 116: 905– 911 [DOI] [PubMed] [Google Scholar]
- 51. Martyn CN, Singh S, Wood PJ. Calcium metabolism in Alzheimer's disease: a case-control study. Gerontology 1989; 35: 153– 157 [DOI] [PubMed] [Google Scholar]
- 52. Nes M, Sem SW, Rousseau B, et al. Dietary intakes and nutritional status of old people with dementia living at home in Oslo. Eur J Clin Nutr 1988; 42: 581– 593 [PubMed] [Google Scholar]
- 53. Sato Y, Honda Y, Hayashida N, Iwamoto J, Kanoko T, Satoh K. Vitamin K deficiency and osteopenia in elderly women with Alzheimer's disease. Arch Phys Med Rehabil 2005; 86: 576– 581 [DOI] [PubMed] [Google Scholar]
- 54. Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer's disease. Bone 1998; 23: 555– 557 [DOI] [PubMed] [Google Scholar]
- 55. Walker MD, McMahon DJ, Inabnet WB, et al. Neuropsychological features in primary hyperparathyroidism: a prospective study. J Clin Endocrinol Metab 2009; 94: 1951– 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int 2008; 19: 1621– 1628 [DOI] [PubMed] [Google Scholar]
- 57. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med 2010; 170: 1135– 1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Corless D, Ellis M, Dawson E, Fraser F. Using activities of daily living assessments to measure the effectiveness of vitamin D supplements in elderly long-stay patients. Br J Occup Ther 1987; 50: 60– 62 [Google Scholar]
- 59. Chandra RK. Effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition 2001; 17: 709– 712 [DOI] [PubMed] [Google Scholar]
- 60. Manger MS, McKenzie JE, Winichagoon P, et al. A micronutrient-fortified seasoning powder reduces morbidity and improves short-term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. Am J Clin Nutr 2008; 87: 1715– 1722 [DOI] [PubMed] [Google Scholar]
- 61. Brannagan TH., III Current treatments of chronic immune-mediated demyelinating polyneuropathies. Muscle Nerve 2009; 39: 563– 578 [DOI] [PubMed] [Google Scholar]
- 62. Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly US population. J Gerontol A Biol Sci Med Sci 2011; 66: 59– 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carter GD. 25-Hydroxyvitamin D assays: the quest for accuracy. Clin Chem 2009; 55: 1300– 1302 [DOI] [PubMed] [Google Scholar]
- 64. Looker AC, Lacher DA, Pfeiffer CM, Schleicher RL, Picciano MF, Yetley EA. Data advisory with regard to NHANES serum 25-hydroxyvitamin D data. Am J Clin Nutr 2009; 90: 695 [DOI] [PubMed] [Google Scholar]
- 65. Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010; 82: 1942– 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nimitphong H, Holick MF. Vitamin D, neurocognitive functioning and immunocompetence. Curr Opin Clin Nutr Metab Care 2011; 14: 7– 14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.