Abstract
α-Actinin2 (Actn2) is a predominant protein in the sarcomere Z disc whose mutation can lead to cardiomyopathy. However, the function of Actn2 in Z-disc assembly and cardiomyopathy in vertebrates remains elusive. We leveraged genetic tools in zebrafish embryos to elucidate the function of Actn2. We identified a single Actn2 homologue expressed in the zebrafish heart and conducted loss-of-function studies by antisense morpholino technology. Although zebrafish Actn2 assembles early into the Z disc, depletion of actn2 did not affect the early steps of sarcomere assembly. Instead, Actn2 is required for Z bodies to register laterally, forming well-aligned Z discs. Presumably as a consequence to this structural defect in the sarcomere, the depletion of Actn2 resulted in reduced cardiac function, primarily characterized as a reduced end-diastolic diameter. The depletion of actn2 also significantly reduced the ventricle chamber size, due to both reduced cardiomyocyte (CM) size and CM number. Interestingly, reduced CM size can be rescued by the cessation of heart contractions. The genetic studies of zebrafish uncovered a function for actn2 in lateral registration of Z body. In actn2 morphant fish, the Z-disc defect sequentially affects cardiac function, which leads to morphological changes in the ventricle through a mechanical force-dependent mechanism.—Yang, J., Xu, X. α-Actinin2 is required for the lateral alignment of Z discs and ventricular chamber enlargement during zebrafish cardiogenesis.
Keywords: cardiac contraction, mechanical force, cardiomyocyte, myofibrillogenesis
A Z disc is a sarcomeric substructure that defines the boundary of a sarcomere, which is the basic contractile unit of striated muscles (1). An increasing number of proteins, including many signaling molecules, have been shown to dock within the Z disc, suggesting that the Z disc is not only a structural node but also a signaling center (2–4). Mutations in Z-disc genes, including CYPHER/ZASP, ACTN2, MYOTILIN, TCAP, and MLP, have been shown to potentiate human cardiomyopathy (2, 5, 6). However, the underlying molecular mechanism through which mutations in these sarcomeric Z-disc genes lead to cardiac remodeling and heart failure remains elusive.
A fundamental question in sarcomere-based cardiac diseases is how mutations in sarcomeric genes lead to sarcomeric structural change and subsequently affect cardiac function and heart morphology. Transparent zebrafish embryos, which develop ex utero, have recently been exploited as an accessible vertebrate model for genetic studies of sarcomeric genes. Fish with mutated sarcomeric genes, including titin and troponin T2 (tnnt2) (7, 8), have been identified in mutagenesis screens, and morphant fish that are depleted for other sarcomeric genes were generated by injection of antisense morpholino (MO) oligos (9–12). These efforts uncovered not only the specific functions of encoded sarcomere proteins in sarcomere assembly, but also their roles in cardiac function and heart morphogenesis. Accumulating evidence suggested that in growing embryonic hearts with constant contraction, the structure, function, and morphological changes are highly coordinated and mutually regulated (13–15). It has been reported that cardiac contraction regulates the enlargement of cardiomyocytes (CMs) during cardiogenesis, whereas intact sarcomeres have an inhibitory effect on heart enlargement (16, 17). Together, these studies established the zebrafish embryo as an in vivo vertebrate model for elucidating the functions of sarcomeric genes, which will facilitate our understanding of the molecular mechanisms underlying sarcomere-based cardiac diseases.
α-Actinin is the predominant component of the Z disc and is estimated to account for ∼20% of the Z-disc mass (2, 18). A highly conserved actin-binding protein belonging to the spectrin superfamily, α-Actinin protein usually forms a rod-shaped molecular dimer with the actin-binding domain and calmodulin-containing domain located at the two ends that cross-link actin filaments into bundles (18, 19). In mammals, there are 4 α-actinin genes encoding proteins that can be grouped into 2 distinct classes. While α-actinin 2 and 3 encode muscle isoforms that are located in the sarcomeric Z disc, α-actinin 1 and 4 encode nonmuscle isoforms that are commonly associated with focal contacts and stress fibers (18). In humans, ACTN2 is a major isoform that is expressed in cardiac and oxidative skeletal muscle, and mutations in ACTN2 have been found in human patients with either hypertrophic or dilated cardiomyopathy (18, 20, 21). Within the Z disc, α-actinin has been shown to bind to a growing list of Z-disc proteins and to play a central role in the intricate protein-protein interactions in the Z-disc network (22). α-Actinin is one of the first proteins to integrate into the Z body during assembly (23, 24). Based on these biochemical and expression data, it has been hypothesized that α-actinin plays a crucial role in Z-disc assembly. However, this concept has not been supported by genetic studies of α-actinin. Drosophila embryos with depletion of the α-actinin homologue develop a normal striated muscle pattern with Z discs (25, 26). Likewise, in Caenorhabditis elegans, dense body-like structures that contain focal adhesion proteins, including integrin, talin, and vinculin, still form when α-actinin is mutated (27). However, these studies were conducted in lower model organisms, and therefore, genetic studies of actn2 in a vertebrate model that better recapitulate human Z-disc structure are needed. Despite a recent genetic study in zebrafish aimed at uncovering distinct functions of actn2 and actn3 (28), the precise function of actinin in Z-disc assembly and cardiogenesis remains elusive.
Here, we cloned zebrafish cardiac actn2 and conducted actn2 loss-of-function studies in zebrafish embryos using MO antisense technology. Our previous study characterized three sequential steps of Z-disc assembly during zebrafish cardiogenesis (9). Using actn2 morphants, we found that Actn2 is only needed at a later step of Z-disc assembly during the lateral alignment of the electron-dense bodies within the Z disc, despite appearing in the Z bodies at the 18-somite (S) stage. The unique Z-disc defect induced by actn2 deficiency leads to a significant reduction in cardiac function, as well as a reduction of the ventricular chamber size. Further investigation suggested that the reduced CM size, which partially accounts for the reduced ventricular chamber size, can be attributed to mechanical force-dependent regulation. Discoveries that are made in zebrafish embryos may illuminate the underlying mechanisms of actn2-mediated cardiomyopathy.
MATERIALS AND METHODS
Zebrafish husbandry
The embryos and adult fish were raised and maintained under standard laboratory conditions. The animal experiments were approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee (protocol A15611).
Molecular cloning
The primer sequences used for polymerase chain reaction (PCR) are listed in Table 1. To generate the Tol2 transposon-based constructs expressing enhanced green fluorescent protein (EGFP)-tagged Actn2 and Tcapa, we used a modified pT2KXIGΔin-egfp plasmid containing the egfp sequence as the backbone (29). The 3-kb titina (ttna) enhancer (DQ649453) was amplified by PCR (ttna enhancer-F/R primers) from zebrafish genomic DNA, and then cloned into a pT2K-egfp plasmid to generate pT2K-ttna. The cDNAs for actn2 (NM_001037573, actn2-F1/R1 primers) and tcapa (XM_679011, tcapa-F1/R1 primers) were amplified and cloned into the pT2K-ttna vector to generate the final constructs.
Table 1.
Primers used for PCR
| Name | Sequence | Purpose |
|---|---|---|
| ttna enhancer-F | GAACTCGAGTCAGTAGTCTGGCATTCCAC | Molecular cloning |
| ttna enhancer-R | GCGGAATTCAAGCTTGCTAGCCCGGGCTACAA | Molecular cloning |
| actn2-F1 | GCGGAATTCATGATGAATCAGATCGAG | Molecular cloning |
| actn2-R1 | GCGGAATTCAAGGTCACTTTCTCCATATAGGG | Molecular cloning |
| tcapa-F1 | GCGGAATTCATGCAGGTTTGCTCAGTCCTG | Molecular cloning |
| tcapa-R1 | GCGGAATTCTTGCCTCTCTGGGCCTCCTGAGACAT | Molecular cloning |
| cmlc2 enhancer-F | GTTCTCGAGCTCGCATTCATCCATCCTTTTCA | Molecular cloning |
| cmlc2 enhancer-R | CCGGAATTCTTCACTGTCTGCTTTGCTGTTGGTCT | Molecular cloning |
| cypher-F1 | AAAAAAGCAGGCTTCACCATGACTTCGTACAACGTG | Molecular cloning |
| cypher-R1 | AGAAAGCTGGGTCCTGAGGCACGTTGAGTAT | Molecular cloning |
| LASP2-F | TTAGGATCCACCATGAGGGTCCCTGTATTTG | Molecular cloning |
| LASP2-R | TTGCTCGAGTGATTAACAAACTCAATGTAATTC | Molecular cloning |
| MYOTILIN-F | TTAGGATCCACCATGTTTAACTACGAACGTC | Molecular cloning |
| MYOTILIN-R | TTGCTCGAGTGAAGTTCTTCACTTTCATAG | Molecular cloning |
| mlp-F1 | TTAGGATCCACCATGCCAAATTGGGGTGGAG | Molecular cloning |
| mlp-R1 | TTGCTCGAGTGCACACTCTCTTCGACCATG | Molecular cloning |
| actn2-F2 | GCTATGAGGAATGGCTGCTGACTG | In situ hybridization |
| actn2-R2 | GGTAATACGACTCACTATAGGTCCTGAATGAGTTGGTGATCTCCC | In situ hybridization |
| actn3a-F | CTTTTATGATGCTGCCACCATCA | In situ hybridization |
| actn3a-R | GGTAATACGACTCACTATAGGGTCAATAGACACATGGCT | In situ hybridization |
| actn3b-F | CTTCTTGTTTTCTTCAAGC | In situ hybridization |
| actn3b-R | GGTAATACGACTCACTATAGGGTGCATTAACTACTATAAG | In situ hybridization |
| actn2-F3 | CTTCCATGTCAGCTGGAAGGATGG | RT-PCR |
| actn2-R3 | GCAGTTTCAGCCTGCTCAGCTC | RT-PCR |
| actn2-F4 | CTGGCATTTGACATTGCTG | RT-PCR |
| actn2-R4 | CACTCCAAGCACCTTACAG | RT-PCR |
| 18S-F | CACTTGTCCCTCTAAGAAGTTGCA | RT-PCR |
| 18S-R | GGTTGATTCCGATAACGAACGA | RT-PCR |
| cypher-F2 | ATTACACCGGGGAGCAAGGC | RT-PCR |
| cypher-R2 | CTTCTGATGAGGAATCACAG | RT-PCR |
| mlp-F2 | CCGCAGCTTCCATAAAACAT | RT-PCR |
| mlp-R2 | TGAGTTTGAGTTCGCAGGTG | RT-PCR |
| lasp2-F | GAGAAAGTGAACTGCCTGGA | RT-PCR |
| lasp2-R | GTCCCTTGCTCTGTTCAAAA | RT-PCR |
| tcapa-F2 | GGGAGGAAAACCCAAATAAGAG | RT-PCR |
| tcapa-R2 | TGGAGAGGACGCACCTGCCA | RT-PCR |
To generate the Tol2 transposon-based constructs expressing EGFP-tagged Cypher, LASP2, MYOTILIN, and Mlp, we used the Tol2 kit generated in Dr. Chi-Bin Chien's laboratory (University of Utah, Salt Lake City, UT, USA; ref. 30). The ttna enhancer and cardiac myosin light chain 2 (cmlc2) enhancer were cloned into the p5E-MCS plasmid to generate a p5E-ttna and p5E-cmlc2 entry clone separately (29). Next, the zebrafish cypher cDNA (BC045908.1) was amplified using cypher-F1/R1 primer pair and cloned into the pDONR221 vector (Invitrogen, Grand Island, NY, USA) by a recombination reaction using the BP clonase mix (Invitrogen). The cDNAs for human LASP2 (BC110453.1, LASP2-F/R primer), human MYOTILIN (BC005376, MYOTILIN-F/R primer), and zebrafish mlp (BC065956, mlp-F1/R1 primer) were amplified by PCR and cloned into the pENTR1A vector (Invitrogen). Finally, a p5E entry clone, a middle entry clone, a p3E entry clone, and the pDest-Tol2poly destination clone were recombined using the Gateway LR Clonase II Plus Enzyme Mix (Invitrogen) to generate the final Tol2-based constructs. The entry plasmids that were chosen to generate each individual construct, including pTol2 (ttna:megfp) are summarized in Supplemental Table S1.
Transient injection analysis and the generation of transgenic fish lines
To analyze the localization of EGFP-tagged Z-disc protein, Tol2-based plasmids were coinjected with 100 ng/μl transposases into the zebrafish embryo at 1-cell stage. Mosaic expression of EGFP in the dissected embryonic zebrafish hearts was observed and imaged using a Zeiss upright microscope equipped with Apotome (Carl Zeiss, Oberkochen, Germany). To generate the fish lines Tg(ttna:megfp), Tg(cmlc2:cypher-egfp), and Tg(ttna:actn2-egfp), the Tol2-based expression vectors were coinjected with 100 ng/μl transposase into zebrafish embryos at the 1-cell stage. EGFP-positive fish were selected to generate founder fish with mosaic expression. Stable transgenic lines were identified by outcrossing founder fish and screening for EGFP-positive embryos.
Whole-mount in situ hybridization
cDNA fragments were amplified by RT-PCR for the following genes: actn2 (actn2-F2/R2 primers), actn3a (actn3a-F/R primers), and actn3b (actn3b-F/R primers). The PCR products were directly used to synthesize riboprobes. Whole-mount in situ hybridization was performed as described previously (31).
MO injection and rescue experiments
Antisense MO oligomers (Gene Tools, Philomath, OR, USA) were prepared and injected into zebrafish embryos as described previously (32). The sequences were as follows: actn2 E1 MO: 5′-AAGGCCAAACTCTCTTTACCTTCCT-3′ (28), actn2 E6 MO: 5′-TTTGAGGACACACTTGTGACCTTGT-3′, and actn2 E7 MO (actn2 MO): 5′-TTTATGCTTATGGTTTACCCTCTGC-3′. To validate the specificity of these MOs, rescue experiments were performed by injecting actn2 MO into 1-cell-stage zebrafish embryos from incrossing Tg(ttna:actn2-egfp) fish or wild-type (WT) fish. Embryos with and without the transgene were determined based on EGFP fluorescence at 24 h postfertilization (hpf).
Western blotting and RT-PCR
Twenty hearts were dissected from WT or actn2 MO fish embryos and homogenized using a Bullet Blender (Next Advance, Averill Park, NY, USA). The sample was denatured at 95°C for 10 min and loaded onto a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel for Western blotting. Anti-actn2 (Novus, Littleton, CO, USA) and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies were used to detect Actn2 and β-actin protein expression levels.
For RT-PCR, total RNA from 10 zebrafish embryos or 15 embryonic zebrafish hearts was extracted using Trizol (Sigma-Aldrich, St. Louis, MO, USA) and an RNeasy mini kit (Qiagen, Valencia, CA, USA). The cDNAs were synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen). The exon-skipping event potentiated by the actn2 MO was detected using actn2-F3/R3 primer pair. The knockdown efficiency of actn2 was assessed by real-time PCR (actn2-F4/R4 primers) and calculated as previously reported (33). Real-time PCR was performed in a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using the iQ SYBR Green Supermix (Bio-Rad). The following primer pairs were used: 18S-F/R, cypher-F2/R2, mlp-F/2R2, myotilin-F/R; lasp2-F/R, tcapa-F/R.
Immunostaining of dissected embryonic hearts and related quantification
The 52-hpf zebrafish embryos were bathed in E3 water containing 20 mM 2,3-butanedione monoxime (BDM) and 0.4% tricaine for 2 min until the fish hearts stopped beating and the heart muscle was completely relaxed. We noted that embryos treated with BDM were comparably relaxed as embryos treated with the relaxing buffer (data not shown). The zebrafish hearts were dissected, stained, and then imaged on a microslide (10, 34). The following antibodies were used: anti-Mef2 (Santa Cruz Biotechnology) at 1:200, anti-β-catenin (Sigma-Aldrich) at 1:200, anti-bromodeoxyuridine (BrdU; Sigma-Aldrich) at 1:50, anti-PCNA (Sigma-Aldrich) at 1:3000, anti-sarcomeric α-actinin (clone EA53; Sigma-Aldrich) at 1:200, F59 [Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA] at 1:10, anti-egfp (Invitrogen) at 1:200, anti-myomesin (DSHB) at 1:20, and Alexa-conjugated secondary antibodies (Invitrogen) at 1:1000. We used Alexa-conjugated phalloidin (Invitrogen) at 1:50 to stain the actin filaments. The heart sample was imaged using a Zeiss LSM 510 Meta Confocal microscope.
The width, interval, and length of Cypher-EGFP bands were measured based on the fluorescence images. For each group, 5–10 well-stained fish hearts were chosen. The distribution of fluorescence density was analyzed by pseudo-line scan using Zeiss AxioVision software. For each heart sample, ≥10 different myofibrils and 3 Z discs on each myofibril were measured to obtain the average Z-disc width or length.
Transmission electron microscopy
The 52-hpf zebrafish embryos were bathed in the relaxing buffer (25 mM imidazole, 7 mM MgAc, 5 mM EGTA, 5 mM creatine phosphate, 100 mM KAc, 0.04 μg/μl Leupeptin, and 5 mM ATP) for 1 h until the hearts stopped beating and cardiac muscles were completely relaxed. Twenty embryonic zebrafish hearts were collected and fixed in Trump's solution overnight at 4°C before being processed at the Mayo Clinic's Electron Microscopy Core Facility and imaged using a Philips CM10 transmission electron microscope (Philips, Amsterdam, The Netherlands); 111 Z discs in 30 CM from 6 WT fish hearts, 65 Z discs in 60 CM from 9 actn2 MO fish hearts, 42 Z discs in 31 CM from 13 blebbistatin-treated fish hearts, and 16 Z discs in 8 CM from 8 blebbistatin-treated actn2 MO fish hearts were scoped in either low or high magnification.
Quantification of heart function and ventricular chamber size
Heart function indices, including the shortening fraction, the end-diastolic diameter, and the end-systolic diameter, were quantified using videos of beating hearts from transparent zebrafish embryos (9). To facilitate the measurement of the ventricular chamber size, fluorescent images of Tg(ttna:megfp) fish were used, and the heartbeat was stopped by treatment with 20 mM BDM and 0.4% tricaine before image acquisition (Supplemental Fig. S3A). When Tg(ttna:megfp) could not be used, we imaged the transparent heart by differential interference contrast (DIC) imaging using a Zeiss Axioplan II microscope. The heart images were analyzed using Zeiss AxioVision software (9).
Measurement of CM size, circularity, proliferation, and apoptosis
To measure the CM size and circularity, immunostaining for Mef2 and β-catenin was performed to label the nuclei and outline the individual CMs. The CM size and circularity were quantified as described previously (16). To identify apoptotic CMs, in situ cell death detection staining (Roche, Indianapolis, IN, USA) was performed on the embryonic heart, which was followed by immunostaining with an anti-Mef2 antibody. Cells that were positive for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and Mef2 staining were counted as apoptotic CMs. The apoptotic index was defined as the ratio between the number of apoptotic CMs (TUNEL+/Mef2+) and the total number of CMs (Mef2+). To identify proliferating CMs, we performed PCNA and Mef2 staining as described before (35). To confirm the result, we also performed BrdU assay followed by Mef2 immunostaining. Briefly, embryos were dechorionated at 44 hpf and bathed in E3 water containing 10 mM BrdU and 15% dimethyl sulfoxide (DMSO) at 6°C to 8°C for 20 min. After further development at 28°C in pure E3 water, the embryonic hearts were dissected at 52 hpf and immunostained using an anti-BrdU antibody (36). The embryonic heart was then fixed in 4% formaldehyde for 20 min, followed by Mef2 immunostaining to identify the CMs. The proliferation index was defined as the ratio between the number of proliferative CMs (BrdU+/Mef2+) and the total number of CMs (Mef2+).
Statistics
The values shown in the graphs are means ± se. At a minimum, 10 fish embryos or 20 cells were analyzed for each group. Student's t test was used to compare the difference between 2 groups, and P < 0.05 was considered to be significant.
RESULTS
Actn2 is the only muscle-specific α-actinin that is expressed in embryonic zebrafish hearts
To identify the zebrafish ortholog of the cardiac actn2 gene, we blast-searched the zebrafish Ensembl genomic database (http://www.ensembl.org) for the human ACTN2 peptide sequence and found 5 candidate zebrafish genes. To identify cardiac α-actinin, we generated riboprobes for actn2, actn3a, and actn3b, 3 candidate genes encoding muscle-specific α-actinins, and assessed their expression profile during embryogenesis using whole-mount in situ hybridization. Consistent with two other independent genome-wide searches of zebrafish α-actinin homologues (28, 37), we only detected expression of the candidate actn2 transcript in the heart starting at the 14-S stage (Supplemental Fig. S1A). The onset of actn2 RNA cardiac expression is consistent with the onset of α-actinin protein expression that is observed in zebrafish hearts at 18 S (9). The actn2 transcript can also be detected in somites from 5 S to 18 S, but the somite expression becomes restricted to the midline of the myotome at 24 hpf and disappears from the somite region altogether by 48 hpf (Supplemental Fig. S1B). Compared to actn2, the transcripts for both actn3a and actn3b were restricted to the somite region and not expressed in the heart (Supplemental Fig. S1C, D). Therefore, expression-based screens from 3 independent groups consistently suggested that actn2 encodes the only muscle-specific α-actinin protein that is expressed in the heart during zebrafish cardiogenesis.
Actn2 assembles into the Z disc early on
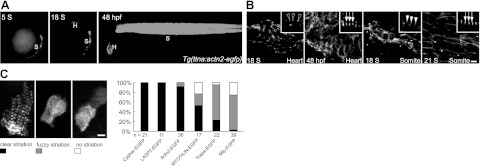
To confirm that actn2 encodes a sarcomeric Z-disc protein, we assessed its subcellular localization by cloning the full-length actn2 cDNA and fusing an EGFP reporter gene to its C terminus. We then generated a transgenic fish line, Tg(ttna:actn2-EGFP), in which a 3.0-kb enhancer identified from the upstream promoter region of the ttna gene drives the expression of EGFP-tagged Actn2 in CM. The ttna enhancer is capable of initiating actn2-GFP expression at 18 S when the early steps of myofibrillogenesis occur in zebrafish hearts (9, 33). Notably, the ttna enhancer also drives somite expression starting at 5 S (Fig. 1A). We found that EGFP fluorescence in Tg(ttna:actn2-EGFP) fish faithfully recapitulates the subcellular expression of endogenous sarcomeric α-actinin that was previously reported by our laboratory (9). In the heart, EGFP fluorescence was detected in the irregularly dotted Z bodies at 18 S; the expression became periodic at 24 S and fused laterally to form broader Z discs at 48 hpf (Fig. 1B and data not shown). In the somites, EGFP fluorescence recapitulates the expression in both the periodic Z bodies in posterior somitic segments at 18 S and the laterally fused Z discs in the more anterior segments at 21 S (Fig. 1B).
Figure 1.
Actn2-GFP recapitulates the localization of endogenous Actn2 and assembles early into the Z disc. A) In Tg(ttna:actn2-egfp) fish, a ttna enhancer drives the expression of EGFP-tagged Actn2 in the somites starting at 5 S and in the heart starting at 18 S. EGFP signal can be detected in both the heart and somites at 48 hpf. H, heart; B) Images of dissected hearts or somites from Tg(ttna:actn2-egfp) fish. Actn2-egfp is localized to Z bodies as irregular dots (empty arrowhead) at 18 S and periodic Z discs (arrow) at 48 hpf in the heart. In the somites, Actn2-egfp forms Z bodies as regular dots (solid arrowhead) at 18 S and striated Z discs (arrow) at 21 S. C) Left panel: examples of 3 types of CMs exhibiting different striation that may coexist in embryos injected with constructs encoding EGFP-tagged Z-disc proteins. Right panel: quantification of the percentage of CMs that exhibit different degrees of striation at 48 hpf. Compared to MYOTILIN-EGFP, Tcapa-EGFP, and Mlp-EGFP, injection of Actn2-EGFP resulted in a higher percentage of CMs exhibiting clear striation. n, CM number. Scale bars = 10 μm (B); 5 μm (C).
To compare the Z-disc assembly of Actn2 with other Z-disc proteins, we cloned cDNAs encoding zebrafish Cypher, Tcap, and Mlp, as well as cDNAs encoding human LASP2 and MYOTILIN (38–42). Injection of the constructs encoding EGFP-tagged fusion proteins (Supplemental Table S1) revealed the Z disc restricted expression pattern of these 5 genes. We observed that the striation of the EGFP fluorescence is heterogeneous in fish hearts, which may reflect the incorporation of the ectopic EGFP-tagged protein into the sarcomere to replace some of the endogenous protein. The EGFP signal forms a sharp striated band in some CM cells, but there is only mild striation or no striation in other cells (Fig. 1C). The quantification of the percentage of cells that contain clearly striated EGFP fluorescence indicated that, together with Cypher and LASP2, Actn2 exhibits better Z-disc localization than MYOTILIN, Mlp, and Tcapa (Fig. 1C). Consistent with previous studies, our in vivo studies supported that Actn2 assembles into the Z disc earlier than Tcap and Mlp (22, 23, 43). Furthermore, these results proved the feasibility of monitoring the assembly of Actn2 and other Z-disc proteins in zebrafish hearts by generating and expressing constructs that encode EGFP-tagged proteins.
Actn2 is required for the lateral alignment of Z bodies but not for the earlier steps of sarcomere assembly
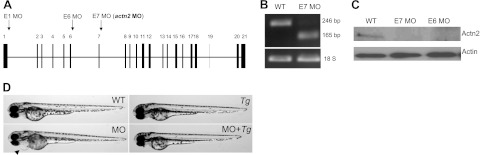
To assess the function of actn2 in sarcomere assembly, we depleted actn2 expression using an antisense MO, actn2 MO, that targeted the splice donor of exon 7 (Fig. 2A). A >99% reduction of actn2 transcript can be achieved by injection of 4 ng actn2 MO, as quantified by real-time RT-PCR. Further PCR analysis suggested that injection of actn2 MO results in an exon-skipping event that deletes exon 7 from the normal actn2 transcript (Fig. 2B). Because exon 7 has 82 nt, it is anticipated that deletion of this exon results in an open reading frameshift in the actn2 transcript. As a consequence, Actn2 protein may be truncated at the neck region, resulting in a 208-aa peptide. However, this truncated product is likely degraded (18), since neither full-length nor truncated version of Actn2 was detected by Western blot analysis using a polyclonal antibody that targets the N-terminal 347 aa of human ACTN2 protein (Fig. 2C).
Figure 2.
actn2 MO effectively knocks down the actn2 gene and induces a heart-specific phenotype. A) Schematic of the gene structure of actn2 and the exons that are targeted by the three splice donor MOs E1 MO, E6 MO, and E7 MO. actn2 MO (bold) refers to E7 MO. B) Injection of actn2 MO induces an exon-skipping event, as revealed by DNA gel electrophoresis after RT-PCR using a primer pair spanning exons 6 and 8. The 246-bp band detected in the WT fish is shifted to 165 bp in the actn2 MO fish, indicating the deletion of exon 7. C) Western blot analysis indicates a strong reduction in Actn2 protein in hearts dissected from embryos that were injected with either E7 MO or E6 MO. D) Lateral views of zebrafish embryos show that embryos injected with actn2 MO exhibit a cardiac-specific phenotype. Pericardial edema (arrowhead) can be rescued by Tg(ttna:actn2-egfp).
We observed cardiac-specific phenotypes due to actn2 deficiency. Mild pericardial edema can be detected at 2 days postfertilization (dpf), and it becomes much more severe by 3 dpf (Fig. 2D). The morphant fish otherwise exhibit normal morphology and swim freely until 7 dpf, when most of the embryos die from severe pericardial edema and a reduced heartbeat. To validate the specificity of the actn2 MO, we conducted a rescue experiment by injecting actn2 MO into Tg(ttna:actn2-egfp) transgenic fish. In contrast to the cardiac defects observed in EGFP-negative embryos, pericardial edema was effectively rescued in EGFP+ embryos containing the actn2 transgene (Fig. 2D). Moreover, we synthesized two additional MOs; one was reported recently that targets the splice donor of exon 1 (28), and the other targets the splice donor of exon 6 (Fig. 2A). Injection of both MOs resulted in the same heart-specific phenotypes as the actn2 MO (Fig. 2C and data not shown).
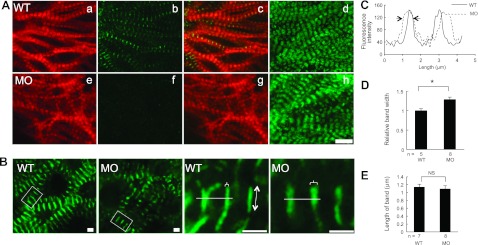
Having proven the efficiency and specificity of the MOs, we evaluated sarcomeric structures in actn2 morphants by staining hearts that were dissected from zebrafish embryos (34). At 72 hpf, sarcomere assembly has already proceeded to a mature stage that is characterized by the formation of a network of bundled myofibrils in the ventricle, as indicated by the thin filament network that was visible using phalloidin staining and the thick filament network that was visible using Myosin immunostaining (Fig. 3Aa, d). Compared to WT fish, the bundled myofibril network is still intact in actn2 morphants, and the striation of both the thick and thin filaments appears normal (Fig. 3Ae, h) in the absence of Actn2 protein (Fig. 3Ab, f). To assess effect on the Z discs, we generated Tg(cmlc2:Cypher-EGFP). In this transgenic fish line, the striated Z discs were labeled by EGFP, which alternated with the M lines as revealed by coimmunostaining with an anti-myomesin antibody (Supplemental Fig. S2A). Although the striations of the Cypher-EGFP bands were normal, these bands appeared thicker in actn2 MO fish hearts (Fig. 3B). Quantification via pseudo-line analysis confirmed that the width, but not the length, of the Z bands was significantly increased in actn2 MO fish heart (Fig. 3C–E). In contrast, both the striation and bandwidth of the M line were normal, as indicated by immunostaining with an anti-myomesin antibody (Supplemental Fig. S2B, C). The interval between Cypher-EGFP bands also remained unchanged, suggesting unaffected sarcomere length (data not shown).
Figure 3.
Depletion of Actn2 leads to broader Z band without affecting other myofibril substructures, including I bands and A bands. A) Images of dissected ventricles from WT and acnt2 MO fish at 48 hpf with phalloidin staining (a, e), immunostaining using an anti-Actn2 antibody (b, f), merged images of phalloidin (red) and Actn2 antibody staining (green) (c, g), and immunostaining using an anti-myosin antibody (d, h). In actn2 morphant fish, Actn2 immunoreactivity is essentially undetectable (b, f), but the striated pattern of thin and thick filaments remain normal. B) Subcellular localization of Cypher in Tg(cmlc2:cypher-egfp) fish is shown in low and high magnification (boxed area in low magnification). Cypher-EGFP exhibits as striated bands in both WT and actn2 MO fish, but the bands are wider in actn2 MO fish heart than in WT. Brackets indicate bandwidth; line with double-ended arrow indicates band length. C) Pseudo-line scan analysis of the fluorescence intensity change along the white line in B. Arrows indicate that actn2 MO fish has wider cypher-GFP band than WT fish. D, E) Measurement of the width (D) and length (E) of Cypher-EGFP bands shows that the width is significantly increased in actn2 MO fish heart, while the length of the bands is not affected. MO, fish injected with actn2 MO; NS, not significant. Scale bars = 10 μm (A); 2 μm (B). *P < 0.05.
To further assess the Z-disc defects, we performed transmission electron microscopy studies. Sarcomeres in actn2 morphants still appear as bundled myofibrils with comparable lateral length (Fig. 4Aa–c). Consistent with the broader Cypher-EGFP band in immunostaining experiments, the lateral registration of the electron-dense Z discs was disturbed. Compared to the 97% straight and complete Z-disc band among 111 Z discs from 6 WT fish hearts (Fig. 4Ad), 62% of Z lines were zigzagged (Fig. 4Ae) and 20% of Z lines were broken into several small pieces among 65 Z discs from 9 actn2 MO fish hearts (Fig. 4Af). As a consequence, the Z-disc width was significantly increased in actn2 MO fish heart (Fig. 4B).
Figure 4.
Depletion of Actn2 leads to lateral alignment defects in the Z discs. A) a–c) Electron micrographs of CMs of 52-hpf embryos at low magnification. Compared to WT fish, actn2 morphants exhibit organized myofibrils but disrupted Z-disc structures (arrows). d–f) High-magnification images of boxed regions panels a–c. In contrast to the laterally aligned Z disc in WT fish (d), the electron-dense Z discs in actn2 morphants are misaligned, exhibiting as either zigzagged lines (e) or dispersed dots (f). Arrowheads indicate dispersed electron-dense dots. Brackets indicate Z-disc width. Scale bars =1 μm (a–c); 200 nm (d–f). B) Measurement of Z-disc width based on electron microscopy data. C) Schematic of the Z-disc defects in actn2 morphants. Injection of actn2 MO blocks the lateral alignment but not the lateral fusion of the Z discs. MO, fish injected with actn2 MO. *P < 0.05.
We examined the expression of Cypher, LASP2, MYOTILIN, Mlp, or Tcapa by RT-PCR and did not detect any significant change of expression for these Z-disc proteins in actn2 MO fish hearts (Supplemental Fig. S2D). We also assessed their Z-disc localization by coinjecting constructs encoding EGFP-tagged proteins with actn2 MO. Depletion of Actn2 does not affect the ability of these Z-disc proteins to form a well-striated Z-disc pattern (Supplemental Fig. S2E). These results suggested that the Z-disc defects were likely a direct consequence of Actn2 depletion, but not sequential events of defects in other Z-disc proteins. Together, our studies suggested that Actn2 is not required for the early steps of sarcomere assembly. Instead, Actn2 plays a role in governing the lateral registration when periodic sarcomeres are fused to wide myofibrils, presumably due to the crosslinking function of Actn2 (ref. 44 and Fig. 4C).
Depletion of Actn2 affects cardiac function and heart morphogenesis
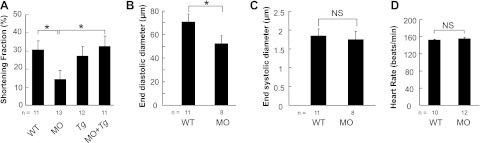
The primary defect in sarcomere structure induced by depletion of Actn2 sequentially affects both cardiac function and heart morphology. The shortening fraction in the ventricle was dramatically reduced in actn2 morphants, a defect that was completely restored by the EGFP-tagged Actn2 transgene (Fig. 5A). Diastolic dysfunction was the primary contributor to the reduced shortening fraction, as indicated by the dramatically reduced end-diastolic diameter but comparable end-systolic diameter in actn2 morphants (Fig. 5B, C). In contrast to the reduced shortening fraction, the heart rate remained normal in actn2 morphant fish (Fig. 5D), suggesting that the disturbed sarcomeres did not affect the calcium wave in CMs or the calcium sensitivity of the sarcomere.
Figure 5.
Injection of actn2 MO leads to reduced cardiac function. A) Injection of actn2 MO reduces the shortening fraction, which is rescued by transgenic actn2 in Tg(ttna:actn2-egfp) fish. B) End-diastolic diameter is reduced in actn2 morphants. C) End-systolic diameter is comparable between WT fish and actn2 morphants. D) Heart rate is not affected by injection of actn2 MO. MO, actn2 MO fish; Tg, Tg(ttna:actn2-egfp) fish; MO+Tg, Tg(ttna:actn2-egfp) fish injected with actn2 MO; n, fish number; NS, not significant. *P < 0.05.
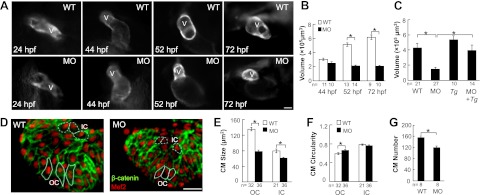
In actn2 morphant embryos, we observed a morphological abnormality in the hearts that manifested as a markedly small ventricle. In normal zebrafish embryos, the ventricle transforms from a primitive tube (24 hpf) to a chamber (44 hpf) that continues to enlarge from 44 to 72 hpf (Fig. 6A). In contrast, the ventricular chamber in actn2 morphants appeared normal until 44 hpf, but failed to enlarge further, as quantified at both 52 and 72 hpf (Fig. 6B). The small ventricle chamber size was completely rescued in the Tg(ttna:actn2-EGFP) transgenic fish that were injected with actn2 MO (Fig. 6C). The specificity of the ventricular chamber size defect and the correlation between the ventricular chamber size and actn2 RNA expression level was supported by consistent phenotypes in the embryos that were injected with either of two additional actn2 MOs (data not shown). When actn2 MO is used for knocking down actn2, the ventricular chamber size was reduced by 31% in the fish with a 92% reduction in actn2 RNA, while the ventricular chamber size was reduced by 71% reduction in the fish with a 99.8% reduction in actn2 RNA.
Figure 6.
Injection of actn2 MO leads to reduced size of the ventricular chamber and individual CMs. A) Ventricle enlargement is halted by actn ctn2 MO. Images show individual fish hearts from living Tg(ttna:megfp) fish embryos with or without actn2 MO injection. Dorsal view and ventral view with the head facing up is shown for embryos at 24 and 44 hpf, respectively; embryos at 52 and 72 hpf are shown in lateral views with the head to the left. V, ventricle. B) Quantification of ventricular volume reveals a significantly reduced ventricular chamber size in MO fish at both 52 and 72 hpf. C) Reduced ventricular chamber sizes in MO fish can be rescued by Tg(ttna:actn2-egfp). D) Image of a ventricle after immunostaining with Mef2 (red) and β-catenin (green) to define the nuclei and the outline of the CMs. Typical OC and IC cells are encircled with solid and dashed lines, respectively. E, F) Quantification of CM size and circularity shows that CM size is significantly reduced in both the OC and IC regions, while CM circularity is only increased in the OC region. G) CM number in the ventricle is reduced in actn2 morphant fish. MO, fish injected with actn2 MO; Tg, Tg(ttna:actn2-egfp) fish; MO+Tg, Tg(ttna:actn2-egfp) fish injected with actn2 MO, OC, outer curvature; IC, inner curvature; n, number of fish (B, C, G) or CMs (E, F). Scale bars =20 μm (A); 40 μm (D). *P < 0.05.
At the cellular level, ventricular chamber enlargement in developing zebrafish hearts can be ascribed to both increased CM size and increased CM number. CM enlargement occurs more significantly in the outer curvature (OC) than the inner curvature (IC) region during development, contributing to the asymmetric growth of the ventricle (16). To reveal the cellular nature of the halted ventricular chamber growth in actn2 morphants, we quantified the CM size and cell number after immunostaining zebrafish embryos using an anti-β-catenin antibody to define the cell border, and an anti-Mef2 antibody to identify individual CMs (Fig. 6D). We detected a significantly reduced CM size in both the OC and IC region and a mildly affected cell shape in the OC region, as indicated by the quantification of CM circularity (Fig. 6E, F). We also observed a reduced CM number in the actn2 morphants, which is a consequence of reduced CM proliferation, as revealed by a BrdU incorporation assay and a PCNA immunostaining assay, and increased CM apoptosis, as revealed by a TUNEL assay (Fig. 6G and Supplemental Fig. S3B–G).
The CM size change incurred by sarcomeric defects in actn2 morphants depends on cardiac contraction
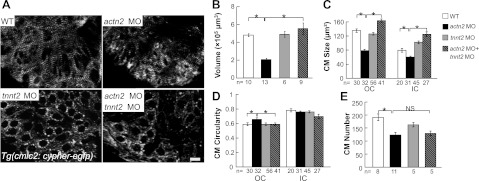
Based on the emerging concept that mechanical force regulates heart morphogenesis (13, 14), and specifically that blood flow and sarcomere integrity regulate regional CM enlargement through opposing forces, (16) we hypothesized that the reduced ventricular chamber size in actn2 morphants may be a sequential morphological consequence that is secondary to misaligned Z discs and altered mechanical forces. To test this hypothesis, we stopped the heartbeat by coinjecting actn2 MO with tnnt2 MO, which has been used to induce silent heart phenotypes, thus disrupting the heart contraction-related mechanical forces that originate from both blood flow and sarcomere integrity (8, 9). In double-morphant fish, the heart contraction was completely stopped, and the myofibril bundling was disrupted, as indicated by the development of dotted Z bodies instead of the wider Z discs that were observed in WT and actn2 morphant fish (Fig. 7A). Notably, the reduced ventricular chamber size in actn2 morphants was restored to a similar size as WT and tnn2 morphant fish (Fig. 7B), suggesting that mechanical forces play a role in actn2 MO-induced ventricular chamber size change. At the cellular level, we found that CM size, but not CM number, was rescued by coinjection of tnnt2 MO (Fig. 7C–E).
Figure 7.
Cessation of heart contraction rescues the ventricle chamber size defect in actn2 MO fish. A) Sarcomere integrity is disrupted by a tnnt2 MO, as revealed by Tg(cmlc2:cypher-egfp). Bundling of the Z discs is disrupted in embryos injected with either tnnt2 MO or tnnt2, actn2 double MO but is intact in WT and actn2 MO fish embryos. Scale bar = 10 μm. B) Quantification of the ventricular volume in embryos at 72 hpf. Ventricular volume is significantly reduced in actn2 morphant fish, but this defect can be rescued by coinjection of a tnnt2 MO. C) Compared to WT fish, both OC and IC cell size are reduced in actn2 morphant fish, but this defect can be rescued by coinjection of a tnnt2 MO. D) Compared to WT fish, the circularity of OC cells is reduced in actn2 MO fish, but this defect can be rescued by coinjection of a tnnt2 MO. E) Compared to WT fish, CM number is significantly reduced in actn2 morphant fish. However, there is no significant rescue of CM number by coinjection of a tnnt2 MO. n, number of fish (B, E) or CMs (C, D); NS, not significant. *P < 0.05.
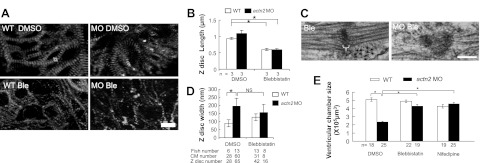
To independently test the possibility that the reduced ventricular chamber size in actn2 morphants depends on mechanical force, we stopped the heart from beating and disrupted sarcomere structure by treating fish embryos with either blebbistatin or nifedipine, two small-molecule compounds that stop the heartbeat through different mechanisms. Blebbistatin is a myosin ATPase inhibitor that stops the heartbeat by blocking active force generation (45), while nifedipine is a calcium channel inhibitor that stops the heart from beating by blocking the calcium wave. Administration of either 8 μM blebbistatin or 60 μM nifedipine completely stopped cardiac contraction and resulted in disturbed sarcomere integrity with disrupted bundling of the Z discs in the myofibril network (Fig. 8A, B). Cessation of contraction did not affect either the misaligned Z disc or the increased Z-disc width in actn2 MO fish heart (Fig. 8C, D), suggesting that the Z-disc defects in actn2 morphants was independent of the contraction. However, both blebbistatin or nifedipine treatment can effectively rescue the reduced ventricular chamber size in actn2 morphants similar to injection of tnnt2 MO (Fig. 8E). Together, our data strongly suggested that the reduced CM size in actn2 morphants was a consequence of mechanical force changes resulting from abnormal cardiac contraction.
Figure 8.
Ventricle chamber size reduction in actn2 morphants is rescued by cessation of contraction via blebbistatin or nifedipine treatment. A) Sarcomere integrity is disrupted in embryos treated with blebbistatin, as revealed by Tg(cmlc2:cypher-egfp). Compared to the long Z discs in DMSO-treated WT or actn2 MO fish, the Z discs in blebbistatin-treated WT or actn2 MO fish is significantly shorter, indicating disrupted Z-disc bundling. Double-ended arrow indicates the Z-disc length. B) Quantification of the Z-disc length. n, number of fish. C) Electron micrographs show that cessation of heart contraction via blebbistatin treatment cannot rescue the disrupted Z disc in actn2 MO fish heart. Bracket indicates Z-disc width. D) Quantification of the Z-disc width, which cannot be rescued by cessation of heart contraction. E) Measurement of the ventricular volume in DMSO and blebbistatin treated fish at 52 hpf. WT and actn2 morphant embryos were bathed in egg water containing 8 μM blebbistatin or 60 μM nifedipine from 37 to 52 hpf. Ventricular volume is significantly reduced in MO fish, but this defect can be rescued by either blebbistatin or nifedipine treatment. MO, actn2 MO-injected fish; Ble, blebbistatin-treated WT fish; MO Ble, blebbistatin-treated actn2 MO fish; NS, not significant. Scale bars = 10 μm (A); 200 nm (C). *P < 0.05.
DISCUSSION
Function of Actn2 in myofibrillogenesis in zebrafish hearts
In this manuscript, we defined actn2 as the only muscle-specific α-actinin in the developing zebrafish heart and uncovered its rather specific function in sarcomeric Z discs. We found that although Actn2 assembles into the sarcomere structure at an early developmental stage, it is not required for the earlier steps of myofibrillogenesis but instead is critical for the lateral registration of the bundled Z bodies. In actn2 MO fish heart, the misalignment defect is restricted to the Z discs, while both the striation and lateral fusion of other sarcomeric substructures, including M lines, I bands, and A bands, are normal. Our results from genetic studies in the vertebrate model of zebrafish are consistent with previous genetic studies of α-actinin in Drosophila and C. elegans (25–27). It is suggested that the cross-linker function of α-actinin is not required for either thin filaments or Titin filaments to anchor into the Z disc. Instead, Actn2 might function to ensure the precise lateral registration of these filaments within the Z disc (Fig. 4C).
Similar lateral alignment defects have been reported in sarcomeres that are depleted of either Cypher or Nexilin, two other Z-disc components (12, 38, 46). Compared to Cypher-knockout mice or nexilin morphant fish, the Z-disc phenotypes in actn2 MO fish heart were more severe, since 22% of Z discs were broken into dispersed dots and the disrupted Z disc could not be rescued by cessation of cardiac contraction. Similar to genetic studies of Cypher and Nexilin (12, 38, 46), we found that α-actinin is not required for the Z-disc localization of many other Z-disc proteins. Since biochemical studies indicate that each Z-disc component binds to several other Z-disc partners (47), it is possible that the intricate Z-disc protein-protein interaction ensure the Z-disc integration of these proteins. However, the distribution of these proteins within the Z disc might become zigzagged or dispersed, as suggested by the broader Cypher-GFP band. These structural changes need further investigation, which will help us to elucidate function of the Z disc in both cardiac contraction and signaling.
Notably, some of our observations differ from a recent genetic study of actn2 in zebrafish embryos (28). In contrast to an enlarged heart with reduced heart rate, as reported in Briggs et al. (28), we noted a smaller ventricle with normal heart rate in fish embryos depleted of Actn2. We have rigorously validated our observation by using an additional two MOs, quantified our results at both organ and cellular level, and confirmed the specificity of our observation by conducting rescue experiments via generating a stable transgenic fish line for Actn2. To exclude the possibility that the discrepancy is simply due to injection of different MOs, we injected the same MO (actn2 E1 MO) used in Briggs et al. (28) and noted the same cardiac phenotypes as shown in our actn2 MO fish. When a higher dose of MO was injected, we also saw somite defects, as reported in Briggs et al. (28). However, the somite defect could not be rescued by Tg(titin:actn2-GFP), which casts concern on the specificity of this phenotype.
Suggestions on the relationship between Z-disc structure and cardiac contraction
The specific Z-disc defects in actn2 morphants provide experimental evidence to support an important function of Z-disc integrity in cardiac contraction. The misaligned Z discs in embryos depleted of Actn2 are expected to impose a direct effect on the primary function of a sarcomere, which is the generation and transmission of mechanical force (2). Normally functioning as a nexus to anchor the thin filaments, the misaligned Z discs affect the transmission of the active force generated by actin-myosin movement. Also important as a nexus to anchor the N terminus of the elastic Titin filament, the misaligned Z discs likely affect the transmission of passive force (48). Due to the combined effects of individual sarcomere dysfunction, the myofibril network presents with an abnormal contractile profile and defective mechanical force, which results in reduced cardiac contraction in actn2 morphant fish.
In addition to having a direct effect on mechanical force generation and transmission, the defective Z-disc structure in actn2 morphants might affect heart function indirectly by disturbing Z-disc-based signaling molecules. For example, the distribution change of Cypher might affect the signaling function of Cypher and block ERK/Stat3 signaling pathway (46). α-Actinin has been shown to directly interact with the serine/threonine protein kinase PKN (49), G-protein-coupled receptor kinases (GRKs; ref. 50), and phospholipase D (PLD; ref. 51). It remains to be investigated whether these signaling molecules mediate the Actn2 deficiency-induced heart contraction defect. Therefore, our actn2 morphant provides an in vivo model to further investigate the relationship between Z-disc structure and cardiac contraction.
Suggestion on the relationship among Z-disc structure, cardiac contraction, and cardiac morphometry
Our genetic studies of actn2 morphant supported the concept that cardiac contraction plays an important role in regulating the enlargement of the ventricular chamber size during zebrafish cardiogenesis (14). More importantly, our data underscored a crucial function of the Z disc in conferring the mechanical force-regulated cardiac morphometric change. There are at least two types of changes in mechanical force on actn2 depletion. First, the disruption of the Z-disc structure affects the intrinsic profile of the myofibril to generate and to transmit mechanical force (52). Second, the reduced heart contraction leads to reduced blood flow, which sequentially affects the fluid pressure against ventricle wall during each contraction (14, 16). The reduced ventricular chamber size in actn2 morphant is likely a combined consequence of both intrinsic and extrinsic mechanical force changes, as well as the potentially defective mechano-signaling due to the misaligned Z discs. Of note, the reduced ventricle size phenotype in actn2 morphants is different from the dilated hearts reported in morphants depleted of Cypher and Nexilin (12, 46), suggesting the unique nature of the Z-disc structural disruption in the actn2 morphants. Thus, a detailed comparison among genetic manipulations of different Z-disc proteins promises to uncover mechanisms on how the Z disc confers mechanical force-regulated CM growth.
Many ACTN2-based cardiomyopathies are of the hypertrophic type with enlarged ventricles, instead of reduced ventricular chamber size noted in actn2 morphants. The discrepancy is likely due to the progressive and hypomorphic nature of adult cardiomyopathies, while the embryonic studies focus on the immediate biological consequence of a near-complete depletion of Actn2. Although not exactly recapitulating pathogenesis in adults, embryonic studies directly address a fundamental question of cardiomyopathy; i.e., the relationship among Z-disc structure, mechanical force, mechanotransduction signaling, and cardiac morphometric change. The extraordinary capacity of a zebrafish embryo to survive a dysfunctional circulation system, even with a halted heartbeat, provides a unique opportunity to make novel discoveries related to this fundamental question. Because abnormal mechanical force and defective mechanical signaling is an important midstep during pathogenesis of many forms of cardiomyopathies, it is anticipated that the knowledge generated using the embryonic zebrafish model will ultimately help us to understand the pathogenesis of actn2- and other Z-disc gene-based cardiomyopathies in humans.
Supplementary Material
Acknowledgments
The authors thank Dr. Yu-huan Shih (Mayo Clinic College of Medicine) for his contributions in conducting the RT-PCR experiments. The authors thank Dr. Chi-Bin Chien (University of Utah, Salt Lake City, UT, USA) for sharing the following plasmids: p5E-MCS, pME-megfp, p3E-poly(A), and pDestTol2pA. The authors thank Dr. Carol Gregorio (University of Arizona, Tucson, AZ, USA) and Dr. Joseph Sanger (SUNY Upstate Medical University, Syracuse, NY, USA) for sharing the LASP2 and MYOTILIN cDNAs, respectively. The authors also thank Beninio Jomok for his help with zebrafish husbandry.
This study was supported by the Mayo Foundation and by a U.S. National Institutes of Health grant (HL81753) to X.X.
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BDM
- 2,3-butanedione monoxime
- BrdU
- bromodeoxyuridine
- CM
- cardiomyocyte
- cmlc2
- cardiac myosin light chain 2
- DIC
- differential interference contrast
- dpf
- days postfertilization
- DMSO
- dimethyl sulfoxide
- EGFP
- enhanced green fluorescent protein
- hpf
- hours postfertilization
- IC
- inner curvature
- MO
- morpholino
- OC
- outer curvature
- PCR
- polymerase chain reaction
- S
- somite
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- WT
- wild-type
REFERENCES
- 1. Clark K. A., McElhinny A. S., Beckerle M. C., Gregorio C. C. (2002) Striated muscle cytoarchitecture: an intricate web of form and function. Annu. Rev. Cell Dev. Biol. 18, 637–706 [DOI] [PubMed] [Google Scholar]
- 2. Frank D., Kuhn C., Katus H. A., Frey N. (2006) The sarcomeric Z-disc: a nodal point in signalling and disease. J. Mol. Med. 84, 446–468 [DOI] [PubMed] [Google Scholar]
- 3. Pyle W. G., Solaro R. J. (2004) At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ. Res. 94, 296–305 [DOI] [PubMed] [Google Scholar]
- 4. Hoshijima M. (2006) Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am. J. Physiol. Heart Circ. Physiol. 290, H1313–H1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keren A., Syrris P., McKenna W. J. (2008) Hypertrophic cardiomyopathy: the genetic determinants of clinical disease expression. Nat. Clin. Pract. Cardiovasc. Med. 5, 158–168 [DOI] [PubMed] [Google Scholar]
- 6. Morimoto S. (2008) Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc. Res. 77, 659–666 [DOI] [PubMed] [Google Scholar]
- 7. Xu X., Meiler S. E., Zhong T. P., Mohideen M., Crossley D. A., Burggren W. W., Fishman M. C. (2002) Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat. Genet. 30, 205–209 [DOI] [PubMed] [Google Scholar]
- 8. Sehnert A. J., Huq A., Weinstein B. M., Walker C., Fishman M., Stainier D. Y. (2002) Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 31, 106–110 [DOI] [PubMed] [Google Scholar]
- 9. Huang W., Zhang R., Xu X. (2009) Myofibrillogenesis in the developing zebrafish heart: a functional study of tnnt2. Dev. Biol. 331, 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z., Huang W., Dahme T., Rottbauer W., Ackerman M. J., Xu X. (2008) Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms. Cardiovasc. Res. 79, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van der Meer D. L., Marques I. J., Leito J. T., Besser J., Bakkers J., Schoonheere E., Bagowski C. P. (2006) Zebrafish cypher is important for somite formation and heart development. Dev. Biol. 299, 356–372 [DOI] [PubMed] [Google Scholar]
- 12. Hassel D., Dahme T., Erdmann J., Meder B., Huge A., Stoll M., Just S., Hess A., Ehlermann P., Weichenhan D., Grimmler M., Liptau H., Hetzer R., Regitz-Zagrosek V., Fischer C., Nurnberg P., Schunkert H., Katus H. A., Rottbauer W. (2009) Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat. Med. 15, 1281–1288 [DOI] [PubMed] [Google Scholar]
- 13. Hove J. R., Koster R. W., Forouhar A. S., Acevedo-Bolton G., Fraser S. E., Gharib M. (2003) Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 [DOI] [PubMed] [Google Scholar]
- 14. Bartman T., Hove J. (2005) Mechanics and function in heart morphogenesis. Dev. Dyn. 233, 373–381 [DOI] [PubMed] [Google Scholar]
- 15. Bartman T., Walsh E. C., Wen K. K., McKane M., Ren J., Alexander J., Rubenstein P. A., Stainier D. Y. (2004) Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2, E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Auman H. J., Coleman H., Riley H. E., Olale F., Tsai H. J., Yelon D. (2007) Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 5, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin Y. F., Swinburne I., Yelon D. (2012) Multiple influences of blood flow on cardiomyocyte hypertrophy in the embryonic zebrafish heart. Dev. Biol. 362, 242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sjoblom B., Salmazo A., Djinovic-Carugo K. (2008) Alpha-actinin structure and regulation. Cell. Mol. Life Sci. 65, 2688–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young P., Ferguson C., Banuelos S., Gautel M. (1998) Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin. EMBO J. 17, 1614–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mills M., Yang N., Weinberger R., Vander Woude D. L., Beggs A. H., Easteal S., North K. (2001) Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: implications for the evolution of functional redundancy. Hum. Mol. Genet. 10, 1335–1346 [DOI] [PubMed] [Google Scholar]
- 21. Chiu C., Bagnall R. D., Ingles J., Yeates L., Kennerson M., Donald J. A., Jormakka M., Lind J. M., Semsarian C. (2010) Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J. Am. Coll. Cardiol. 55, 1127–1135 [DOI] [PubMed] [Google Scholar]
- 22. Wang J., Shaner N., Mittal B., Zhou Q., Chen J., Sanger J. M., Sanger J. W. (2005) Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil. Cytoskeleton 61, 34–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stout A. L., Wang J., Sanger J. M., Sanger J. W. (2008) Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging. Cell Motil. Cytoskeleton 65, 353–367 [DOI] [PubMed] [Google Scholar]
- 24. Du A., Sanger J. M., Sanger J. W. (2008) Cardiac myofibrillogenesis inside intact embryonic hearts. Dev. Biol. 318, 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roulier E. M., Fyrberg C., Fyrberg E. (1992) Perturbations of Drosophila alpha-actinin cause muscle paralysis, weakness, and atrophy but do not confer obvious nonmuscle phenotypes. J. Cell Biol. 116, 911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jani K., Schock F. (2007) Zasp is required for the assembly of functional integrin adhesion sites. J. Cell Biol. 179, 1583–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moulder G. L., Cremona G. H., Duerr J., Stirman J. N., Fields S. D., Martin W., Qadota H., Benian G. M., Lu H., Barstead R. J. (2010) alpha-actinin is required for the proper assembly of Z-disk/focal-adhesion-like structures and for efficient locomotion in Caenorhabditis elegans. J. Mol. Biol. 403, 516–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta V., Discenza M., Guyon J. R., Kunkel L. M., Beggs A. H. (2012) alpha-Actinin-2 deficiency results in sarcomeric defects in zebrafish that cannot be rescued by alpha-actinin-3 revealing functional differences between sarcomeric isoforms. FASEB J. 26, 1892–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang R., Xu X. (2009) Transient and transgenic analysis of the zebrafish ventricular myosin heavy chain (vmhc) promoter: An inhibitory mechanism of ventricle-specific gene expression. Dev. Dyn. 238, 1564–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007) The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- 31. Sun X., Zhang R., Lin X., Xu X. (2008) Wnt3a regulates the development of cardiac neural crest cells by modulating expression of cysteine-rich intestinal protein 2 in rhombomere 6. Circ. Res. 102, 831–839 [DOI] [PubMed] [Google Scholar]
- 32. Nasevicius A., Ekker S. C. (2000) Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220 [DOI] [PubMed] [Google Scholar]
- 33. Seeley M., Huang W., Chen Z., Wolff W. O., Lin X., Xu X. (2007) Depletion of zebrafish titin reduces cardiac contractility by disrupting the assembly of Z-discs and A-bands. Circ. Res. 100, 238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang J., Xu X. (2012) Immunostaining of dissected zebrafish embryonic heart. J. Vis. Exp. 59, e3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun X., Hoage T., Bai P., Ding Y., Chen Z., Zhang R., Huang W., Jahangir A., Paw B., Li Y. G., Xu X. (2009) Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One 4, e6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poss K. D., Nechiporuk A., Hillam A. M., Johnson S. L., Keating M. T. (2002) Mps1 defines a proximal blastemal proliferative compartment essential for zebrafish fin regeneration. Development 129, 5141–5149 [DOI] [PubMed] [Google Scholar]
- 37. Holterhoff C. K., Saunders R. H., Brito E. E., Wagner D. S. (2009) Sequence and expression of the zebrafish alpha-actinin gene family reveals conservation and diversification among vertebrates. Dev. Dyn. 238, 2936–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou Q., Chu P. H., Huang C., Cheng C. F., Martone M. E., Knoll G., Shelton G. D., Evans S., Chen J. (2001) Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J. Cell Biol. 155, 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang R., Yang J., Zhu J., Xu X. (2009) Depletion of zebrafish Tcap leads to muscular dystrophy via disrupting sarcomere-membrane interaction, not sarcomere assembly. Hum. Mol. Genet. 18, 4130–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knoll R., Hoshijima M., Hoffman H. M., Person V., Lorenzen-Schmidt I., Bang M. L., Hayashi T., Shiga N., Yasukawa H., Schaper W., McKenna W., Yokoyama M., Schork N. J., Omens J. H., McCulloch A. D., Kimura A., Gregorio C. C., Poller W., Schaper J., Schultheiss H. P., Chien K. R. (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111, 943–955 [DOI] [PubMed] [Google Scholar]
- 41. Garvey S. M., Miller S. E., Claflin D. R., Faulkner J. A., Hauser M. A. (2006) Transgenic mice expressing the myotilin T57I mutation unite the pathology associated with LGMD1A and MFM. Hum. Mol. Genet. 15, 2348–2362 [DOI] [PubMed] [Google Scholar]
- 42. Moncman C. L., Wang K. (2002) Targeted disruption of nebulette protein expression alters cardiac myofibril assembly and function. Exp. Cell Res. 273, 204–218 [DOI] [PubMed] [Google Scholar]
- 43. Sanger J. W., Wang J., Holloway B., Du A., Sanger J. M. (2009) Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell Motil. Cytoskeleton 66, 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dabiri G. A., Turnacioglu K. K., Sanger J. M., Sanger J. W. (1997) Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc. Natl. Acad. Sci. U. S. A. 94, 9493–9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jou C. J., Spitzer K. W., Tristani-Firouzi M. (2010) Blebbistatin effectively uncouples the excitation-contraction process in zebrafish embryonic heart. Cell. Physiol. Biochem. 25, 419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zheng M., Cheng H., Li X., Zhang J., Cui L., Ouyang K., Han L., Zhao T., Gu Y., Dalton N. D., Bang M. L., Peterson K. L., Chen J. (2009) Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum. Mol. Genet. 18, 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lange S., Ehler E., Gautel M. (2006) From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol. 16, 11–18 [DOI] [PubMed] [Google Scholar]
- 48. Linke W. A. (2008) Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc. Res. 77, 637–648 [DOI] [PubMed] [Google Scholar]
- 49. Mukai H., Toshimori M., Shibata H., Takanaga H., Kitagawa M., Miyahara M., Shimakawa M., Ono Y. (1997) Interaction of PKN with alpha-actinin. J. Biol. Chem. 272, 4740–4746 [DOI] [PubMed] [Google Scholar]
- 50. Freeman J. L., Pitcher J. A., Li X., Bennett V., Lefkowitz R. J. (2000) alpha-Actinin is a potent regulator of G protein-coupled receptor kinase activity and substrate specificity in vitro. FEBS Lett. 473, 280–284 [DOI] [PubMed] [Google Scholar]
- 51. Park J. B., Kim J. H., Kim Y., Ha S. H., Yoo J. S., Du G., Frohman M. A., Suh P. G., Ryu S. H. (2000) Cardiac phospholipase D2 localizes to sarcolemmal membranes and is inhibited by alpha-actinin in an ADP-ribosylation factor-reversible manner. J. Biol. Chem. 275, 21295–21301 [DOI] [PubMed] [Google Scholar]
- 52. Luther P. K. (2009) The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling. J. Muscle Res. Cell. M. 30, 171–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.