Abstract
Nicotinic acetylcholine receptors (nAChRs) containing α6 and/or α4 subunits modulate the release of dopamine. However, few compounds can effectively discriminate between ligand-binding sites that contain α6 vs. α4 nAChR subunits. Using a chimeric (α6/α4) subunit, we showed that α-conotoxin BuIA binds the extracellular rat α6β2 vs. α4β2 interface with ∼60,000-fold selectivity. Chimeras containing residues from the α6 subunit were inserted into the homologous position of the α4 subunit to identify critical sequence segments. The region between residues 184 and 207 in the α6 subunit accounted for the potency difference. Chimeras within this region followed by point mutations were constructed for further definition. α6 Lys185, Thr187, and Ile188 form a triad of key residues that influence BuIA binding; when these 3 α6 residues were inserted into the α4 subunit, there was an ∼2000-fold increase in toxin potency. We used a crystal structure of BuIA bound to the acetylcholine-binding protein together with the structure of the Torepedo marmorata nAChR to build a homology model of BuIA bound to the interface between α6 and β2 subunits. The results indicate that the triad of α6 residues lies outside the C loop and is distantly located from bound BuIA (>10 Å). This suggests that alterations in potency are not caused by the direct interaction between the triad and BuIA. Instead, alterations in C-loop 3-dimensional structure and/or flexibility may account for differential potency. Thr198 and Tyr205 also contributed to BuIA potency. In addition, Thr198 caused BuIA potency differences between the closely related α6 and α3 subunits. Together, the findings provide insight into differences between the α6 and other α subunits that may be exploited by α-conotoxins to achieve binding selectivity.—Kim, H.-W., McIntosh, J. M. α6 nAChR subunit residues that confer α-conotoxin BuIA selectivity.
Keywords: nicotinic, Conus, C loop, chimera, channel
Neuronal nicotinic acetylcholine receptors (nAChRs) are a family of ligand-gated ion channels that are expressed in many regions of the central and the peripheral nervous systems (1). In vertebrates, 9 α (α2-α10) subunits and 3 β (β2-β4) subunits have been cloned and studied (2). Functional nAChRs are assembled as either homo- or heteropentameric channels from a combination of various α and β subunits. These various nAChR subunit combinations display unique pharmacological properties, allowing different physiological roles. Receptor expression levels and location also vary, enabling individual nAChR subtypes to subserve distinct functions. α4, β2, and α7 are the most abundantly expressed subunits, while other subunits are expressed in tissue-specific ways.
In particular, expression of the α6 subunit is concentrated in catecholaminergic regions, including locus coeruleus, ventral tegmental area, and substantia nigra (3–6). α6* (asterisk indicates the presence of additional subunits) nAChRs modulate dopamine release and are involved in motor behaviors. In primate and rodent animal models of Parksinon's disease, dopamine neuron injury produced by 1-methyl 4 phenyl 1,2,3,6-tetrahydropyridine (MPTP) leads to selective loss of α6* nAChRs (7). In addition, α6* nAChRs are preferentially lost in human Parkinson's disease (8, 9). Therefore, α6* nAChRs represent novel targets for the treatment of neurodegenerative disorders characterized by dopaminergic dysfunction, such as Parkinson's disease (9–11).
α6* nAChRs have also recently been shown to be critical to self-administration of nicotine in mice (12–15). The more ubiquitously expressed α4 subunit is often colocalized in tissue expressing the α6 subunit (3). Unfortunately, current drugs show poor discrimination for α6* vs. α4* nAChRs. Thus, from both a pathophysiological and pharmacological basis, it is highly desirable to gain mechanistic insight into how ligands may discriminate between α6* and α4* nAChRs.
α-Conotoxins are a family of small, disulfide-rich peptides isolated from predatory marine snails known as Conus. These peptides target nAChRs of their prey, and some of these peptides also potently act at mammalian nAChRs (16). α-Conotoxin BuIA (BuIA), from Conus bullatus (17) is a 2-disulfide bridge peptide that belongs to the A superfamily of Conus toxins. Previous work has defined the primary structure of BuIA, which is unusual compared to other α-conotoxins in that BuIA has a 4/4 Cys spacing (18). In the present work, we determined the structural features of the α6 nAChR subunit that confer high affinity for BuIA. BuIA potently binds to the N-terminal extracellular portion of the α6 nChR subunit. The residues that allow discrimination between the α6 subunit vs. the α4 subunit were identified.
MATERIALS AND METHODS
Mutagenesis and construction of chimeric nAChR subunits
Chimeric and mutant subunits were constructed by various PCR strategies. Native rat α4, β2, and β3 clones were previously constructed and characterized (19). Because the α6 subunit does not form functional receptors with the β2 subunit, we used a functional surrogate formed by splicing the N-terminal extracellular ligand-binding region of the α6 subunit with the remaining portion of the α4 subunit, as described previously (20, 21). Numbering of residues of each subunit was based on that previously proposed for the muscle nAChR (22). Rat α6-207-α4 in the pSGEM2 vector was a generous gift from Roger Papke (University of Florida, Gainesville, FL, USA); other chimeric subunits were constructed by PCR. Briefly, each fragment to be joined was amplified using Vent DNA Polymerase (New England BioLabs, Beverly, MA, USA) with long primers overlapping both sequences and gene-specific primers for each respective end. The two fragments were purified from the agarose gel slice using a Qiaquick Gel Extraction kit (Qiagen, Valencia, CA, USA). Purified fragments were then fused and amplified with 2 gene-specific primers for each end. The amplified chimeric subunit was then ligated into the pSGEM2 vector developed by Dr. Michael Hollmann (Ruhr University, Bochum, Germany). We also used the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) for single and multiple point mutations. The DNA sequences of constructed chimeric subunits and point mutants were confirmed by DNA sequencing. The rat (α6/α3) chimera and α6T198Qα3 were constructed as described previously (19, 23). Two other point mutants, (α6D187Eα3)β2β3 and (α6F203Lα3)β2β3 were constructed, as described by methods described above for the α4 point mutants.
The notation for these chimeras is the length of the α6 sequence at the N-terminal portion, followed by the remaining α4 sequence, which makes up the C-terminal portion. For example, α6-139-α4 is composed of the α6 sequence through residue 139 with the subsequent sequence composed of the remaining α4 sequence. Chimeras were also constructed in which the α6 sequence replaced a portion of the homologous α4 sequence. The notation for these chimeras is α4 followed by the sequence from α6 that has been substituted shown in parenthesis For example, α4-α6(185-188)-α4 indicates that residues of α4 from 185 to 188 are substituted by corresponding α6 sequences. For the point mutant, the mutated amino acid is listed followed by the original amino acid sequence name and number. For example, α4 R188I indicates an α4 subunit in which the 188th residue Arg is substituted by Ile.
Synthesis and injection of cRNAs into Xenopus laevis oocytes
M7 G(5′)ppp(5′)G capped cRNA was transcribed in vitro from linearized template DNA using mMessage mMachine (Ambion, Austin, TX, USA). Clones of rat nAChR subunits were used to produce cRNA for injection into X. laevis oocytes, as described previously (24). To express nAChRs in oocytes, 5 ng of each nAChR subunit was injected.
Electrophysiology
Oocytes were used 2 to 5 d after injection. A 30-μl cylindrical oocyte recording chamber fabricated from Sylgard was gravity-perfused with ND96 solution with atropine (ND96A; 96.0 mM NaCl, 2.0 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 1 μM atropine, and 5 mM HEPES, pH 7.1-7.5) at a rate of ∼2 ml/min (25). All toxin solutions also contained 0.1 mg/ml BSA to reduce nonspecific adsorption of peptide. Acetylcholine (ACh)-gated currents were measured with a 2-electrode voltage-clamp amplifier (model OC-725B; Warner Instruments, Hamden, CT, USA), and data were captured as described previously (26). The membrane potential of the oocytes was clamped at −70 mV. To apply a pulse of ACh to the oocytes, the perfusion fluid was switched to one containing ACh for 1 s. This was done automatically at intervals of 1 min. The shortest time interval was chosen such that reproducible control responses were obtained with no observable desensitization. The concentration of ACh was 100 μM for all nAChRs. All ACh pulses contained no toxin, for it was assumed that little if any bound toxin washed away in the brief time (<1 s) it takes for the responses to peak. In our recording chamber, the bolus of ACh does not project directly at the oocyte but rather enters tangentially, swirls, and mixes with the bath solution. The average peak amplitude of three control responses just preceding exposure to toxin was used to normalize the amplitude of each test response to obtain a percentage response or percentage block. Each data point of a dose-response curve represents the average ± se value of measurements from ≥3 oocytes. Dose-response curves were fit to the equation percentage response = 100/(1 + ([toxin]/IC50)nH, where nH is the Hill slope determined with Prism software (GraphPad Software, San Diego, CA, USA). If only 3 different concentrations were used for constructing a concentration response curve, nH was constrained to a value of 1.0.
Molecular modeling
The toxin-bound form of rat α4-α6(185-188)-α4β2 extracellular domain was built by homology to the structure of the Torpedo marmorata AChR [Protein Data Bank (PDB) ID: 2BG9] as the template. The sequence alignment and homology modeling were performed by Swiss-Model (27). BuIA in the toxin-bound form with acetylcholine binding protein (AChBP) from the mollusk (PDB ID: 4EZ1) was copied onto the homology model. The toxin-bound model structure was solvated in a cubical periodic box with 42,904 water molecules and 0.1 M NaCl. Then, the total system was further equilibrated for 5 ns under the constant pressure (1 atm) and temperature (298.15 K) conditions, after 1000 steps of energy minimization and 60 ps gradual heating to 298.15 K. Energy minimization and MD simulations were performed by NAMD2.8 (28) with CHARMM27 force field (29, 30). The constructed model was visualized by Visual Molecular Dynamics (31).
RESULTS
BuIA potently blocks (α6/α4)β2β3 but not α4β2 nAChRs
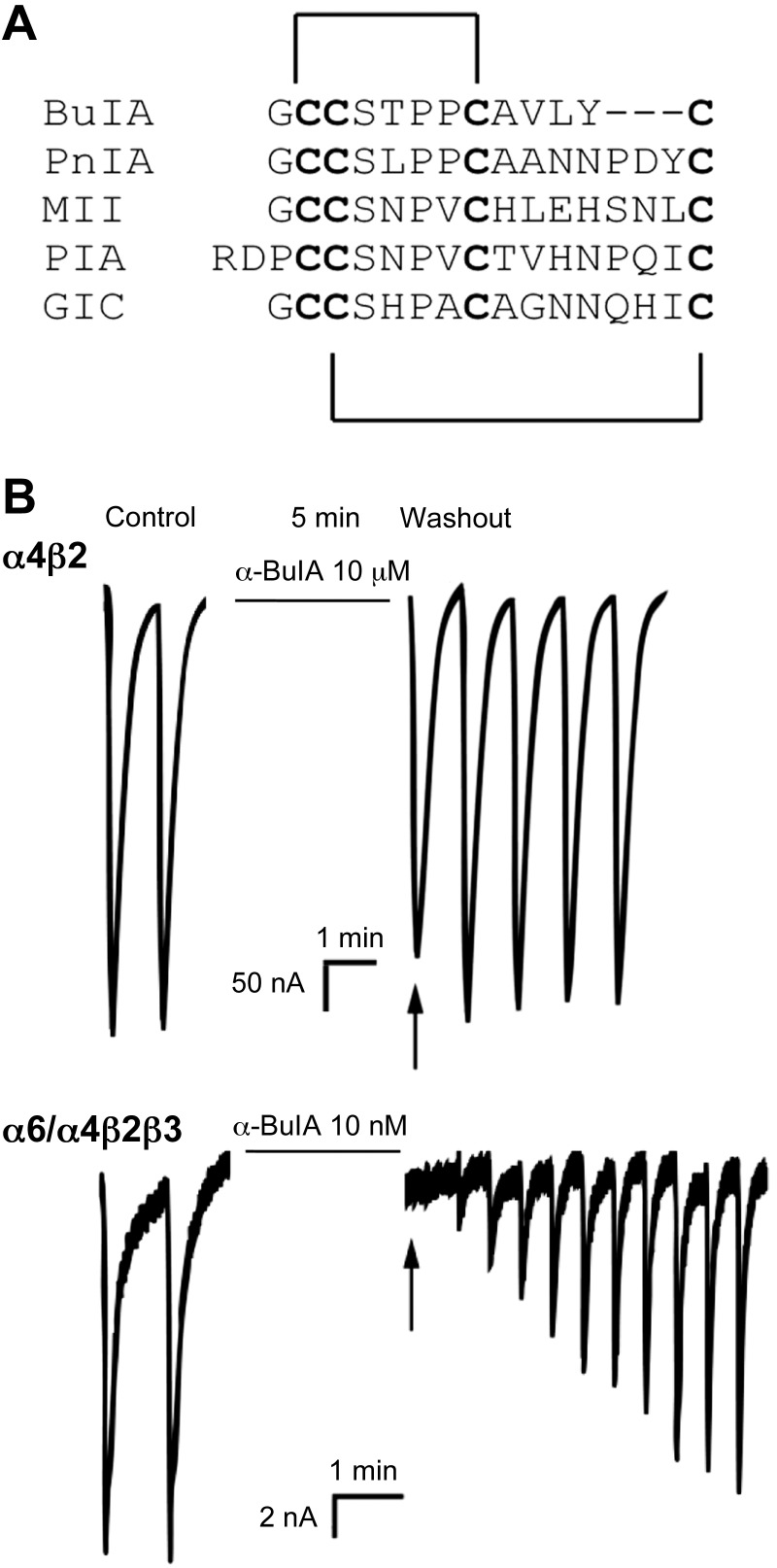
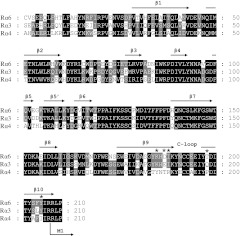
BuIA is a 2-disulfide bridge peptide (Fig. 1A) cloned from C. bullatus. BuIA was previously shown to bind to residues in the nAChR β subunit, allowing discrimination between β2* and β4* nAChRs (17). In the present study, BuIA was tested on the (α6/α4)β2β3 nAChR that contains the extracellular ligand binding domain of the α6 subunit (see Materials and Methods) and found to be highly potent (IC50=0.43 nM); in contrast, BuIA had little effect at concentrations tested on the major high-affinity nicotine-binding nAChR subtype α4β2 (IC50>20 μM; Fig. 1B and Table 1). We also assessed the effect of BuIA on the (α6/α4)β2 receptor without the presence of the β3 subunit. Consistent with previous observations, we usually observed no functional expression. That is, the β3 subunit greatly increases functional expression of α6-containing receptors (21). However, occasionally, small ACh-evoked currents (∼2 nA) were observed. Functional expression of the (α6/α4)β2 nAChR was too low and too rare to construct a full-dose response curve. However, BuIA (10 nM) blocked 92.6 ± 2.6 of the current consistent with the IC50 observed for the (α6/α4)β2β3 nAChRs. Thus, the effect, if any, of the β3 subunit on BuIA binding appears to be modest. Thus, BuIA also has the capacity to distinguish between ligand binding sites from different nAChR α subunits at an interface with the β3 subunit. As expected, the binding determinants lie in the extracellular ligand binding domain. In this N-terminal ligand binding region, α6 and α4 subunits display 77% amino acid sequence similarity (Fig. 2).
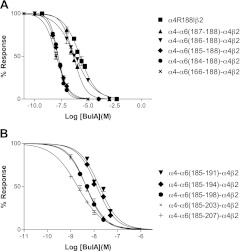
Figure 1.
BuIA differentially blocks α4β2 and (α6/α4)β2β3 nAChRs. A) Peptide sequence alignment of α-conotoxins. Disulfide connectivity shown. Note the differences in amino acids between cysteines among the toxins. B) Oocytes expressing α4β2 nAChRs (top panel) and (α6/α4)β2β3 nAChRs (bottom panel) were voltage clamped at −70 mV and exposed to a 1-s pulse of 100 μM ACh 1×/min as described in Materials and Methods. In each series, the first 2 responses are controls; each oocyte was then incubated in a solution containing the indicated concentration of BuIA for 5 min. Toxin was then washed out, and responses to ACh were again measured. BuIA was more potent in blocking (α6/α4)β2β3 than α4β2 nAChRs (note 1000-fold difference in toxin-concentration). Block by BuIA of (α6/α4)β2β3 nAChRs was more slowly reversible than block of α4β2 nAChRs.
Table 1.
Effect of BuIA on α4–α6 chimeric nAChRs
| nAChR | IC50 (nM) | CI (nM) | ACh-evoked current |
|---|---|---|---|
| α4β2 | >20,000 | +++ | |
| α4R188I β2 | 1,830 | 1400–2400 | ++ |
| α4-α6(187–188)-α4β2 | 441 | 349–556 | ++ |
| α4-α6(186–188)-α4β2 | 636 | 498–813 | ++ |
| α4-α6(185–188)-α4β2 | 15.7 | 14.1–17.5 | ++ |
| α4-α6(184–188)-α4β2 | 15.9 | 14.7–17.1 | ++ |
| α4-α6(185–191)-α4β2 | 16.9 | 13.8–20.8 | + |
| α4-α6(185–194)-α4β2 | 13.0 | 11.2–15.1 | + |
| α4-α6(166–188)-α4β2 | 11.0 | 9.10–13.2 | ++ |
| α4-α6(185–198)-α4β2 | 4.49 | 4.06–4.96 | + |
| α4-α6(185–203)-α4β2 | 4.64 | 4.20–5.11 | + |
| α4-α6(185–207)-α4β2 | 2.13 | 1.82–2.48 | + |
| α4-α6(184–207)-α4β2 | 1.98 | 1.67–2.31 | + |
| α4-α6(166–207)-α4β2 | 2.49 | 2.37–2.63 | ++ |
| α4-α6(100–207)-α4β2 | 2.24 | 1.91–2.62 | + |
| α4-α6(59–207)-α4β2 | 1.90 | 1.66–2.17 | ++ |
| (α6/α4)β2β3 | 0.43 | 0.26–0.71 | + |
IC50, concentration of toxin that produced 50% block; CI, 95% confidence interval. ACh-evoked current indicates the amount of current evoked by ACh in an oocyte expressing the indicated receptor. +, usually <200 nA; ++, usually 200–2000 nA; +++, usually >2000 nA.
Figure 2.
Amino acid sequence comparison of the extracellular domain of nAChR α subunits. Amino acid sequences of the extracellular domain of rat α3, α4, and α6 nAChR subunits are aligned. Solid arrows indicate the corresponding β-sheet structure; the C-loop region is shown connecting 2 β-sheet structures. Asterisks indicate amino acid residues that influenced BuIA binding.
Many N-terminal α6 subunit chimeras have no or poor levels of functional expression when coinjected with the β2 subunit
To ascertain the residues that confer higher affinity for the α6 vs. α4 subunit, a series of chimeras were made that had progressively longer amounts of N-terminal α6 subunit sequence inserted into the corresponding region of the α4 subunit. These chimeras were coinjected with the β2 subunit in Xenopus oocytes and the response to an ACh was assessed. Most chimeric α subunits produced little or no current in response to ACh when expressed with β2 subunits; these same chimeric α subunits did produce functional channels with β4 subunits (data not shown). The chimeras that, when coinjected with β2, did not show a response to ACh included α6-42-α4; α6-139-α4, and α6-202-α4. Interestingly, α6-22- α4 was functionally expressed with β2, indicating that some of the rat α6 residues between position 22 and 42 are responsible for inhibiting functional expression. These results are consistent with a previous report that a human (α6/α4) chimeric nicotinic receptor, in which the extracellular domain was α6 and the remainder α4, did not functionally express with human β2 but did express with human β4 subunits both in Xenopus oocytes and human embryonic kidney (HEK)-293 cells (32).
Residues 184-207 of the nAChR α6 subunit confer high affinity for the binding of BuIA
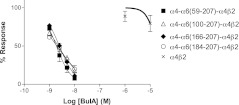
To overcome functional expression difficulties, we instead replaced amino acids of the α4 subunit with corresponding α6 residues beginning with residue 59. Each of these chimeras produced functional channels when expressed with the β2 subunit, and the IC50 of BuIA was assessed. BuIA potently blocked receptors formed by α4-α6(59-207)-α4β2, where α4 residues 59-207 were replaced by α6 residues. BuIA also blocked, with approximately equivalent potencies, chimeras formed with progressively fewer substituted α6 residues, including the chimera where only α6 residues 184-207 were replaced (Fig. 3 and Table 1). This suggests that residues downstream of residue 184 in the α6 subunit are primarily responsible for high BuIA potency at the α6 compared to the α4 nAChR ligand-binding domain.
Figure 3.
Concentration-response analysis of BuIA on rat chimeric α4 nAChRs. Rat α4 sequences were replaced by corresponding to α6 sequences. Note that α4-α6(184-207)-α4β2 has ∼7700-fold higher affinity than α4β2 nAChR.
Residues within the α6 sequence segment 184-188 confer high discrimination for nAChR subunit α6 vs. α4
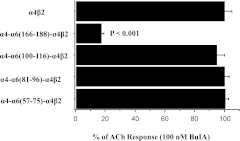
To determine the microdomain of the α6 sequence that confers high potency for BuIA, a series of rat (α6/α4) chimeric subunits were constructed in which small-sequence segments of α6 were used to replace α6 residues. After comparison of amino acid sequence similarity, we identified 4 regions that may affect binding affinity. Chimeras in which α4 residues 57-75, 81-96, or 100-116 were replaced with α6 residues and coexpressed with the rat β2 subunit were not blocked by 100 nM BuIA. In contrast, 100 nM BuIA blocked the ACh response of the α4-α6(166-188)-α4β2 nAChR (Fig. 4). Concentration-response analysis indicated that α4-α6(166-188)-α4β2 was ∼1,800-fold more sensitive to BuIA than wild-type α4β2 nAChRs. (Fig. 5 and Table 1). In addition, BuIA blocked the α4-α6(184-188)-α4β2 nAChR with a potency that was similar to that of α4-α6(166-188)-α4β2 (Fig. 5A and Table 1). Thus, the amino acids within the 184-188 region are critical with respect to differentiating the ligand binding site of α6 vs. α4 nAChR subunits.
Figure 4.
BuIA IC50 values for receptors formed by chimeric α subunits. Chimeric rat α subunits were constructed from α6 and α4 sequences. Responses to 100 μM ACh were measured after a 5-min application of 100 nM BuIA. Responses of the chimeras are normalized to the wild-type α4β2. Note that substitution of α4 residues 166-188 into the α6 subunit increased affinity for BuIA.
Figure 5.
Concentration-response analysis of BuIA on rat chimeric α4 nAChRs. A) Series of chimeras were constructed to test the influence of α6 residues 184-188. Note that α4-α6(185-188)α4β2 was 950-fold more sensitive than α4β2 to block by BuIA. B) Chimeras with further extension of the α6 sequence within the 185-207 region were tested. Note that T198 and Y205 residues of the α6 subunit also influence binding to BuIA.
Three critical residues within the 184-188 region
All 5 amino acid residues are different between the α6 and α4 subunits within the microdomain of residues 184-188 (Fig. 2). Therefore, we tested BuIA on a chimera in which these 5 α6 residues were substituted into the homologous region of the α4 subunit at position 184-188. First, we constructed a point mutation at position 188 and found that BuIA blocked α4R188Iβ2 ∼16-fold more potently than wild-type α4β2, indicating that I188 of the α6 subunit is important for binding (Table 1). We then produced additional chimeras that extended the mutations, residue by residue, back to position 184. The α4-α6(187-188)-α4β2 chimera decreased the IC50 value relative to wild-type α4β2 by 68-fold indicating D187 is also important for interacting with BuIA. α4-α6(186-188)-α4β2 produced no further change in toxin potency; in contrast α4-α6(185-188)α4β2 was 950-fold more sensitive to block by BuIA, indicating the importance of K185. Extension of the chimera to position 184, α4-α6(184-188)-α4, produced no further change in toxin potency.
Molecular modeling
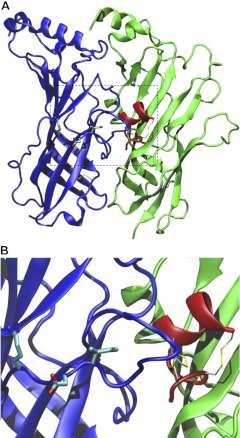
The acetylcholine binding protein serves as a surrogate for the extracellular ligand-binding domain of the nAChR. The AChBP can be expressed and purified in quantities that allow X-ray crystallography studies. Several conotoxins have been cocrystalized with the AChBP, including BuIA (33–36). We used the cocrystal structure of BuIA together with the 4-Å resolution structure of the membrane-associated Torpedo nAChR (22) to create a homology model of BuIA bound to an α6β2 nAChR interface (Fig. 6A). The triad of α6 residues Lys185, Asp187, and Ile188 appear to be located at the end of the C loop and do not contact BuIA (Fig. 6A). Rather, it is the tip of the C loop that may make direct contact with the toxin. In addition, the distance between the toxin and the triad is > 10 Å (Fig. 6B). These results suggest that the critical 3 residues of the α6 subunit do not interact with BuIA directly. Instead, structural alterations in the binding pocket formed by the C loop may explain the difference in BuIA potency.
Figure 6.
Structural model of the pentameric toxin-bound rat α4-α6(185-188)-α4β2 Extracellular domain is represented by ribbon in top view (A; viewed from the extracellular side to the intracellular side of the receptor) and side view (B; viewed parallel to the membrane surface). The α subunit is shown in blue. BuIA, represented by red ribbon, is located next to the α subunit. Conserved disulfide bonds and α6 residues (K185, D187, I188) are represented by thin and thick sticks, respectively. In the stick representation, carbon, oxygen, nitrogen, and sulfur atoms are in cyan, red, blue, and yellow, respectively. Structural model was taken after 5-ns equilibration and was visualized by Visual Molecular Dynamics.
α6 residues C-terminal to residue 188 also contribute to toxin potency
Although the residues in the region of 185-188 alter toxin potency by 950-fold, these residues do not fully account for the difference in potency between the (α6/α4)β2β3 and α4β2 nAChRs. Therefore, we substituted additional extracellular α6 sequences downstream of residue 188 since the α4-α6(184-207)-α4 chimeric subunit had ∼8-fold higher BuIA affinity (IC50=1.98 nM) than the α4-α6(185-188)-α4 subunit (IC50=15.9 nM), suggesting that the region between residues 189-207 also contributed to toxin potency. Four additional chimeric α subunits α4-α6(185-191)-α4, α4-α6(185-194)-α4, α4-α6(185-198)-α4, and α4-α6(185-203)-α4 were constructed and tested (Fig. 5B). Among them α4-α6(185-198)-α4β2 significantly shifted the IC50 value down to 4.5 nM compared with ∼16 nM for α4-α6(185-191)-α4β2 and α4-α6(185-194)-α4β2 (Fig. 5B). This result suggests that T198 also plays an important role for binding with BuIA. In addition, all 5 toxin chimeras that extend to residue 207 had an approximately 2-fold lower IC50 than α4-α6(185-203)-α4 (Table 1), indicating that Y205 also influences BuIA binding.
T198 also contributes to the difference in BuIA binding to α6 vs. α3 subunits
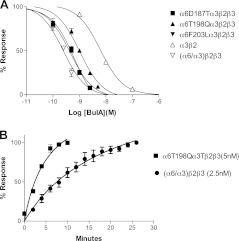
T198 has also been implicated in the binding of an analog of α-conotoxin MII, MII[S4A, E11A, L15A], which is selective for (α6/α3)β2β3 vs. α3β2 nAChRs (19). BuIA shows modest selectivity for (α6/α3)β2β3 vs. α3β2; therefore, we examined whether T198 played a role in this selectivity difference. Amino acid alignment shows that in the region 184-207, only 3 amino acids differ between rat α6 and α3 nAChR subunits. Therefore, we constructed and tested point mutants in which α6 residues were substituted for the corresponding α3 residues in this region. The α6T198Qα3 subunit had an ∼2-fold decrease of toxin IC50 (Fig. 7A and Table 2), whereas α6D187Eα3 and α6F203Lα3 did not show a significant change in IC50. In addition, the α6T198Qα3 mutant had a significantly faster off-rate than (α6/α3)β2β3 (Fig. 7B). Thus, as with α-conotoxin MII[S4A, E11A, L15A], T198 of the α6 subunit appears to be important in the BuIA preference for the α6 vs. α3 ligand-binding domain.
Figure 7.
Concentration-response analysis of BuIA on point mutants of α6/α3 nAChRs. A) There are 3 amino acid differences between rat α6 and α3 within the 184-203 regions. To investigate how these differences affect toxin binding, 3 corresponding point mutants were constructed and expressed in Xenopus oocytes coinjected with rat β2 and β3 subunits. Responses to a 1-s pulse of ACh (100 μM) with and without perfused toxin were measured. Among the 3-point mutants, (α6T198Qα3)β2β3 had a higher BuIA IC50 compared to wild-type (α6/α3)β2β3. B) Comparison of kinetics of unblock by BuIA. Recovery from block was measured by recording the response to a 1-s pulse of ACh (100 μM) every 2 min after 90% block by 5-min perfusion of toxin. Note that BuIA has a ∼5-fold faster off-rate from the (α6T198Qα3)β2β3 mutant. ACh responses were calculated as a percentage of control.
Table 2.
Effect of BuIA on α6–α3 chimeric nAChRs
| nAChR | IC50 (nM) | CI (nM) | ACh-evoked current |
|---|---|---|---|
| (α6/α3)β2β3 | 0.29 | 0.19–0.39 | + |
| (α6D187Eα3)β2β3 | 0.41 | 0.33–0.49 | + |
| (α6T198Qα3)β2β3 | 0.98 | 0.82–1.18 | + |
| (α6F203Lα3)β2β3 | 0.42 | 0.33–0.53 | + |
| α3β2 | 2.28 | 0.84–6.14 | ++ |
IC50, concentration of toxin that produced 50% block; CI, 95% confidence interval. ACh-evoked current indicates denotes the amount of current evoked by ACh in an oocyte expressing the indicated receptor. +, usually <200 nA; ++, usually 200–2000 nA; +++, usually >2000 nA.
DISCUSSION
In this study, we showed that BuIA blocks (α6/α4)β2β3 nAChRs with high potency, IC50 = 0.43 nM. In contrast, BuIA has ∼50,000-fold less activity at α4β2 nAChRs (IC50>20 μM). Using subunit chimeras and point mutations, we identified the amino acid residues that confer BuIA selectivity for the α6 vs. α4 extracellular ligand-binding site. We determined that the region 185-205 of the α6 subunit, which contains the nAChR β9, C-loop, and β10 region, is primarily responsible for the interaction with BuIA. Five amino acid residues (K185, T187, I188, T198, and Y205) were identified as critical for selective BuIA binding.
Conus is a genus of predatory mollusks that envenomates marine organisms, including vertebrate fish. The selectivity of conotoxins has led to their investigation as novel therapeutics (37, 38). One peptide, ziconotide, has become a U.S. Food and Drug Administration-approved drug for treatment of chronic pain (39). Among the manifold venom components, peptides acting on nAChRs are prominent and used to disable or immobilize prey (40). The ∼500 species of Conus feed on organisms from 5 different phyla. This extensive heterogeneity likely helps explain the diversity of nAChR subtypes targeted by Conus toxins. Though we do not view BuIA as a drug candidate, understanding of structure activity relationships of conotoxins, in general, can lead to further modifications that imbue drug-like properties, such as oral bioavailability (41–44).
ACh acts at the N-terminal extracellular portions of α and β subunits that form a ligand-binding interface. Competitive antagonists, such as α-conotoxins, prevent ACh from binding, generally by interacting with both nAChR α and β subunits (45, 46). In this report, we show that BuIA selectively binds the α6β2 interface vs. α4β2 interface. By use of an α6/α4 chimera that differs from the α4 subunit only in the extracellular ligand binding domain, BuIA was shown to block the α6-containing chimeric receptor with a subnanomolar IC50 and with a potency 4 orders of magnitude greater compared to the α4β2 nAChR. A critical binding segment was determined by using chimeras with increasingly smaller portions of the α6 subunit. Point mutagenesis was used to identify 5 residues in the α6 subunit β9 and β10 strands that flank the C loop and determine BuIA selectivity (Fig. 2). The combined effect of Lys185, Asp187, Ile188, Thr198, and Tyr205 of the α6 subunit accounts for ∼11,000-fold lower IC50 than the wild-type α4β2 nAChR.
The difference between the IC50 of the α4-α6(185-207)-α4β2 nAChR (2.1 nM) and the (α6/α4)β2β3 nAChR that contains the entire α6 N-terminal extracellular region (0.43 nM) may be due to the aggregate effect of residues N-terminal of position 185 in the α-subunit. Alternatively, the difference may be due to allosteric effects of the structural β3 subunit necessary for functional expression of the α6/α4 chimera.
The nAChR α3 subunit closely resembles the α6 subunit. An analog of α-conotoxin MII, MII [S4A, E11A, L15] selectively binds the α6 vs. α3 subunit by ∼1000-fold (47). Three residues were determined to be critical for this selectivity, namely, Glu152, Asp187, and Thr198 (19). Interestingly, as with BuIA, the majority of residues important for MII [S4A, E11A, L15] selectivity reside in the β9 strand. Thus, the β9 strand may be particularly important for subtype discrimination by α-conotoxins. In contrast, E155 of the β8 strand is important for MII [S4A, E11A, L15] selectivity for α6 vs. α3 subunits. E155 of α6 is substituted by Lys in α3. However, Lys is also present in the homologous position of the α4 subunit, yet the Glu to Lys substitution is not important for α6 vs. α4 selectivity by BuIA. D187 of the α6 subunit is replaced by Glu in the α3 subunit, contributing to selectivity of α6 vs. α3 by MII[S4A, E11A, L15]. Thr is present in the homologous position of the α4 subunit and contributes to BuIA selectivity for α6 vs. α4.
α6 T198 is important for MII[S4A, E11A, L15] discrimination of α6 vs. α3, and likewise also contributes to BuIA discrimination of α6 vs. α4. BuIA showed some preference for 6 α vs. 3α (∼8 fold). Therefore, we tested whether T198 was also important for this discrimination and found that Gln substitution as found in the α3 subunit decreased BuIA potency. The difference in affinity is largely accounted for by a difference in off-rate (Fig. 6B). Thus, T198 appears to be common denominator in α-conotoxin subtype discrimination of nAChR α-subunits. Y205 is located far from the ligand binding pocket near the boundary with the transmembrane region MI. Thus, the effect on potency by this residue is very likely indirect. It is possible that the hydrophobic bulky side chain of Y205 also affects C-loop topology. Both α6 and α3 subunits have this Tyr, while BuIA-insensitive α4 and α2 subunits have Ile or Val, respectively.
When acetylcholine agonists bind the ligand-binding interface, a conformational change in the receptor is propagated to the transmembrane region of the nAChR leading to channel opening. A molluskan protein that binds ACh forms a pentamer with striking homology to the N-terminal ligand-binding domain of the nAChR. Crystallographic studies of AChBP have provided surrogate structures for understanding nAChR structure (34). When the structures of AChBP bound to nicotinic agonist are compared to the apo AChBP, it is evident that large conformational changes occur in the C loop (34, 48). When agonist is bound, the C loop moves ∼4 Å to close the ligand-binding pocket. In contrast, when α-conotoxins are bound to the AChBP, the C loop opens wider than found in the apo form (34, 35). Molecular dynamics modeling suggests that that nAChRα subunit β9 and β10 strands slide in response to C-loop closure and ultimately contribute to motion in the M2-M3 linker and subsequent channel opening (49). Three of the critical α6 residues for BuIA selectivity are present in the α subunit β9 strand, and 2 are located in the β10 strand (Fig. 2). The identified residues in the α6 vs. α4 β9 and β10 strands may confer subtle differences to the geometry of the C loop that consequently affect BuIA binding. Homology modeling of BuIA bound to the α6β2 interface is consistent with this interpretation.
Discriminating between α4 and α6 ligand-binding interfaces has pathophysiological implications. α4 and α6 nAChR subunits are implicated in the molecular mechanisms of smoking addiction and Parkinson's disease. Intriguingly, in both of these disorders, α4β2* and α6β2* nAChRs are differentially affected by exogenous insult. In the case of nicotine exposure, the expression α4β2* nAChRs is substantially up-regulated in response to the addicting alklyloid nicotine found in tobacco products (50). In contrast, α6β2* nAChRs down-regulate in response to nicotine (51). In animal models of Parkinson's disease, MPTP exposure leads to selective loss of α6β2* vs. α4β2* nAChRs in dopaminergic terminals (51, 52). Likewise, in human Parkinson's disease, there is early loss of α6β2* vs. α4β2* nAChRs in the striatum (53, 54). Nicotinic agonists devoid of toxic side effects are being sought as treatment options for smoking cessation and Parkinson's disease. Selectively targeting the α6 nAChR is one consideration.
In summary, BuIA has high discrimination for α6 vs. α4 nAChR subunits. Five residues that account for the selectivity of BuIA for the α6 vs. α4 subunit have been identified. Although α-conotoxins are competitive antagonists, all but T198 of the identified nAChR residues lie sufficiently far from the BuIA ligand binding site that they are unlikely to make direct interaction with BuIA. Instead, the data indicate that the exquisite selectivity of BuIA is achieved by exploiting differences in sequence strands that border the critical C loop.
Acknowledgments
Coordinates for the crystal structure were generously provided by T. Talley (Idaho State University), Albert S. Reger and Choel Kim (Baylor College of Medicine, Houston, TX, USA), and Palmer Taylor (University of California, San Diego, CA, USA). Molecular model was constructed by Myunggi Yi (Pukyong National University).
This work was supported by the U.S. National Institutes of Health, grants GM103801 and GM84677.
Footnotes
- ACh
- acetylcholine
- AChBP
- acetylcholine binding protein
- BuIA
- α-conotoxin BuIA
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- nAChR
- nicotinic acetylcholine receptor
REFERENCES
- 1. Albuquerque E. X., Pereira E. F., Alkondon M., Rogers S. W. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le Novere N., Corringer P. J., Changeux J. P. (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456 [DOI] [PubMed] [Google Scholar]
- 3. Le Novere N., Zoli M., Changeux J. P. (1996) Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur. J. Neurosci. 8, 2428–2439 [DOI] [PubMed] [Google Scholar]
- 4. Han Z. Y., Le Novere N., Zoli M., Hill J. A., Jr., Champtiaux N., Changeux J. P. (2000) Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur. J. Neurosci. 12, 3664–3674 [DOI] [PubMed] [Google Scholar]
- 5. Quik M., Polonskaya Y., Gillespie A., Jakowec M., Lloyd G. K., Langston J. W. (2000) Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J. Comp. Neurol. 425, 58–69 [DOI] [PubMed] [Google Scholar]
- 6. Azam L., Winzer-Serhan U. H., Chen Y., Leslie F. M. (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol. 444, 260–274 [DOI] [PubMed] [Google Scholar]
- 7. Quik M., Polonskaya Y., Kulak J. M., McIntosh J. M. (2001) Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. J. Neurosci. 21, 5494–5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bordia T., Grady S. R., McIntosh J. M., Quik M. (2007) Nigrostriatal damage preferentially decreases a subpopulation of α6β2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol. Pharmacol. 72, 52–61 [DOI] [PubMed] [Google Scholar]
- 9. Quik M., McIntosh J. M. (2006) Striatal α6* nicotinic acetylcholine receptors: potential targets for Parkinson's disease therapy. J. Pharmacol. Exp. Ther. 316, 481–489 [DOI] [PubMed] [Google Scholar]
- 10. McCallum S. E., Parameswaran N., Bordia T., McIntosh J. M., Grady S. R., Quik M. (2005) Decrease in alpha3*/alpha6* nicotinic receptors but not nicotine-evoked dopamine release in monkey brain after nigrostriatal damage. Mol. Pharmacol. 68, 737–746 [DOI] [PubMed] [Google Scholar]
- 11. Yang K. C., Jin G. Z., Wu J. (2009) Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology. Acta Pharmacol. Sin. 30, 740–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pons S., Fattore L., Cossu G., Tolu S., Porcu E., McIntosh J. M., Changeux J. P., Maskos U., Fratta W. (2008) Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 28, 12318–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson K. J., McIntosh J. M., Brunzell D. H., Sanjakdar S. S., Damaj M. I. (2009) The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J. Pharmacol. Exp. Ther. 331, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brunzell D. H., Boschen K. E., Hendrick E. S., Beardsley P. M., McIntosh J. M. (2010) alpha-Conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology 35, 665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Exley R., Maubourguet N., David V., Eddine R., Evrard A., Pons S., Marti F., Threlfell S., Cazala P., McIntosh J. M., Changeux J. P., Maskos U., Cragg S. J., Faure P. (2011) Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc. Natl. Acad. Sci. U. S. A. 108, 7577–7582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azam L., McIntosh J. M. (2009) Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 30, 771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azam L., Dowell C., Watkins M., Stitzel J. A., Olivera B. M., McIntosh J. M. (2005) Alpha-conotoxin BuIA, a novel peptide from Conus bullatus, distinguishes among neuronal nicotinic acetylcholine receptors. J. Biol. Chem. 280, 80–87 [DOI] [PubMed] [Google Scholar]
- 18. Shiembob D. L., Roberts R. L., Luetje C. W., McIntosh J. M. (2006) Determinants of alpha-conotoxin BuIA selectivity on the nicotinic acetylcholine receptor beta subunit. Biochemistry 45, 11200–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azam L., Yoshikami D., McIntosh J. M. (2008) Amino acid residues that confer high selectivity of the alpha6 nicotinic acetylcholine receptor subunit to alpha-conotoxin MII[S4A,E11A,L15A]. J. Biol. Chem. 283, 11625–11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papke R. L., Dwoskin L. P., Crooks P. A., Zheng G., Zhang Z., McIntosh J. M., Stokes C. (2008) Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology. 54, 1189–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuryatov A., Olale F., Cooper J., Choi C., Lindstrom J. (2000) Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology 39, 2570–2590 [DOI] [PubMed] [Google Scholar]
- 22. Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 23. Dowell C., Olivera B. M., Garrett J. E., Staheli S. T., Watkins M., Kuryatov A., Yoshikami D., Lindstrom J. M., McIntosh J. M. (2003) Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J. Neurosci. 23, 8445–8452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cartier G. E., Yoshikami D., Gray W. R., Luo S., Olivera B. M., McIntosh J. M. (1996) A new alpha-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J. Biol. Chem. 271, 7522–7528 [DOI] [PubMed] [Google Scholar]
- 25. Luo S., Kulak J. M., Cartier G. E., Jacobsen R. B., Yoshikami D., Olivera B. M., McIntosh J. M. (1998) alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J. Neurosci. 18, 8571–8579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo S., McIntosh J. M. (2004) Iodo-α-conotoxin MI selectively binds the α/δ subunit interface of muscle nicotinic acetylcholine receptors. Biochemistry. 43, 6656–6662 [DOI] [PubMed] [Google Scholar]
- 27. Arnold K., Bordoli L., Kopp J., Schwede T. (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 [DOI] [PubMed] [Google Scholar]
- 28. Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kale L., Schulten K. (2005) Scalable molecular dynamics with NAMD. J. Comp. Chem. 26, 1781–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mackerell A. D., Jr., Feig M., Brooks C. L., 3rd (2004) Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comp. Chem. 25, 1400–1415 [DOI] [PubMed] [Google Scholar]
- 30. MacKerell A. D., Bashford D., Bellott, Dunbrack R. L., Evanseck J. D., Field M. J., Fischer S., Gao J., Guo H., Ha S., Joseph-McCarthy D., Kuchnir L., Kuczera K., Lau F. T. K., Mattos C., Michnick S., Ngo T., Nguyen D. T., Prodhom B., Reiher W. E., Roux B., Schlenkrich M., Smith J. C., Stote R., Straub J., Watanabe M., Wiórkiewicz-Kuczera J., Yin D., Karplus M. (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 [DOI] [PubMed] [Google Scholar]
- 31. Humphrey W., Dalke A., Schulten K. (1996) VMD–visual molecular dynamics. J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
- 32. Evans N. M., Bose S., Benedetti G., Zwart R., Pearson K. H., McPhie G. I., Craig P. J., Benton J. P., Volsen S. G., Sher E., Broad L. M. (2003) Expression and functional characterisation of a human chimeric nicotinic receptor with α6β4 properties. Eur. J. Pharmacol. 466, 31–39 [DOI] [PubMed] [Google Scholar]
- 33. Talley T. T., Olivera B. M., Han K. H., Christensen S. B., Dowell C., Tsigelny I., Ho K. Y., Taylor P., McIntosh J. M. (2006) Alpha-conotoxin OmIA is a potent ligand for the acetylcholine-binding protein as well as α3β2 and α7 nicotinic acetylcholine receptors. J. Biol. Chem. 281, 24678–24686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansen S. B., Sulzenbacher G., Huxford T., Marchot P., Taylor P., Bourne Y. (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 24, 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Celie P. H., Kasheverov I. E., Mordvintsev D. Y., Hogg R. C., van Nierop P., van Elk R., van Rossum-Fikkert S. E., Zhmak M. N., Bertrand D., Tsetlin V., Sixma T. K., Smit A. B. (2005) Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an alpha-conotoxin PnIA variant. Nat. Struct. Mol. Biol. 12, 582–588 [DOI] [PubMed] [Google Scholar]
- 36. Dutertre S., Ulens C., Buttner R., Fish A., van Elk R., Kendel Y., Hopping G., Alewood P. F., Schroeder C., Nicke A., Smit A. B., Sixma T. K., Lewis R. J. (2007) AChBP-targeted alpha-conotoxin correlates distinct binding orientations with nAChR subtype selectivity. EMBO J. 26, 3858–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halai R., Craik D. J. (2009) Conotoxins: natural product drug leads. Nat. Prod. Rep. 26, 526–536 [DOI] [PubMed] [Google Scholar]
- 38. Lewis R. J. (2009) Conotoxin venom peptide therapeutics. Adv. Exp. Med. Biol. 655, 44–48 [DOI] [PubMed] [Google Scholar]
- 39. Schmidtko A., Lotsch J., Freynhagen R., Geisslinger G. (2010) Ziconotide for treatment of severe chronic pain. Lancet 375, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 40. Olivera B. M., Quik M., Vincler M., McIntosh J. M. (2008) Subtype-selective conopeptides targeted to nicotinic receptors: Concerted discovery and biomedical applications. Channels (Austin) 2, 143–152 [DOI] [PubMed] [Google Scholar]
- 41. Clark R. J., Jensen J., Nevin S. T., Callaghan B. P., Adams D. J., Craik D. J. (2010) The engineering of an orally active conotoxin for the treatment of neuropathic pain. Angew. Chem. Int. Ed. Engl. 49, 6545–6548 [DOI] [PubMed] [Google Scholar]
- 42. Kasheverov I. E., Utkin Y. N., Tsetlin V. I. (2009) Naturally occurring and synthetic peptides acting on nicotinic acetylcholine receptors. Curr. Pharm. Des. 15, 2430–2452 [DOI] [PubMed] [Google Scholar]
- 43. Muttenthaler M., Nevin S. T., Grishin A. A., Ngo S. T., Choy P. T., Daly N. L., Hu S. H., Armishaw C. J., Wang C. I., Lewis R. J., Martin J. L., Noakes P. G., Craik D. J., Adams D. J., Alewood P. F. (2010) Solving the alpha-conotoxin folding problem: efficient selenium-directed on-resin generation of more potent and stable nicotinic acetylcholine receptor antagonists. J. Am. Chem. Soc. 132, 3514–3522 [DOI] [PubMed] [Google Scholar]
- 44. Luo S., Akondi K. B., Zhangsun D., Wu Y., Zhu X., Hu Y., Christensen S., Dowell C., Daly N. L., Craik D. J., Wang C. I., Lewis R. J., Alewood P. F., Michael McIntosh J. (2010) Atypical alpha-conotoxin LtIA from Conus litteratus targets a novel microsite of the alpha3beta2 nicotinic receptor. J. Biol. Chem. 285, 12355–12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mordvintsev D. Y., Polyak Y. L., Kuzmine D. A., Levtsova O. V., Tourleigh Y. V., Kasheverov I. E. (2006) A model for short alpha-neurotoxin bound to nicotinic acetylcholine receptor from Torpedo californica. J. Mol. Neurosci. 30, 71–72 [DOI] [PubMed] [Google Scholar]
- 46. Dutertre S., Ulens C., Buttner R., Fish A., van Elk R., Kendel Y., Hopping G., Alewood P. F., Schroeder C., Nicke A., Smit A. B., Sixma T. K., Lewis R. J. (2007) AChBP-targeted alpha-conotoxin correlates distinct binding orientations with nAChR subtype selectivity. EMBO J. 26, 3858–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McIntosh J. M., Azam L., Staheli S., Dowell C., Lindstrom J. M., Kuryatov A., Garrett J. E., Marks M. J., Whiteaker P. (2004) Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol. Pharmacol. 65, 944–952 [DOI] [PubMed] [Google Scholar]
- 48. Celie P. H., van Rossum-Fikkert S. E., van Dijk W. J., Brejc K., Smit A. B., Sixma T. K. (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 [DOI] [PubMed] [Google Scholar]
- 49. Cheng X., Wang H., Grant B., Sine S. M., McCammon J. A. (2006) Targeted molecular dynamics study of C-loop closure and channel gating in nicotinic receptors. PLoS Comput. Biol. 2, e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buisson B., Bertrand D. (2001) Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J. Neurosci. 21, 1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lai A., Parameswaran N., Khwaja M., Whiteaker P., Lindstrom J. M., Fan H., McIntosh J. M., Grady S. R., Quik M. (2005) Long-term nicotine treatment decreases striatal alpha 6* nicotinic acetylcholine receptor sites and function in mice. Mol. Pharmacol. 67, 1639–1647 [DOI] [PubMed] [Google Scholar]
- 52. Quik M., Vailati S., Bordia T., Kulak J. M., Fan H., McIntosh J. M., Clementi F., Gotti C. (2005) Subunit composition of nicotinic receptors in monkey striatum: effect of treatments with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or L-DOPA. Mol. Pharmacol. 67, 32–41 [DOI] [PubMed] [Google Scholar]
- 53. Quik M., Bordia T., Forno L., McIntosh J. M. (2004) Loss of alpha-conotoxinMII- and A85380-sensitive nicotinic receptors in Parkinson's disease striatum. J. Neurochem. 88, 668–679 [DOI] [PubMed] [Google Scholar]
- 54. Gotti C., Moretti M., Bohr I., Ziabreva I., Vailati S., Longhi R., Riganti L., Gaimarri A., McKeith I. G., Perry R. H., Aarsland D., Larsen J. P., Sher E., Beattie R., Clementi F., Court J. A. (2006) Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer's disease, Parkinson's disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol. Dis. 23, 481–489 [DOI] [PubMed] [Google Scholar]