Abstract
Adenosine A2A receptor (A2AR) stimulation promotes wound healing and is required for the development of fibrosis in murine models of scleroderma and cirrhosis. Nonetheless, the role of A2AR in the formation of scars following skin trauma has not been explored. Here, we examined the effect of pharmacological blockade of A2AR, with the selective adenosine A2AR-antagonist ZM241385 (2.5 mg/ml), in a murine model of scarring that mimics human scarring. We found that application of the selective adenosine A2AR antagonist ZM241385 decreased scar size and enhanced the tensile strength of the scar. Within the scar itself, collagen alignment and composition (marked reduction in collagen 3), but not periostin, biglycan, or fibronectin accumulation, was improved by application of ZM241385. Moreover, A2AR blockade reduced the number of myofibroblasts and angiogenesis but not macrophage infiltration in the scar. Taken together, our work strongly suggests that pharmacological A2AR blockade can be used to diminish scarring while improving the collagen composition and tensile strength of the healed wound.—Perez-Aso, M., Chiriboga, L., Cronstein, B. N. Pharmacological blockade of adenosine A2A receptors diminishes scarring.
Keywords: ZM241385, collagen, splinted wounds
Cutaneous wound repair is a complex process involving blood clotting, inflammation, new tissue formation, and, ultimately, tissue remodeling. These processes are controlled by cell-cell and cell-matrix interactions and by a wide variety of different growth factors and cytokines (1). Wound healing can be divided into 3 overlapping phases: inflammation, proliferation, and scar maturation. Inflammation sets in within minutes of a skin injury. The proliferation phase proceeds typically over the next 5 to 14 d, when fibroblasts, macrophages, and vascular tissues coordinately enter the wound to begin formation of granulation tissue (2). Fibroblasts and myofibroblasts lay down collagen-rich connective tissue, and myofibroblasts also contribute to wound contraction. Finally, the healing wound enters a maturational phase, during which granulation tissue continues to be remodeled. During this phase, synthesis of structural proteins, such as collagen, continues to be elevated for 6 to 12 mo (3), although the scar only ever reaches, at most, 70% of the tensile strength of unwounded skin (4).
Collagen is the principal building block of connective tissue. In normal skin, collagen type I (Col1) and collagen type III (Col3) exist in a ratio of ∼4:1. In hypertrophic and immature scars, the percentage of Col3 may be as high as 33%, altering the Col1:Col3 ratio to 2:1 (5). By 3 wk after injury, although there is no net increase in collagen content, collagen fibers are remodeled into a more organized lattice structure, and the wound progressively continues to increase in tensile strength. The majority of the Col3 fibers laid down early in the healing process are replaced by Col1 (5).
Aside from the psychological and social detriments associated with prominent scars, there are many other problems with excessive scar tissue, including restricted joint mobility, impaired growth, and loss of organ function (6–7). Scar formation results from dysfunction in remodeling of the skin, resulting in excessive deposition and misalignment of extracellular matrix proteins (8). Scarring is a challenge to study, because animal models for scarring do not recapitulate the complex situation of human hypertrophic scars or keloids. The lack of insight into the pathophysiology of hypertrophic scar formation has been further promoted by the absence of a reliable animal model (9), and rodent models have been criticized because the major mechanism of wound closure is contraction, whereas in humans reepithelialization and granulation tissue formation are the major mechanisms (10). But recently, it has been shown that mechanical loading early in the proliferation phase of wound healing produces hypertrophic scars by inhibiting cellular apoptosis (11) and that splinting wounds in mice allows healing to occur by the processes of granulation and epithelialization, while minimizing the effects of contraction (10, 12).
Adenosine is present in most biological fluids and is elevated during tissue or organ stress, when it acts as a potent endogenous modulator of inflammation and tissue repair (13, 14). We have previously reported that adenosine activation of the adenosine A2A receptors (A2ARs), which belong to the superfamily of G-protein-coupled receptors (GPCRs), promotes dermal fibrosis, as both A2AR antagonism and knockdown protect mice from developing bleomycin-induced dermal fibrosis (15), and A2AR antagonism protects from dermal fibrosis in a model of elevated tissue adenosine (16). To our knowledge, the present work represents the first description of the role of A2AR in scar progression. Because hypertrophic scarring is characterized by fibroblast proliferation and transformation and by excessive deposition of extracellular matrix components (17, 18) and shares several other features of fibrosis (19), we hypothesized that A2AR blockade with highly selective and bioavailable nonxanthine selective A2AR antagonist ZM241385 (Supplemental Fig. S1 and refs. 20, 21) may reduce scar formation in healing wounds in mice that were splinted open with silicon stents to reduce wound contraction and permit healing by secondary intention (12). Here, we report that A2AR blockade prevents Col3 overproduction and misalignment, as well as excessive increase of myofibroblasts and angiogenesis, thereby ameliorating and reducing scar formation in a murine model of human wound healing.
MATERIALS AND METHODS
Wound model
C57/BL6J male 6-wk-old mice were anesthetized using an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The dorsal surface was shaved with an electric clipper followed by a depilatory agent. A doughnut-shaped splint fashioned from a 0.5-mm-thick silicone sheet (Grace Bio-Laboratories, Bend, OR, USA) was sutured so that the wound was centered within the splint. Two wounds were patterned with a sterile 12-mm punch biopsy tool on the dorsum of the mouse, one on each side of the midline, while the skin between both wounds was used as the unwounded control. Next, an occlusive dressing (Tegaderm; 3M, St. Paul, MN, USA) was placed to cover the wounds. To prevent mice from damaging the silicone ring, a 3-cm straw was glued to the lower part of the ring. The animals were housed individually in the institutional animal facility. Since wound closure in mice takes 12–15 d and there is a 10- to 14-d lag phase before tensile strength begins to rapidly increase (22), silicone rings were removed after 10 d. After 14 d, the A2AR antagonist or vehicle was applied every 2 d thereafter. For application of vehicle or active agent, the animals were anesthetized, and the left wound was topically treated with the vehicle (3% carboxymethylcellulose and 7.4% DMSO), while the right wound was treated with ZM241385 (2.5 mg/ml in vehicle), covering the wound with a bandage. When needed (3 times on average), the hair of the animals was removed by depilatory before treatment. The mice were euthanized 1 mo after the day of surgery. Scars were then excised, bisected, and fixed in 10% formalin to undergo routine histological processing, or homogenized to extract proteins and mRNA or for the hydroxyproline assay. All protocols were approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Scar image analysis
Scar size was determined from digital photographs taken with a ruler at the same level of the scar. The area was analyzed by tracing the margin with a fine-resolution computer and calculating pixel area using SigmaScan Pro Image Analysis 5.0.0 (Aspire Software International, Leesburg, VA, USA). The actual area in square millimeters was extrapolated from the millimeters per pixel determined by comparison to the ruler in the same photomicrograph.
Hydroxyproline assay
Tissue specimens were hydrolyzed in 12 N HCl at 120°C. Then, 200 μl of dextran-charcoal solution (1% charcoal and 0.05% dextran 500T) was mixed with 800 μl of water and centrifuged at 2000 rpm for 10 min; 100 μl of a 1:50 dilution of the supernatant was mixed with 250 μl of chloramine solution (1.3% chloramine-T, 10% propanol, and 80% citrate-acetate buffer) for 20 min at room temperature, followed by the addition of 250 μl Ehrlich solution and incubation at 60°C for 20 min. Absorbance was measured at 550 nm. Standard curves (0–100 μg/ml) were generated using reagent hydroxyproline as a standard. Results were expressed as micrograms of hydroxyproline per microgram of protein content measured in the same solution by UV absorbance at 280 nm.
Western blot analysis
Skin samples were dissected out and homogenized in T-PER extraction reagent (Thermo Scientific, Barrington, IL, USA) with protease and phosphatase inhibitors. Skin homogenates (15 μg) were subjected to Western blot analysis with the primary antibodies Col1 (1:500; Southern Biotechnology, Birmingham, AL, USA), Col3 (1:500; Southern Biotechnology) and β-actin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Quantitative RT-PCR
Total RNA was extracted and purified using the RNeasy fibrous tissue minikit (no. 74704; Qiagen, Valencia, CA, USA), according to manufacturer's protocol. Relative quantification of gene expression was performed using real-time RT-PCR on the Mx3005P real-time PCR system (Stratagene; Agilent Technologies, Santa Clara, CA, USA) with SYBR Green (600548; Agilent Technologies), according to the manufacturer's protocol. The following primers were used in real-time PCR amplification: actin, forward 5-TGCGTGACATCAAAGAGAAG-3 and reverse 5-CGGATGTCAACGTCACACTT-3; Col1, forward 5′-GACGCCATCAAGGTCTACTG-3′ and reverse 5′-ACGGGAATCCATCGGTCA-3′; and Col3, forward 5′-CTGTAACATGGAAACTGGGGAAA-3′ and reverse 5′-CCATAGCTGAACTGAAAACCACC-3′. mRNA abundance was determined relative to that of actin.
Morphometric dermal measurements
Skin thickness was measured using skin calipers on 12-mm punch biopsies obtained from the back. Dermal thickness was measured from hematoxylin and eosin (H&E) skin cross sections with the SigmaScan Pro 5 software. Breaking strength of the skin was measured using a forceps clamped to one side of the 12-mm skin biopsy and a tensiometer (Mark-10 Series EG Digital Force Gauge; Mark-10 Corp., Copiague, NY, USA) coupled to another forceps clamped to the furthermost extreme of the biopsy. The point of maximal stress before tearing of the biopsy was recorded.
Histology, immunocytochemistry, and image analysis
Paraffin sections were stained with H&E, picrosirius red (PSR; method of Puchtler; ref. 23) or immunohistochemistry with antibodies α-smooth muscle actin (α-SMA; 1:400; Abcam, Cambridge, MA, USA), periostin (1:200; Santa Cruz Biotechnology), biglycan (1:200; Abcam), fibronectin (1:200; Santa Cruz Biotechnology), CD-68 (1;10; Abd Serotech, Raleigh, NC, USA) or CD-31 (1:20; BD Pharmingen, San Diego, CA, USA). Endogenous peroxidase activity was blocked with hydrogen peroxide. Tissue sections were digested with alkaline endopeptidase for 12 min at 42°C, and primary antibodies were incubated overnight at room temperature. Primary antibody was detected with secondary anti-rabbit (Santa Cruz Biotechnology) or anti-rat (1:20; BD Pharmingen) followed by Fast 3′3′-diaminobenzidine (Sigma) development, with hematoxylin nuclear counterstaining. Appropriate negative controls were included with the study sections. Slides were acquired using a Leica SCN400 Digital Slide Scanner (Leica Microsystems, Wetzlar, Germany). Data were quantified by a second observer in a blinded fashion. For PSR, polarized images were collected with a Nikon DS-Fi1 camera (Nikon, Tokyo, Japan), and images were quantified with SigmaScan Pro 5 software. The same red (hue 0, saturation 84 to hue 20, saturation 100) or green (hue 11, saturation 1 to hue 80, saturation 25) color threshold was applied to sections of the same size for all the images, and the area for each color was then calculated and normalized to the sum areas for the same mouse.
Statistical analysis
Statistical differences were determined using t test or repeated-measures ANOVA carried out using GraphPad software (GraphPad, San Diego, CA, USA) on a PC. The α nominal level was set at 0.05 in all cases. A value of P < 0.05 was considered significant.
RESULTS
Pharmacological blockade of A2AR with ZM241385 improves the morphological characteristics of scarring
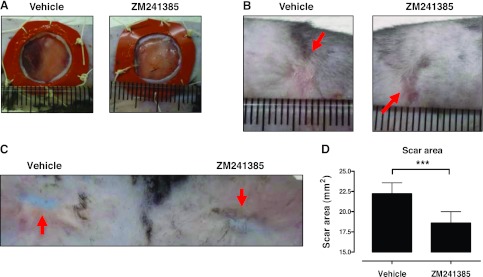
We have previously reported that A2AR activation promotes dermal fibrosis, as both A2AR antagonism and knockdown protect from bleomycin-induced dermal fibrosis (15), and A2AR antagonism protects from dermal fibrosis in a model of elevated tissue adenosine levels (16). However, the long-term consequences of A2AR blockade on scar formation have not yet been studied for lack of a reliable model. Recently, Galiano et al. (10) found that splinting full-thickness wounds in C57/BL6 mice with a silicone ring impedes contraction and allows healing to occur by new tissue deposition, resulting in scar formation, a condition that closely parallels human wound healing and scarring. We therefore created two full-thickness wounds of 12 mm diameter extending through the panniculus carnosus on the mouse dorsum, and contraction of the wound was blocked by suture of silicone splints to the edges of the wounds (Fig. 1A). As shown in Fig. 1B–D, topical application of the A2AR antagonist ZM241385, starting 2 wk after wounding, markedly reduced the area of the scar, as compared to those formed in untreated wounds in the same mice. In addition to diminishing the area of the scar, there was an increase in pigmented hair growth in the scars of the ZM241385-treated wounds (Fig. 1B, C). Interestingly, splinted wounds in mice lacking A2ARs do not heal (experiments terminated after 20 d; unpublished results), probably a result of poor granulation formation (24); thus, scarring could not be studied in these mice.
Figure 1.
Application of ZM241385 diminishes scar size. A) Wounds (12 mm) were splinted on the day of surgery, as described in Materials and Methods. B) Representative images of 1-mo scars (arrows) following ZM241385 or vehicle treatment starting at d 14. C) Representative images showing the dermal aspect of the scar (arrows). D) Quantification of scar area was performed as described in Materials and Methods. Data are expressed as means ± se; n = 30. ***P = 0.0005; 2-tailed paired t test.
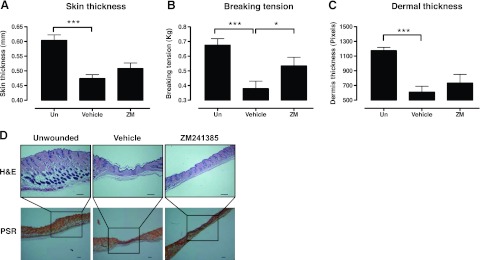
To further characterize the effect of the adenosine receptor antagonist on scar formation, we performed morphometric analyses of the scars. Skin thickness (Fig. 2A) was dramatically decreased in all of the scars, although thinning was slightly, but not significantly, prevented by A2AR blockade. Breaking tension (Fig. 2B) of the scars was determined as an indication of the tensile strength of the healed scars, and, when compared to the unwounded skin, scars had a dramatic decrease in tensile strength, a decrease that was significantly abrogated by treatment with ZM241385. Dermal thickness (Fig. 2C) was measured in tissue cross sections, and treatment with ZM241385 partially, but not significantly, prevented the decrease in dermal thickness observed in the scars. Scar location was ascertained in PSR-stained sections, as this histochemical stain of collagen shows different polarization colors depending on collagen fiber thickness and alignment (25).
Figure 2.
ZM241385 alters scar characteristics. A, B) Skin thickness (A) and breaking tension (B) were analyzed as described in Materials and Methods. C, D) Dermal thickness (C) was determined in cross sections of skin (D). Scale bars = 200 μm. Data represent means ± SE of 6–10 animals/condition. *P < 0.05, ***P < 0.001; ANOVA with Newman-Keuls posttest.
ZM241385 treatment prevents collagen accumulation and increases the Col1:Col3 ratio
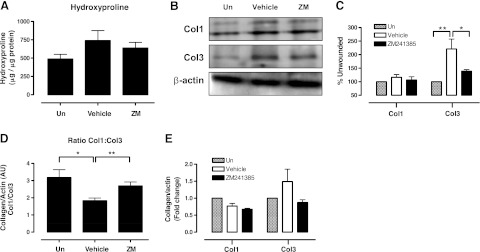
The ratio of Col1 to Col3 in normal skin is ∼4:1, but it diminishes to 2:1 in hypertrophic and immature scars as the Col3 percentage is increased; thus, the ratio of Col1 to Col3 may be used as an indicator of remodeling, scarring, and fibrosis (5). We performed a study of the collagen composition in the scars 1 mo after wound formation. We first measured the hydroxyproline content in the skin (total collagen content; Fig. 3A) and noted a modest increase in collagen content of scars when compared to unwounded skin, which was prevented by ZM241385. Similarly, there was a minimal increase in Col1 in scars, and ZM241385 reduced the Col1 content, although none of these differences was statistically significant (Fig. 3B, C). In contrast, Col3 was markedly increased in scars, and ZM241385 treatment significantly blocked the increase in Col3 in the scars. Thus, as described previously (5), the ratio of Col1:Col3 in normal, unwounded skin was 3.2 ± 0.5, which fell to 1.8 ± 0.2 in the untreated scars. The fall in the ratio of Col1:Col3 in scarred skin was almost completely reversed in the scars treated with the A2AR antagonist ZM241385, to 2.7 ± 0.2 (Fig. 3D). Total mRNA levels in dermis from these animals revealed minimal change in Col1 message in scars and a nonsignificant increase in Col3 mRNA in scars that was blocked in the ZM241385-treated scars (Fig. 3E).
Figure 3.
A2AR blockade prevents excessive accumulation of collagen 3 in the scar. A) Hydroxyproline levels in ZM241385-treated scars. B) Representative Western blot images of Col1 and Col3. C) Quantification of Western blots from Col1 and Col3 in scars and mean levels (after normalization to β-actin). *P < 0.05, **P < 0.01; ANOVA with Newman-Keuls posttest. D) Col1:Col3 ratio was calculated from collagen/β-actin arbitrary units. Statistical significance of differences was determined by ANOVA (P=0.022) and unpaired t test. *P < 0.05, **P < 0.01. E) mRNA levels of Col1 and Col3 analyzed by real time RT-PCR, normalized to actin levels. Data represent means ± se of determinations on 6–10 animals.
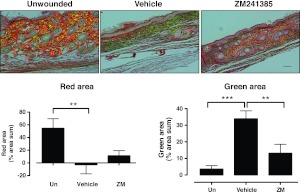
We further analyzed the collagen content of scars by histochemical staining with PSR, which, under polarizing microscopy, imparts intense yellow to red birefringence to thicker, more densely packed fibrils and weaker greenish birefringence, which is present in more loosely packed thin fibrils (26, 27). As shown in Fig. 4, untreated scars showed a predominantly weak and greenish birefringence, whereas there was a marked increase in red birefringence with a decrease in green birefringence in the ZM241385-treated cars. These findings are consistent with the observation that treatment of scars with an A2AR antagonist improves collagen quality in the scar by diminishing Col3 accumulation without significantly reducing Col1 content. The resulting dense collagen is stronger and more capable of withstanding trauma, as demonstrated by increased breaking tension.
Figure 4.
A2AR blockade prevents disordered collagen deposition in scars. After fixation and embedding in paraffin, sections were cut and stained with PSR staining, and the slide was reviewed in white light and in polarizing light, as described in Materials and Methods. Photomicrographs of representative sections viewed under polarizing light were taken and analyzed using SigmaScan software. Scale bars = 100 μm. Data bars represent means ± se of determinations carried out on slides from 8 animals/condition. **P < 0.01, ***P < 0.001; ANOVA with Newman-Keuls posttest.
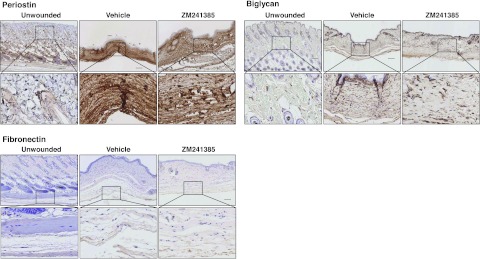
ZM241385 treatment does not modify periostin, biglycan, and fibronectin accumulation in the scar
To determine whether A2AR blockade modifies different matrix proteins other than collagens, we examined the effect of ZM241385 treatment on periostin, biglycan, and fibronectin expression, previously demonstrated to be increased during skin scarring (28–30). As expected and as shown in Fig. 5, periostin was dramatically increased in the scar, and biglycan and fibronectin were also increased, but none of these effects were prevented by ZM241385 application.
Figure 5.
A2AR blockade does not modify scar-promoted increase of periostin, biglycan, and fibronectin. Immunohistochemistry was performed to determine periostin, biglycan, and fibronectin expression; photomicrographs of sections were taken at ×100 and ×400. Scale bars = 100 μm (top panels); 10 μm (bottom panels).
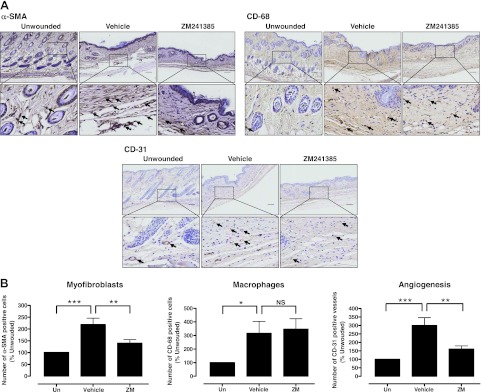
ZM241385 partially prevents increased myofibroblast and endothelial cell accumulation in the scar
Myofibroblasts are present in certain normal tissues, in healing wounds, and in tissues affected by other fibrosing diseases (31–33). There is growing evidence that the fibroblast/myofibroblast is the cell type most responsible for interstitial matrix accumulation and consequent structural deformations associated with fibrosis. During wound healing and progressive fibrotic events, fibroblasts transform into myofibroblasts, acquiring smooth muscle features, most notably the expression of α-SMA, the most widely used myofibroblast marker (34) used to identify fibrotic progression, and synthesis of mesenchymal cell-related matrix proteins (35). As shown in Fig. 6, we observed an increase in the α-SMA+ myofibroblast population in scars that was prevented by A2AR blockade. Similarly, there is increased monocyte infiltration in areas of fibrotic skin (36), and it has been suggested that the enhanced presence of monocyte/macrophages promotes the transformation of fibroblasts to myofibroblasts (37). As with myofibroblasts, we observed an increase in monocyte/macrophage infiltration in scars, as identified by the specific macrophage marker CD68 (38), but blockade of A2ARs did not change monocyte/macrophage infiltration. Another characteristic of healing scars is increased angiogenesis, and we found a marked increase in CD31+ endothelial cells in the untreated scars. Consistent with our previous observation that A2AR activation stimulates both angiogenesis and vasculogenesis (24, 39–41), in wounds, we found that blockade of these receptors diminishes the number of CD31+ endothelial cells in scars.
Figure 6.
A2AR blockade alters the cell population and matrix of scars. A) Immunohistochemistry was performed to determine α-SMA, CD-68, and CD-31 expression; photomicrographs of sections were taken at ×100 and ×400. Scale bars = 100 μm (top panels); 10 μm (bottom panels). B) Images were analyzed and α-SMA+ and CD-68+ cells and CD-31+ vessels were counted on defined areas of sections. Data represent means ± se of sections from 13 animals/condition. NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001; ANOVA with Newman-Keuls posttest.
DISCUSSION
The mechanisms involved in normal skin repair and wound healing, including cellular migration and proliferation, extracellular matrix deposition, and remodeling, have been extensively studied (42–44). At 3 wk after wounding, collagen production and degradation become equal, so there is no net collagen gain, but remodeling of the wound begins, and Col1 provides the predominant tensile strength to the scar until the later phase of maturation. Thus, scar formation results from the process of wound repair and incomplete remodeling leading to excessive deposition and misalignment of extracellular matrix proteins (8). We report here that blockade of A2ARs with topical application of ZM241385 after 2 wk from the day of wounding diminishes scar formation, at least in part, by shifting collagen production from Col3 and preventing myofibroblast and endothelial cell accumulation in the forming scar remodeling.
All of the cellular subtypes involved in wound healing (macrophages, epidermal cells, fibroblasts, and microvascular endothelial cells) express functional adenosine receptors (45). We have previously reported that stimulation of A2AR increases the rate of excisional wound closure (24, 46) and that A2AR-deficient mice suffer from disordered wound healing with poor granulation tissue and matrix formation (24), strongly suggesting that A2AR is beneficial for wound closure. However, we also found that A2AR stimulation promotes fibrosis, as A2AR deletion and blockade protect mice from bleomycin-induced dermal fibrosis (15), and A2AR antagonism protects mice from dermal fibrosis in a model of elevated tissue adenosine (16). Thus, we hypothesized that A2AR may contribute to scar progression and that A2AR blockade would therefore reduce chronic scarring, a hypothesis confirmed by our results. Because splinted wounds do not heal in mice lacking A2ARs, due to poor granulation tissue formation (ref. 24 and unpublished results), we could not study this model in mice lacking A2ARs, in which wound closure, healing, and granulation tissue formation are deregulated, therefore masking the effect of ZM241385 on scar formation.
Although we have previously shown that A2ARs play an active role in collagen production by human dermal fibroblasts (15), the use of animal models that recapitulate human scarring has been criticized because rodent scarring occurs mainly by contraction, whereas in humans wound repair involves primarily reepithelialization and granulation tissue formation (10). To better model wound healing in humans, splinting wounds in rodents diminishes the role of contraction, permitting wound repair to occur by the processes of granulation and epithelialization (10, 12). After wounding, there is a 10- to 14-d lag phase before the tensile strength of the wound begins to rapidly increase over the next 4 wk, after which the wound has reached ∼70% of the undamaged tissue (22). Thus, the conditions studied here more closely approximate wound healing and scar formation in humans, and the effects of A2AR blockade are more likely to simulate their effects in human scar formation.
We observed that A2AR blockade significantly reduced scar size but increased skin breaking tension, suggesting that A2AR antagonism promotes thickening and strengthening of the dermis and epidermis in the scar. There was a minimal increase in the total collagen content of the scar, as compared to normal skin, in untreated mice, and A2A receptor blockade prevented the increase in collagen accumulation, as measured by hydroxyproline content. More complete analysis of the collagen in the wounds of the untreated mice demonstrated that there was a modest increase in Col1 content but a marked increase in Col3 content and A2AR blockade selectively inhibited Col3 accumulation in the scars. These findings were confirmed by the histochemical stains of the scars, which demonstrated significant accumulation in loosely packed thin fibrils in scars, which was prevented by A2AR blockade. Moreover, we found a marked reduction in the ratio of Col1:Col3 in scarred skin that was similar to the changes previously reported (5) and which was mostly reversed in the A2A antagonist-treated animals. Thus, blockade of A2ARs alters not only the size of the scar but its composition and the strength of the skin as well. We also analyzed different extracellular matrix proteins, periostin and biglycan, which have been previously reported to regulate collagen fibrillogenesis and alter the biomechanical properties of connective tissues, and fibronectin, all three of which are up-regulated in scarring (28–30). As expected, we observed an increase of all three proteins, which was not prevented by A2AR blockade. This observation indicates that the effects of the A2AR on fibroblast function in scars is confined to collagen synthesis, as previously demonstrated in vitro (47) and in vivo (24, 46) without affecting production of other extracellular matrix proteins that regulate collagen fibrillogenesis and skin integrity.
It has been shown previously that myofibroblasts increase in number as a result of mechanical tension due to splinting of full-thickness wounds (48), and these cells are largely responsible for the contractures observed in hypertrophic scars (49). It has also been suggested that CD68-positive macrophages that infiltrate healing wounds may play a role in the transformation of fibroblasts to myofibroblasts (37) and are associated with areas of fibrotic skin (36). We also detected an increased number of myofibroblasts and infiltration of CD-68+ macrophages in the evolving scar and application of the A2AR antagonist ZM241385 diminished myofibroblast but not macrophage infiltration in the scar, suggesting, therefore, that A2AR blockade prevents scarring and therefore reduces the myofibroblast content, but this effect is not likely due to a reduction of chronic inflammation.
It is widely accepted that wound healing depends on angiogenesis (50). Although the majority of studies examining the function of angiogenesis in wound healing have focused on its overall effect on wound closure or reepithelialization, it is not known how angiogenesis specifically affects collagen production and remodeling during wound repair (51), although accumulating data strongly suggest that VEGF-mediated angiogenesis plays a significant role in skin keloid formation (52, 53). Moreover, angiogenesis inhibitors modify dermal matrix deposition (54), and reduced levels of angiogenesis are found in scarless fetal wounds (55, 56). In our study, the endothelial cell marker CD31 indicates an increase in angiogenesis in the scar, which is prevented by ZM241385, and because adenosine, acting at both A2ARs and A2BRs, stimulates angiogenesis and vasculogenesis (57), it is likely that inhibition of adenosine receptor-dependent angiogenesis contributes to the diminished scar size and altered composition of the scar.
In summary, we analyzed the effect of pharmacological blockade of A2A receptors with the selective A2A antagonist ZM241385 on scar size, extracellular matrix protein structure, tensile strength, and skin thickness, as well as on representative fibrosis markers in a murine model that mimics human scar progression, and we found that A2AR blockade diminishes scar size and prevents dysfunctional matrix remodeling by precluding excessive myofibroblasts and angiogenesis increase and improving collagen alignment and the Col1:Col3 ratio, thereby increasing the strength of the scar and preventing progression of fibrosis. These findings suggest that topical administration of an A2AR antagonist during early matrix remodeling will diminish scar size and promote restoration of skin integrity in patients.
Supplementary Material
Acknowledgments
The authors thank the Histopathology Core of the New York University (NYU) Langone Medical Center.
This work was supported by grants from the U.S. National Institutes of Health (AR56672, AR56672S1, and AR54897) and the NYU–Health and Hospitals Corp. Clinical and Translational Science Institute (UL1TR000038), and by the NYU Cancer Institute Center support grant (U.S. National Cancer Institute grant 5P30CA0016087-33).
B.N.C. holds patents on use of A2AR agonists to promote wound healing and use of A2AR antagonists to inhibit fibrosis, use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone, use of adenosine A1 and A2BR antagonists to treat fatty liver, and use of A2AR agonists to prevent prosthesis loosening. B.N.C. is a consultant for Bristol-Myers Squibb, Novartis, CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, Data and Safety Monitoring Board), Endocyte, Protalex, Allos, Inc., Savient, Gismo Therapeutics, Antares Pharmaceutical, and Medivector.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- A2AR
- adenosine A2A receptor
- α-SMA
- α-smooth muscle actin
- Col1
- collagen type I
- Col3
- collagen type III
- PSR
- picrosirius red
REFERENCES
- 1. Wankell M., Munz B., Hubner G., Hans W., Wolf E., Goppelt A., Werner S. (2001) Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 20, 5361–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhett J. M., Ghatnekar G. S., Palatinus J. A., O'Quinn M., Yost M. J., Gourdie R. G. (2008) Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 26, 173–180 [DOI] [PubMed] [Google Scholar]
- 3. Mignatti P., Rifken D. B., Welgus H. G., Parks W. C. (1996) Proteinases and tissue remodeling. In The Molecular and Cellular Biology of Wound Repair (Clark R. A. F., ed) pp. 427–474, New York, Plenum Press [Google Scholar]
- 4. Clark R. A. F. (1996) Wound repair: overview and general considerations. In The Molecular and Cellular Biology of Wound Repair (Clark R. A. F., ed) pp. 3–50, New York, Plenum Press [Google Scholar]
- 5. Stadelmann W. K., Digenis A. G., Tobin G. R. (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am.J. Surg. 176, 26S–38S [DOI] [PubMed] [Google Scholar]
- 6. Martin P. (1997) Wound healing–aiming for perfect skin regeneration. Science 276, 75–81 [DOI] [PubMed] [Google Scholar]
- 7. Singer A. J., Clark R. A. (1999) Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 [DOI] [PubMed] [Google Scholar]
- 8. Yates C. C., Krishna P., Whaley D., Bodnar R., Turner T., Wells A. (2010) Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am. J. Pathol. 176, 1743–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheridan R. L., Tompkins R. G. (2004) What's new in burns and metabolism. J. Am. Coll. Surg. 198, 243–263 [DOI] [PubMed] [Google Scholar]
- 10. Galiano R. D., Michaels J., 5th, Dobryansky M., Levine J. P., Gurtner G. C. (2004) Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 12, 485–492 [DOI] [PubMed] [Google Scholar]
- 11. Aarabi S., Bhatt K. A., Shi Y., Paterno J., Chang E. I., Loh S. A., Holmes J. W., Longaker M. T., Yee H., Gurtner G. C. (2007) Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21, 3250–3261 [DOI] [PubMed] [Google Scholar]
- 12. Wetterau M., George F., Weinstein A., Nguyen P. D., Tutela J. P., Knobel D., Cohen Ba O., Warren S. M., Saadeh P. B. (2011) Topical prolyl hydroxylase domain-2 silencing improves diabetic murine wound closure. Wound Repair Regen. 19, 481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasko G., Cronstein B. N. (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 25, 33–39 [DOI] [PubMed] [Google Scholar]
- 14. Ohta A., Sitkovsky M. (2001) Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 [DOI] [PubMed] [Google Scholar]
- 15. Chan E. S., Fernandez P., Merchant A. A., Montesinos M. C., Trzaska S., Desai A., Tung C. F., Khoa D. N., Pillinger M. H., Reiss A. B., Tomic-Canic M., Chen J. F., Schwarzschild M. A., Cronstein B. N. (2006) Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 54, 2632–2642 [DOI] [PubMed] [Google Scholar]
- 16. Fernandez P., Trzaska S., Wilder T., Chiriboga L., Blackburn M. R., Cronstein B. N., Chan E. S. (2008) Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am. J. Pathol. 172, 1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellemare J., Roberge C. J., Bergeron D., Lopez-Valle C. A., Roy M., Moulin V. J. (2005) Epidermis promotes dermal fibrosis: role in the pathogenesis of hypertrophic scars. J. Pathol. 206, 1–8 [DOI] [PubMed] [Google Scholar]
- 18. Ghahary A., Shen Y. J., Scott P. G., Gong Y., Tredget E. E. (1993) Enhanced expression of mRNA for transforming growth factor-beta, type I and type III procollagen in human post-burn hypertrophic scar tissues. J. Lab. Clin. Med. 122, 465–473 [PubMed] [Google Scholar]
- 19. Tredget E. E., Shankowsky H. A., Pannu R., Nedelec B., Iwashina T., Ghahary A., Taerum T. V., Scott P. G. (1998) Transforming growth factor-beta in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. Plast. Reconstr. Surg. 102, 1317–1328; discussion 1329–1330 [DOI] [PubMed] [Google Scholar]
- 20. Poucher S. M., Keddie J. R., Brooks R., Shaw G. R., McKillop D. (1996) Pharmacodynamics of ZM 241385, a potent A2a adenosine receptor antagonist, after enteric administration in rat, cat and dog. J. Pharm. Pharmacol. 48, 601–606 [DOI] [PubMed] [Google Scholar]
- 21. Poucher S. M., Keddie J. R., Singh P., Stoggall S. M., Caulkett P. W., Jones G., Coll M. G. (1995) The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 115, 1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madden J. W., Peacock E. E., Jr. (1968) Studies on the biology of collagen during wound healing. I. Rate of collagen synthesis and deposition in cutaneous wounds of the rat. Surgery 64, 288–294 [PubMed] [Google Scholar]
- 23. Kiernan J. A. (1999) Histological and Histochemical Methods: Theory and Practice, Butterworth Heinemann, Oxford; Boston [Google Scholar]
- 24. Montesinos M. C., Desai A., Chen J. F., Yee H., Schwarzschild M. A., Fink J. S., Cronstein B. N. (2002) Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am. J. Pathol. 160, 2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dayan D., Hiss Y., Hirshberg A., Bubis J. J., Wolman M. (1989) Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 93, 27–29 [DOI] [PubMed] [Google Scholar]
- 26. Junqueira L. C., Montes G. S., Krisztan R. M. (1979b) The collagen of the vertebrate peripheral nervous system. Cell Tissue Res. 202, 453–460 [DOI] [PubMed] [Google Scholar]
- 27. Montes G. S., Krisztan R. M., Shigihara K. M., Tokoro R., Mourao P. A., Junqueira L. C. (1980) Histochemical and morphological characterization of reticular fibers. Histochemistry 65, 131–141 [DOI] [PubMed] [Google Scholar]
- 28. Elliott C. G., Wang J., Guo X., Xu S. W., Eastwood M., Guan J., Leask A., Conway S. J., Hamilton D. W. (2012) Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J. Cell Sci. 125, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kozma E. M., Olczyk K., Glowacki A., Bobinski R. (2000) An accumulation of proteoglycans in scarred fascia. Mol. Cell. Biochem. 203, 103–112 [DOI] [PubMed] [Google Scholar]
- 30. Sidgwick G. P., Bayat A. (2012) Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J. Eur. Acad. Dermatol. Venereol. 26, 141–152 [DOI] [PubMed] [Google Scholar]
- 31. Grinnell F. (2000) Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 10, 362–365 [DOI] [PubMed] [Google Scholar]
- 32. Ronnov-Jessen L., Petersen O. W., Bissell M. J. (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev. 76, 69–125 [DOI] [PubMed] [Google Scholar]
- 33. Serini G., Gabbiani G. (1999) Mechanisms of myofibroblast activity and phenotypic modulation. Exp. Cell Res. 250, 273–283 [DOI] [PubMed] [Google Scholar]
- 34. Hinz B. (2007) Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127, 526–537 [DOI] [PubMed] [Google Scholar]
- 35. Barnes J. L., Gorin Y. (2011) Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 79, 944–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibson S. E., Farver C. F., Prayson R. A. (2006) Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch. Pathol. Lab. Med. 130, 209–212 [DOI] [PubMed] [Google Scholar]
- 37. Abe K., Ozono Y., Miyazaki M., Koji T., Shioshita K., Furusu A., Tsukasaki S., Matsuya F., Hosokawa N., Harada T., Taguchi T., Nagata K., Kohno S. (2000) Interstitial expression of heat shock protein 47 and alpha-smooth muscle actin in renal allograft failure. Nephrol. Dial. Transplant. 15, 529–535 [DOI] [PubMed] [Google Scholar]
- 38. Di Gregorio G. B., Yao-Borengasser A., Rasouli N., Varma V., Lu T., Miles L. M., Ranganathan G., Peterson C. A., McGehee R. E., Kern P. A. (2005) Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54, 2305–2313 [DOI] [PubMed] [Google Scholar]
- 39. Desai A., Victor-Vega C., Gadangi S., Montesinos M. C., Chu C. C., Cronstein B. N. (2005) Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol. Pharmacol. 67, 1406–1413 [DOI] [PubMed] [Google Scholar]
- 40. Montesinos M. C., Shaw J. P., Yee H., Shamamian P., Cronstein B. N. (2004) Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am. J. Pathol. 164, 1887–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leibovich S. J., Chen J. F., Pinhal-Enfield G., Belem P. C., Elson G., Rosania A., Ramanathan M., Montesinos C., Jacobson M., Schwarzschild M. A., Fink J. S., Cronstein B. (2002) Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am. J. Pathol. 160, 2231–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cha J., Falanga V. (2007) Stem cells in cutaneous wound healing. Clin. Dermatol. 25, 73–78 [DOI] [PubMed] [Google Scholar]
- 43. Pierce G. F., Mustoe T. A. (1995) Pharmacologic enhancement of wound healing. Annu. Rev. Med. 46, 467–481 [DOI] [PubMed] [Google Scholar]
- 44. Romer J., Bugge T. H., Pyke C., Lund L. R., Flick M. J., Degen J. L., Dano K. (1996) Plasminogen and wound healing. Nat. Med. 2, 725. [DOI] [PubMed] [Google Scholar]
- 45. Valls M. D., Cronstein B. N., Montesinos M. C. (2009) Adenosine receptor agonists for promotion of dermal wound healing. Biochem. Pharmacol. 77, 1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Montesinos M. C., Gadangi P., Longaker M., Sung J., Levine J., Nilsen D., Reibman J., Li M., Jiang C. K., Hirschhorn R., Recht P. A., Ostad E., Levin R. I., Cronstein B. N. (1997) Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 186, 1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Che J., Chan E. S., Cronstein B. N. (2007) Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol. Pharmacol. 72, 1626–1636 [DOI] [PubMed] [Google Scholar]
- 48. Hinz B., Mastrangelo D., Iselin C. E., Chaponnier C., Gabbiani G. (2001) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 159, 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ehrlich H. P., Desmouliere A., Diegelmann R. F., Cohen I. K., Compton C. C., Garner W. L., Kapanci Y., Gabbiani G. (1994) Morphological and immunochemical differences between keloid and hypertrophic scar. Am. J. Pathol. 145, 105–113 [PMC free article] [PubMed] [Google Scholar]
- 50. Werner S., Grose R. (2003) Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835–870 [DOI] [PubMed] [Google Scholar]
- 51. Wilgus T. A., Ferreira A. M., Oberyszyn T. M., Bergdall V. K., Dipietro L. A. (2008) Regulation of scar formation by vascular endothelial growth factor. Lab. Invest. 88, 579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu Y., Zhang Q., Ann D. K., Akhondzadeh A., Duong H. S., Messadi D. V., Le A. D. (2004) Increased vascular endothelial growth factor may account for elevated level of plasminogen activator inhibitor-1 via activating ERK1/2 in keloid fibroblasts. Am. J. Physiol. Cell Physiol. 286, C905–C912 [DOI] [PubMed] [Google Scholar]
- 53. Gira A. K., Brown L. F., Washington C. V., Cohen C., Arbiser J. L. (2004) Keloids demonstrate high-level epidermal expression of vascular endothelial growth factor. J. Am. Acad. Dermatol. 50, 850–853 [DOI] [PubMed] [Google Scholar]
- 54. Bloch W., Huggel K., Sasaki T., Grose R., Bugnon P., Addicks K., Timpl R., Werner S. (2000) The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J. 14, 2373–2376 [DOI] [PubMed] [Google Scholar]
- 55. Whitby D. J., Ferguson M. W. (1991) Immunohistochemical localization of growth factors in fetal wound healing. Dev. Biol. 147, 207–215 [DOI] [PubMed] [Google Scholar]
- 56. Ihara S., Motobayashi Y., Nagao E., Kistler A. (1990) Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development 110, 671–680 [DOI] [PubMed] [Google Scholar]
- 57. Feoktistov I., Biaggioni I., Cronstein B. N. (2009) Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb. Exp. Pharmacol. 193, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.