Abstract
Spinophilin (SPL), a multidomain scaffolding protein known to modulate the activity of different G-protein-coupled receptors, regulates various central nervous system (CNS) functions. However, little is known about the role of SPL expressed in peripheral cell types including pancreatic β cells. In this study, we examined the ability of SPL to modulate the activity of β-cell M3 muscarinic acetylcholine receptors (M3Rs), which play an important role in facilitating insulin release and maintaining normal blood glucose levels. We demonstrated, by using both in vitro and in vivo approaches (mouse insulinoma cells and SPL-deficient mice), that SPL is a potent negative regulator of M3R-mediated signaling and insulin release. Additional biochemical and biophysical studies, including the use of bioluminescence resonance energy transfer technology, suggested that SPL is able to recruit regulator of G-protein signaling 4 (RGS4) to the M3R signaling complex in an agonist-dependent fashion. Since RGS4 is a member of the RGS family of proteins that act to reduce the lifetime of activated G proteins, these findings support the concept that the inhibitory effects of SPL on M3R activity are mediated by RGS4. These data suggest that SPL or other G-protein-coupled receptor-associated proteins may serve as novel targets for drug therapy aimed at improving β-cell function for the treatment of type 2 diabetes.—Ruiz de Azua, I., Nakajima, K.-I., Rossi, M., Cui, Y., Jou, W., Gavrilova, O., Wess, J. Spinophilin as a novel regulator of M3 muscarinic receptor-mediated insulin release in vitro and in vivo.
Keywords: G-protein-coupled receptors, knockout mice, receptor regulation, signal transduction
Spinophilin (SPL) is a widely expressed multidomain scaffolding protein that modulates many important functions of the central nervous system (CNS; refs. 1, 2). Accumulating evidence suggests that SPL can directly interact with various G-protein-coupled receptors (GPCRs), including different muscarinic (3, 4) and adrenergic (5–7) receptor subtypes to regulate the efficiency and/or selectivity of GPCR signaling. For example, studies with the α2A-adrenergic receptor have shown that SPL binding to this receptor subtype interferes with the ability of the activated receptor to recruit proteins of the arrestin family (7, 8), which function as key regulators of GPCR signaling and trafficking (9, 10). Biochemical studies (11) indicate that SPL can also interact with different members of the regulator of G-protein signaling (RGS) protein family (these proteins accelerate the GTPase activity of activated G-protein α-subunits).
The physiological relevance of SPL regulation of GPCR activity has been demonstrated in studies focusing on various functions of the CNS, including μ-opioid receptor-mediated analgesic and behavioral responses to opiates (12, 13) and α2A-adrenergic receptor-mediated sedation (7). However, little is known about the potential physiological roles of SPL in peripheral tissues and cell types, including pancreatic β cells (2).
In the present study, we examined the ability of SPL to modulate the function of M3 muscarinic acetylcholine receptors (M3Rs) expressed by pancreatic β cells. Following acetylcholine binding, M3Rs couple to G proteins of the Gq family (14, 15), triggering the activation of phospholipase Cβ and the generation of the second messengers inositol trisphosphate (IP3; note that IP3 promotes the release of calcium from intracellular stores) and diacylglycerol (DAG). Several recent studies suggest that β-cell M3Rs play a key role in facilitating insulin release and maintaining normal glucose homeostasis in vivo (16, 17). For example, studies with mutant mice lacking M3Rs selectively in their pancreatic β cells displayed impaired glucose tolerance and significantly reduced insulin release (16). Consistent with this phenotype, transgenic mice overexpressing M3Rs in their pancreatic β cells showed greatly improved glucose tolerance and enhanced insulin release under different experimental conditions. Moreover, these transgenic mice were resistant to diet-induced glucose intolerance and hyperglycemia (16). Mutant mice that expressed a constitutively active mutant M3R in their β cells showed a very similar phenotype (17).
Taken together, these findings strongly suggest that chronic activation of β-cell M3Rs may represent a useful approach to boost insulin output in the long-term treatment of type 2 diabetes (T2D). However, drugs that can selectively activate the M3R subtype are not available at present. Thus we decided to identify M3R-associated proteins that might serve as potential new targets to enhance signaling through β-cell M3Rs for therapeutic purposes.
In the present study, we found, by using both in vitro and in vivo approaches, including the use of SPL-deficient (SPL−/−) mice, that SPL is a potent negative regulator of M3R-mediated insulin release. Additional biochemical and biophysical experiments strongly support a model in which SPL acts as an adaptor protein that recruits RGS4 to the M3R signaling complex, thus limiting the lifetime of M3R-activated G proteins.
This is the first report demonstrating a role for SPL in regulating β-cell function. Given the importance of β-cell M3Rs in maintaining normal blood glucose levels, our findings highlight the significant functional role of M3R (GPCR)-associated proteins in modulating β-cell biology. Such proteins may prove useful as targets for novel classes of drugs aimed at improving β-cell function for therapeutic purposes.
MATERIALS AND METHODS
Materials
Oxotremorine-M (OXO-M), ammonium iodide, atropine sulfate, bethanechol chloride, ADP, arginine vasopressin (AVP), and exendin-4 were purchased from Sigma-Aldrich (St. Louis, MO, USA), and glucagon-like-peptide 1 (GLP-1; aa 7–36) amide was obtained from Bachem (King of Prussia, PA, USA). [3H]-N-methylscopolamine ([3H]NMS; 79–83 Ci/mmol) was from PerkinElmer Life Sciences (Downers Grove, IL, USA). Tissue culture media were from Invitrogen (Carlsbad, CA, USA).
The SPL and SPL-eGFP (18) expression plasmids were kind gifts of Dr. Qin Wang (University of Alabama, Birmingham, AL, USA). The RGS4-Venus plasmid (19) was generously provided by Drs. Kazuharu Furutani and Yoshihisa Kurachi (Osaka, University, Osaka, Japan). Plasmids coding for rat GRK2 and Venus-tagged arrestin-2 (20) were gifts by the laboratory of Dr. Vsevolod Gurevich (Vanderbilt University, Nashville, TN, USA). A human RGS4 expression construct (in the pcDNA 3.1+ vector; RGS040TN00) was obtained from the Missouri Univerrsity of Science and Technology cDNA Resource Center (Rolla, MO, USA).
SP−/− mice
The generation of SP−/− mice has been described previously (21). SP−/− mice and their wild-type (WT) littermates were generated by crossing heterozygous SPL+/− mice. Mice were housed in a specific pathogen-free barrier facility (4–5 mice/cage), maintained on a 12-h light-dark cycle. Mice were fed ad libitum with a standard mouse chow [4% (w/w) fat content; Zeigler, Gardners, PA, USA]. All animal studies were approved by the Animal Care and Use Committee of the U.S. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH).
MIN6 cell culture and siRNA-mediated gene knockdown
MIN6 cells (a kind gift from Dr. Abner Notkins, U.S. National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA) were cultured as described previously (22). For gene-silencing studies, MIN6 cells (∼1×106 cells) were electroporated with 60 nmol of siRNA (Amaxa, Walkersville, MD, USA) according to the manufacturer's instructions. Duplex siRNAs for mouse SPL and RGS4, and scrambled control siRNAs were obtained from Ambion (Austin, TX, USA; for details, see Supplemental Data). About 48 h after electroporations, MIN6 cells were subjected to various assays (see below).
Calcium assays (MIN6 cells)
About 48 h after electroporation of MIN6 cells with different siRNAs, OXO-M-dependent increases in intracellular calcium levels were determined by the use of FLIPR technology (Molecular Devices, Sunnyvale, CA, USA). All measurements were carried out in 96-well plates, as described in detail previously (23). OXO-M concentration-response curves were analyzed by using Prism 4.0 software (GraphPad Software, San Diego, CA, USA), yielding Emax and EC50 values.
Insulin release assays (MIN6 cells)
MIN6 cells that had been treated with different siRNAs were incubated with increasing concentrations of OXO-M at 37°C for 1 h (in Krebs-Ringer bicarbonate/HEPES buffer; 16.7 mM glucose). Insulin release was determined by measuring insulin concentrations in the incubation medium using an insulin ELISA kit (Crystal Chem, Downers Grove, IL, USA). Emax and EC50 values were obtained from OXO-M concentration-response curves using GraphPad Prism 4.0 software.
Western-blot analysis
The effectiveness of siRNA-mediated knockdown of SPL and RGS4 expression was determined by standard Western blotting techniques. SPL and RGS4 expression was visualized by using an anti-SPL monoclonal antibody (1:5,000 dilution; this antibody was a kind gift from Dr. Patrick B. Allen (Yale University, New Haven, CT, USA; ref. 21) or an anti-RGS4 polyclonal antibody (1:10,000 dilution; ref. 24), respectively. Horseradish peroxidase-labeled secondary antibodies (Amersham Biosciences, Piscataway, NJ, USA) were used to detect the primary antibodies via chemiluminescence. Immunoreactive proteins were visualized by using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA). The blots were stripped and reprobed using an anti-β-actin polyclonal antibody (1:1000 dilution; Cell Signaling, Danvers, MA, USA).
Agonist-induced M3R internalization in MIN-6 cells
MIN-6 cells were electroporated with SPL-1 siRNA or scrambled control siRNA (Con-2) as described above. Electroporated cells were then transferred into 6-well plates (∼1.5×106 cells/well). Cells were grown for 48 h and then incubated with OXO-M (100 μM) for 90 min at 37°C. Following agonist treatment, cells were washed once with PBS and then scraped off the dishes with ice-cold DMEM (900 μl/well). Subsequently, cells were incubated for 2 h at 4°C in binding buffer (25) with a saturating concentration (2 nM) of the membrane-impermeable muscarinic radioligand [3H]NMS (total volume: 0.5 ml). Nonspecific binding was determined in the presence of 10 μM atropine. Protein concentrations were measured using a bicinchoninic acid kit (Pierce), according to the manufacturer's protocol. Data were analyzed by using GraphPad Prism 4.0 software.
Bioluminescence resonance energy transfer (BRET) assays
COS-7 cells were seeded into 6-well tissue culture dishes at a density of ∼300,000 cells/well. About 24 h later, cells were cotransfected with constant amounts of plasmid DNAs coding for M3R-Luc (50 ng) and increasing amounts of SPL-eGFP (200–2000 ng), either in the absence or presence of a cotransfected RGS4 expression construct (200 ng; BRET saturation experiments). In another set of BRET studies, cells were cotransfected with constructs coding for M3R-Luc (50 ng), RGS4-V (1200 ng), and SPL (600 ng) or with plasmids coding for M3R-Luc (200 ng), GRK2 (200 ng), arrestin-2-V (800 ng), and SPL (800 ng). In these latter experiments, cells were treated with increasing concentrations of OXO-M for 30–45 min at room temperature. In each experiment, vector DNA (pcDNA 3.1) was added to keep the total amount of transfected DNA constant (M3R-Luc/SPL-eGFP BRET saturation experiments: 2.55 μg; RGS4-V and arrestin-2-V recruitment studies: 1.85 and 4.4 μg, respectively). After ∼48 h, cells were trypsinized, transferred to microcentrifuge tubes, and centrifuged at 110 g for 5 min at room temperature. Cell pellets were resuspended in 900 μl of PBS supplemented with glucose (1 mg/ml), ascorbic acid (1 mM), and EDTA-free complete protease inhibitor (Roche Applied Science, Indianapolis, IN, USA). Subsequently, 45 μl aliquots were added to individual wells of a white opaque 96-well plate (PerkinElmer Life Sciences, Waltham, MA, USA). In a subset of experiments (see above), cells were treated with increasing concentrations of OXO-M for 30–45 min at room temperature. Total fluorescence was first measured via excitation at 485 nm and monitoring emission at 535 nm. Coelenterazine-h (Promega, Madison, WI, USA) was then added to each well at a final concentration of 5 μM, and emissions were measured at 530 and 460 nm for the SPL-eGFP/M3R-Luc combination and at 530 and 480 nm for the RGS4-V/M3R-Luc combination. Total luminescence was subsequently measured in the absence of filters. All measurements were performed using the Mithras LB 940 plate reader (Berthold Technologies, Oak Ridge, TN, USA). The BRET ratio was defined as the ratio of emission at 530 nm to emission at 460 nm (eGFP) or 480 nm (Venus) after the addition of coelenterazine-h. In the M3R-Luc/SPL-eGFP BRET saturation experiments, NetBRET ratios were calculated as BRET ratios obtained with cells coexpressing M3R-Luc and SPL-eGFP-BRET ratios obtained with cells expressing M3R-Luc alone. BRET50 values were determined as the ratio of acceptor to donor emission that generated a half-maximal NetBRET signal. In the RGS4-V recruitment assays, NetBRET ratios were calculated as follows: BRET ratio (M3R-Luc+RGS4-V+SPL) − BRET ratio (M3R-Luc+SPL). In the arrestin-2-V recruitment assays, NetBRET ratios were expressed as a fraction of the maximal observed BRET ratio (=1). Data were analyzed using GraphPad Prism 4.0 software.
Static islet insulin secretion assay
Pancreatic islets were isolated from SPL−/− and WT mice (5- to 6-mo-old male littermates), and static islet insulin secretion assays were carried out as described in detail previously (16). In brief, batches of 10 islets were incubated in 12-well cell culture plates for 1 h in a CO2 incubator at 37°C in the presence or absence of different GPCR ligands (glucose concentration: 3.3 or 16.7 mM). The medium was collected for insulin measurements, and the remaining islet insulin was extracted by sonication in acid/ethanol. The amount of insulin secreted during the incubation period was normalized to the total insulin content of each well (islets plus medium). Islet insulin content was measured by using an acid-ethanol method, as described in detail previously (26). Insulin concentrations were determined via an ELISA kit (Crystal Chem).
In vivo physiological studies with SPL−/− mice and WT littermates
Intraperitoneal glucose tolerance tests (IGTT) were carried out with mice that had been subjected to overnight (10–12 h) food withdrawal. Blood samples were taken from the tail vein before (0 min) and 15, 30, 60, and 120 min after glucose administration (2 mg/g body weight). Blood glucose levels were determined using an automated blood glucose reader (Glucometer Contour; Bayer, Tarrytown, NY, USA). Plasma insulin concentrations were determined via ELISA (Crystal Chem).
For insulin tolerance (sensitivity) tests, human insulin (0.75 U/kg; Eli Lilly, Indianapolis, IN, USA) was administered intraperitoneally to freely fed mice (at 3 PM). Blood glucose measurements were carried out using blood samples taken from the tail vein.
Body composition was measured using the Echo 3-in-1 NMR analyzer Bruker Minispec mq10 (Echo Medical Systems, Houston, TX, USA).
Bethanechol and exendin-4 treatment of SPL−/− mice and WT littermates
A single dose of bethanechol chloride (2 μg/g s.c.) or exendin-4 (12 nmol/kg i.p.) was administered to freely fed SPL−/− mice and WT littermates (14-wk-old female mice; n=4/group). Blood was collected at different time points (0, 5, 30, and 60 min) to measure blood glucose and plasma insulin levels (see previous paragraph). All experiments were carried out during the light cycle between 9 and 11 AM.
Statistics
Data are expressed as means ± se for the indicated number of observations. For comparisons between 2 groups, the paired or unpaired Student's t test (2-tailed) was used, as appropriate. For multiple comparisons, the 1-way ANOVA was applied. A value of P < 0.05 was considered statistically significant.
RESULTS
SPL inhibits M3R activity in insulinoma cells
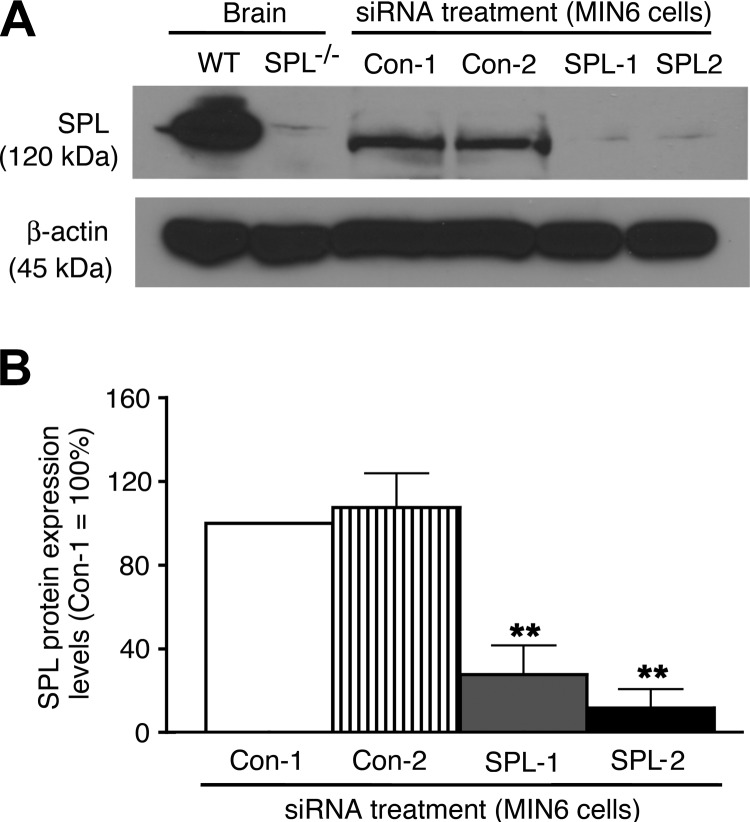
We recently demonstrated that MIN6 mouse insulinoma cells express functional M3Rs and that agonist activation of these receptors leads to concentration-dependent increases in intracellular calcium levels [Ca2+]i and insulin release (27). Western blotting studies demonstrated that SPL expression is readily detectable in MIN6 cells (Fig. 1A). Thus, MIN6 cells represent an excellent in vitro model system to study the potential effects of SPL on M3R-mediated regulation of β-cell function.
Figure 1.
Effective knockdown of SPL expression in MIN6 cells by using siRNA technology. A) Representative Western blot indicating SPL expression after treatment of MIN6 cells with 2 different SPL siRNAs (SPL-1 and SPL-2) and 2 different scrambled control siRNAs (Con-1 and Con-2). For control purposes, brain lysates from SPL−/− mice and WT littermates were also included in this analysis (first 2 lanes). Forty micrograms of total protein was loaded per well. B) Quantification of immunoblotting results. SPL protein expression levels are expressed relative to those found with cells treated with Con-1 siRNA (=100%). Data are given as means ± se of 4 independent experiments. **P < 0.01 vs. Con-2 siRNA-treated cells.
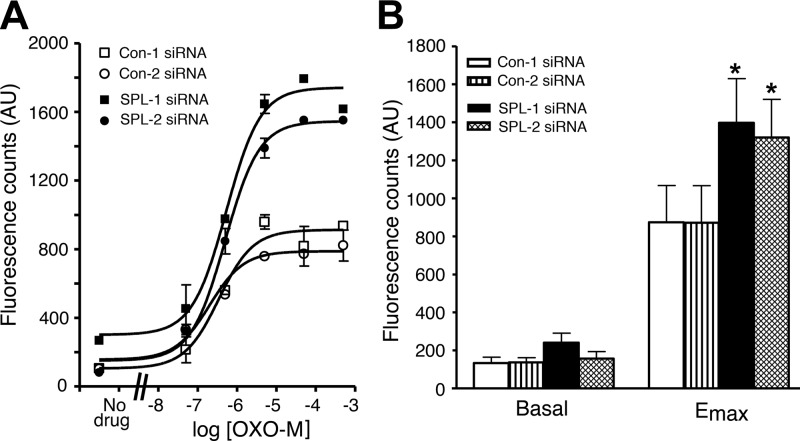
Initially, we electroporated MIN6 cells with 2 different SPL siRNAs (SPL-1 and SPL-2). Western blotting studies demonstrated that use of either of these 2 siRNAs resulted in a highly effective knockdown of SPL protein expression (remaining SPL levels: ∼10–25%), as compared with control cells treated with scrambled control siRNAs (Con-1 and Con-2; Fig. 1). Strikingly, after SPL knockdown, treatment of cells with the muscarinic agonist OXO-M led to a concentration-dependent augmentation of M3R-mediated increases in [Ca2+]i, as compared with cells treated with scrambled control siRNA (Fig. 2A). SPL knockdown had no significant effect on basal [Ca2+]i levels but resulted in a ∼50% increase in maximum OXO-M-stimulated [Ca2+]i levels (Emax), as compared with control cells (Fig. 2B; P<0.05). OXO-M EC50 values ranged from ∼3 to 10 μM and were not significantly affected by the knockdown of SPL expression.
Figure 2.
Knockdown of SPL expression in MIN6 cells results in enhanced M3R-mediated increases in [Ca2+]i. MIN6 cells were electroporated with 2 different scrambled control siRNAs (Con-1 and Con-2) and 2 different SPL siRNAs (SPL-1 and SPL-2). A) Representative concentration-response curves for muscarinic agonist OXO-M-induced increases in [Ca2+]i. B) Summary of basal and maximum agonist-stimulated [Ca2+]i levels derived from OXO-M concentration-response curves. AU, arbitrary units. Data are expressed as means ± se of 4 independent experiments, each carried out in duplicate. *P < 0.05 vs. Con-1 siRNA-treated cells.
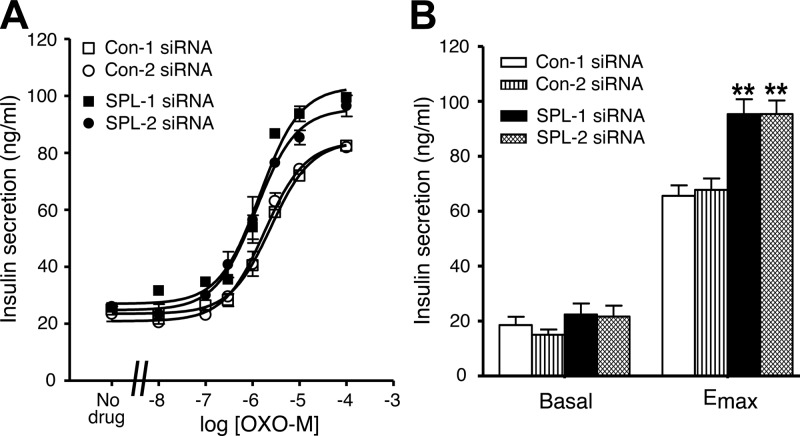
Consistent with the [Ca2+]i measurements, SPL knockdown in MIN6 cells led to significant increases in OXO-M-mediated insulin secretion (all insulin release experiments were carried out in the presence of 16.7 mM glucose; Fig. 3A). SPL knockdown had no significant effect on basal insulin release but resulted in a ∼30% increase in OXO-M Emax values (P<0.01 vs. OXO-M Emax values obtained with cells treated with either of the 2 control siRNAs; Fig. 3B). OXO-M EC50 values ranged from ∼2 to 4 μM and did not differ significantly between SPL-deficient and control cells.
Figure 3.
Knockdown of SPL expression in MIN6 cells augments M3R-mediated insulin release. MIN6 cells were electroporated with 2 different scrambled control siRNAs (Con-1 and Con-2) and 2 different SPL siRNAs (SPL-1 and SPL-2). A) Representative concentration-response curves for muscarinic agonist OXO-M-induced increases in insulin secretion. MIN6 cells were incubated with increasing concentrations of OXO-M in the presence of 16.7 mM glucose. B) Summary of basal insulin release and Emax values derived from OXO-M concentration-response curves. Data are expressed as means ± se of 4 independent experiments, each carried out in triplicate. **P < 0.01 vs. Con-1 siRNA-treated cells.
Taken together, these data clearly demonstrate that SPL represents a negative regulator of M3R signaling in MIN6 insulinoma cells.
SPL and RGS4 as components of the same pathway inhibiting M3R function in MIN6 cells
We previously demonstrated that RGS4, similar to SPL, acts as a potent negative regulator of M3R function in pancreatic β cells, both in vitro and in vivo (27). Electrophysiological studies (11) indicated that SPL can inhibit signaling through the α1A-adrenergic receptor by recruiting RGS2 to the ligand-activated receptor/G-protein complex. We therefore tested the hypothesis that the inhibitory effect of SPL on M3R activity may require the presence of RGS4.
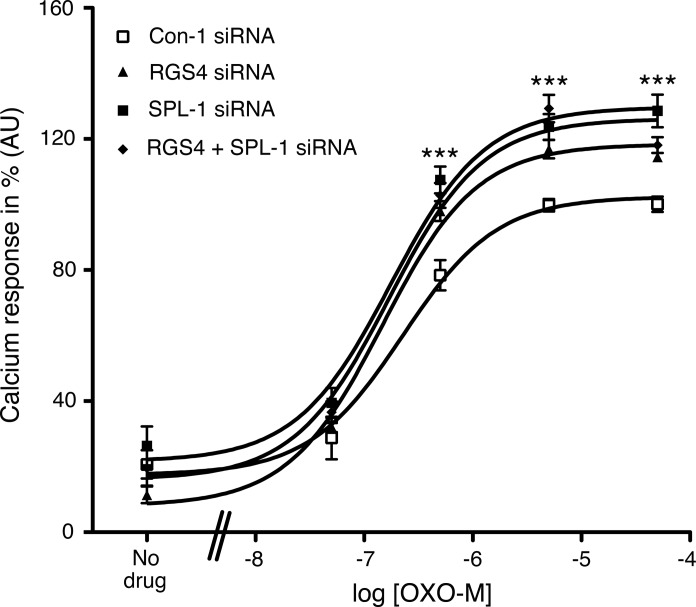
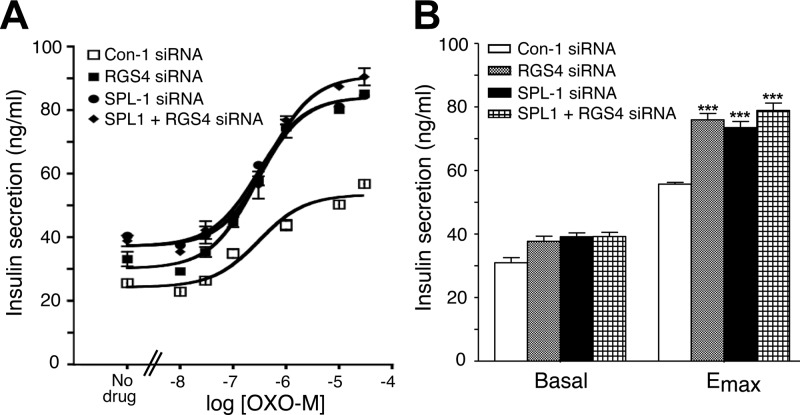
Figure 4 shows that siRNA-mediated knockdown of RGS4 expression in MIN6 cells resulted in a significant augmentation of OXO-M-mediated increases in [Ca2+]i (∼20-30% increase in Emax; P<0.01 vs. control cells), consistent with the outcome of a previous study (27). The magnitude of this effect was similar to that observed after siRNA-mediated knockdown of SPL expression (Fig. 4). The RGS4 siRNA used for these experiments reduced RGS4 protein expression to ∼25% of control levels (data not shown), as demonstrated previously (27). Notably, simultaneous knockdown of both SPL and RGS4 expression resulted in OXO-M-induced calcium responses that were similar to those observed after knockdown of either SPL or RGS4 alone (Fig. 4).
Figure 4.
Effect of single and combined knockdown of RGS4 and SPL expression in MIN6 cells on M3R-mediated increases in [Ca2+]i. MIN6 cells were electroporated with RGS4 and SPL-1 siRNAs, either individually or in combination (Con-1=scrambled control siRNA). OXO-M-induced changes in [Ca2+]i were measured via the use of FLIPR technology. In each individual experiment, the maximum response obtained with cells treated with Con-1 siRNA was set equal to 100%. Data are expressed as means ± se of 3 independent experiments. ***P < 0.001 for RGS4/SPL-1 siRNA-treated cells vs. Con-1 siRNA-treated cells.
In agreement with the [Ca2+]i measurements, siRNA-mediated RGS4 knockdown mimicked the stimulatory effects of siRNA-mediated SPL knockdown on M3R-mediated insulin secretion (Fig. 5). Simultaneous knockdown of both SPL and RGS4 expression in MIN6 cells enhanced OXO-M-mediated insulin release in a fashion similar to that observed after knockdown of either SPL or RGS4 alone (Fig. 5). Thus, the outcomes of the RGS4/SPL-knockdown studies are consistent with a model in which SPL and RGS4 are components of the same cellular pathway that inhibits M3R activity in insulinoma cells.
Figure 5.
Effect of single and combined knockdown of RGS4 and SPL expression in MIN6 cells on M3R-mediated insulin release. A) Representative concentration-response curves for OXO-M-induced increases in insulin secretion. MIN6 cells were electroporated with RGS4 and SPL-1 siRNAs, either individually or in combination (Con-1=scrambled control siRNA). Cells were incubated with increasing concentrations of OXO-M in the presence of 16.7 mM glucose. B) Summary of basal and maximum insulin release (Emax) derived from OXO-M concentration-response curves. Data are expressed as means ± se of 5 independent experiments. ***P < 0.001 vs. Con-1 siRNA-treated cells.
SPL recruits RGS4 to the agonist-activated M3R (BRET experiments)
To monitor muscarinic agonist-dependent M3R/SPL/RGS4 interactions in intact cells, we employed BRET. Since BRET studies require the use of tagged proteins and MIN6 cells are difficult to transfect with plasmid DNA, we carried out the these experiments using transfected COS-7 cells.
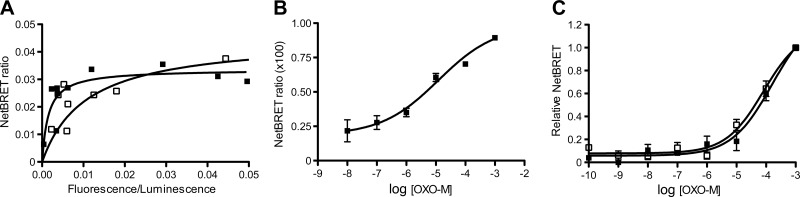
As a BRET donor, we used a human M3R construct fused at its C terminus to the RLuc8 coding sequence (M3R-Luc). We previously demonstrated that the M3R-Luc construct has similar ligand binding and functional properties as the WT M3R (25). To examine whether M3R-Luc was able to interact with SPL, we performed BRET saturation experiments using COS-7 cells coexpressing M3R-Luc and a modified version of SPL fused at its C terminus to eGFP (SPL-eGFP). Previous work showed that the eGFP tag does not interfere with normal SPL localization and function (18). In the BRET saturation studies, the amount of transfected M3R-Luc DNA (BRET donor) was held constant, while the amount of cotransfected SPL-eGFP DNA (BRET acceptor) was gradually increased. The resulting BRET data could be best fitted by a classical saturation isotherm in which NetBRET ratios increased as a hyperbolic function of the concentration of the acceptor (Fig. 6A), indicative of a specific interaction between M3R-Luc and SPL-eGFP. This interaction was characterized by a BRET50 value of 0.0102 ± 0.0068 (ratio of acceptor emission/donor emission that generates a half-maximal BRET signal; arbitrary units; n=3). BRET50 values are considered a measure of the relative affinity between specific BRET donor and acceptor proteins (25, 28, 29). Under the same experimental conditions, agonist treatment (OXO-M; 100 μM) of COS-7 cells coexpressing M3R-Luc and SPL-eGFP resulted in a ∼4-fold shift to the left of BRET saturation curves (BRET50 value=0.0028±0.0018; n=3; Fig. 6A). This observation supports the concept that M3R activation promotes M3R/SPL interactions. OXO-M (100 μM) treatment of COS-7 cells that coexpressed RGS4 together with M3R-Luc and SPL-eGFP yielded a BRET50 value that was not significantly different from the one observed in the absence of cotransfected RGS4 (0.0021±0.0002; n=4), suggesting that RGS4 does not further enhance the affinity of the M3R/SPL interaction or, alternatively, does not affect the proximity and relative orientation of the M3R/SPL complex (as inferred by BRET).
Figure 6.
BRET experiments. A) BRET saturation experiments demonstrate the formation of M3R-SPL complexes. BRET saturation studies were performed using COS-7 cells expressing a constant amount of M3R-Luc along with increasing amounts of SPL-eGFP. BRET data were obtained either in the absence of ligand (open squares) or in the presence of the muscarinic agonist, OXO-M (100 μM; solid squares). Data from a representative experiment are shown (see Materials and Methods for experimental details). Two additional experiments gave similar results. B) SPL promotes the interaction of M3R with RGS4 in an agonist-dependent fashion. BRET studies were carried out using COS-7 cells coexpressing M3R-Luc with a Venus-tagged version of RGS4 (RGS4-V) or with COS-7 cells coexpressing M3R-Luc, RGS4-V, and SPL. Cells were incubated with increasing concentrations of OXO-M. BRET measurements showed that OXO-M treatment had no significant effect on the BRET signals observed with cells coexpressing M3R-Luc and RGS4-V (data not shown; n=3). In contrast, OXO-M treatment of cells coexpressing M3R-Luc, RGS4-V, and SPL led to concentration-dependent increases in BRET signals. BRET signals are given as NetBRET ratios calculated by subtracting baseline BRET ratios obtained in the absence of SPL (M3R-Luc+RGS4-V) from BRET ratios obtained in the presence of SPL (M3R-Luc+RGS4-V+SPL). Data are given as means ± se of 3 independent experiments, each carried out in duplicate. C) SPL does not interfere with M3R-mediated recruitment of arrestin. BRET studies were carried out using COS-7 cells coexpressing M3R-Luc and a Venus-tagged version of arrestin-2 (arrestin-2-V; open squares) or with COS-7 cells coexpressing M3R-Luc, arrestin-2-V, and SPL (solid squares). Cells were incubated with increasing concentrations of OXO-M. In each individual experiment, maximum NetBRET values were set equal to 1. Data are given as means ± se of 3 independent experiments.
We next wanted to examine whether SPL was able to recruit RGS4 to the M3R in an agonist-dependent fashion. Initially, we incubated COS-7 cells coexpressing M3R-Luc and a modified version of RGS4 containing an N-terminal Venus tag (RGS4-V; BRET acceptor; ref. 19) with increasing concentrations of OXO-M. BRET studies showed that OXO-M treatment had no significant effect on the magnitude of the observed BRET signals (data not shown; n=3). In contrast, OXO-M treatment of COS-7 cells coexpressing M3R-Luc, RGS4-V, and SPL led to concentration-dependent increases in BRET signals (NetBRET ratios; Fig. 6B). The resulting concentration-response curve was characterized by an OXO-M EC50 value of 4.6 ± 3.0 μM (n=3). This observation supports a model in which SPL acts as an adaptor protein recruiting RGS4 to the M3R in an agonist-dependent fashion. However, we cannot completely exclude the possibility that the observed agonist effects involve an SPL-dependent reorientation between M3R-Luc and RGS4-V within a signaling complex involving various receptor-associated proteins.
SPL does not interfere with M3R-mediated recruitment of arrestins
Previous studies have shown that SPL interferes with the binding of arrestins to the α2A-adrenergic receptor (7). Members of the arrestin protein family play key roles in GPCR desensitization but can also function as scaffolding proteins to initiate new signaling pathways (30). We therefore carried out additional BRET studies to examine the potential effects of SPL on M3R/arrestin interactions. BRET techniques are widely used to examine the recruitment of arrestins to activated GPCRs in live cells (31–33). Arrestin-2 is a member of the arrestin family that is expressed in virtually all cell types, including pancreatic β cells (34). BRET studies with COS-7 cells coexpressing M3R-Luc and a modified version of arrestin-2 containing an N-terminal Venus tag (arrestin-2-V; BRET acceptor; ref. 20) demonstrated that the M3R was able to bind arrestin-2 in an agonist-dependent fashion (OXO-M EC50 value: 43.2±16.9 μM; n=3; Fig. 6C). Coexpression of SPL with M3R-Luc and arrestin-2-V had no significant effect on the intensities of the observed BRET signals (OXO-M EC50 value: 51.1±25.1 μM; n=3; Fig. 6C). These findings suggest that SPL does not compete with arrestin-2 for binding to the agonist-activated M3R, at least not under the experimental conditions of the BRET assay used for this analysis.
SPL does not affect cell surface M3R expression and agonist-induced M3R internalization in MIN6 cells
Studies with α2A-adrenergic (6) and μ-opioid (12) receptors indicated that SPL can affect cell surface GPCR expression levels. We therefore examined whether SPL had a similar effect on M3Rs expressed endogenously by MIN6 cells. Cell surface M3R expression levels were assessed by incubating MIN6 cells with a saturating concentration (2 nM) of the membrane-impermeable muscarinic antagonist [3H]-N-methylscopolamine ([3H]-NMS). We found that siRNA-mediated knockdown of SPL expression had no significant effect on cell surface M3R expression levels (number of [3H]-NMS binding sites, fmol/mg membrane protein: SPL-1 siRNA, 444±125; Con-2 siRNA, 483±90; n=3).
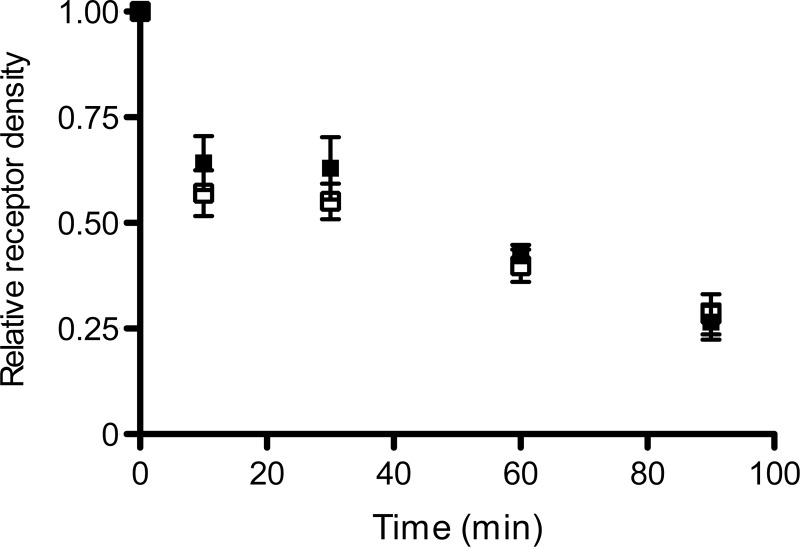
Wang et al. (7) demonstrated that SPL modulates agonist-induced α2A-adrenergic receptor sequestration, probably by interfering with receptor/arrestin interactions. We found that siRNA-mediated knockdown of SPL expression had no significant effect on agonist (OXO-M)-induced M3R internalization in MIN6 cells (Fig. 7). Taken together, these data indicate SPL does not interfere with cell surface M3R localization or agonist-induced M3R internalization.
Figure 7.
Knockdown of SPL expression in MIN6 cells has no significant effect on agonist-induced M3R internalization. MIN6 cells were electroporated with SPL-1 siRNA or scrambled control siRNA (Con-2), as described in detail in Materials and Methods. After 2 d, cells were treated with the agonist OXO-M (100 μM) at 37°C for the indicated periods of time. Cell surface M3R expression was determined by incubating cells with a saturating concentration (2 nM) of [3H]NMS, a membrane-impermeable muscarinic radioligand. In each individual experiment, M3R densities before agonist addition were set equal to 100% (see text for absolute receptor densities). Data are given as means ± se of 3 independent experiments.
SPL knockdown in MIN6 cells enhances calcium responses after agonist stimulation of other Gq-coupled receptors
Besides the M3R, pancreatic β cells and MIN6 cells express several other GPCRs that are linked to G proteins of the Gq family, including the P2Y1/6 receptor subtypes (agonist: ADP; ref. 35) and the V1b vasopressin receptor (agonist: AVP; ref. 36). To investigate whether SPL was able to modulate the activity of P2Y1/6 and V1b vasopressin receptors, we studied ADP- and AVP-meditated increases in [Ca2+]i in MIN6 cells. Following siRNA-mediated SPL knockdown, both ligands triggered concentration-dependent increases in [Ca2+]i, as compared with cells treated with scrambled control siRNA (Supplemental Fig. S1). While basal [Ca2+]i levels remained unaffected, SPL knockdown led to significant increases in ADP- or AVP-stimulated [Ca2+]i Emax levels as compared with control cells (Supplemental Fig. S1). ADP and AVP EC50 values were not significantly affected by the knockdown of SPL expression (ADP, control-1 siRNA: 1.14±0.78 μM; ADP, SPL-1 siRNA: 0.51±0.17 μM; AVP, control-1 siRNA: 0.18±0.02 μM; AVP, SPL-1 siRNA: 0.13±0.02 μM; n=3). These data indicate that SPL deficiency augments calcium responses in MIN6 cells following activation of different Gq-coupled receptors (M3R, P2Y1/6, and V1b vasopressin receptor subtypes).
M3R-mediated stimulation of insulin release is enhanced in SPL-deficient pancreatic islets
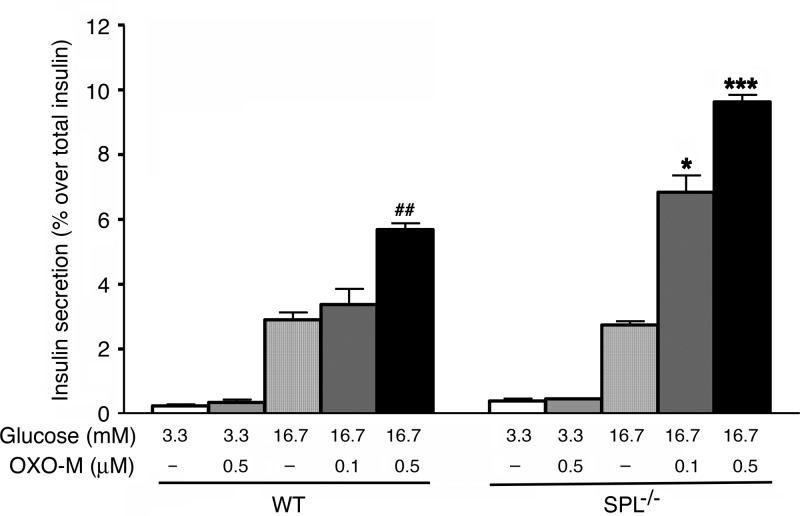
To examine whether SPL also acts as a negative regulator of M3R function in native β cells, we carried out insulin release studies using isolated pancreatic islets from SPL−/− mice and their WT littermates. In the presence of a low concentration of glucose (3.3 mM), OXO-M (0.5 μM) had no significant effect on basal insulin release in islets from either WT or SPL−/− mice (Fig. 8). In the presence of a stimulatory concentration of glucose (16.7 mM), the addition of 0.5 μM OXO-M (but not of 0.1 μM OXO-M) led to a significant augmentation of glucose-stimulated insulin secretion (GSIS) in WT islets, as reported previously (16). Strikingly, the OXO-M (0.5 μM)-mediated enhancement of GSIS was increased by ∼2-fold in SPL-deficient islets, as compared with WT islets (Fig. 8). Moreover, even the low concentration of OXO-M (0.1 μM) that had no effect on GSIS in WT islets led to a pronounced augmentation of GSIS in SPL−/− islets (Fig. 8).
Figure 8.
Augmentation of muscarinic agonist-mediated increases in glucose-dependent insulin release in pancreatic islets derived from SPL−/− mice. Isolated pancreatic islets prepared from adult WT and SPL−/− mice (males) were incubated for 1 h at 37°C in Krebs solution containing the indicated glucose concentrations, either in the absence or in the presence of the muscarinic agonist, OXO-M (0.1 and 0.5 μM). Amount of insulin secreted into the medium during the 1 h incubation period was normalized to the total insulin content of each well (islets plus medium). Data are expressed as means ± se of 4 independent experiments, each carried out in triplicate. *P < 0.05, ***P < 0.001 vs. corresponding WT values; ##P < 0.01 vs. WT treated with 16.7 mM glucose alone.
The total insulin content of islets prepared from SPL−/− mice was not significantly different from that of WT control islets (46.7±3.0 vs. 53.1±3.6 ng/islet, respectively; 18 independent batches of islets from different groups of mice were tested per strain). This observation excludes the possibility that changes in islet insulin content contributed to the enhanced efficacy of OXO-M to augment GSIS in SPL−/− islets.
We also incubated pancreatic islets from WT and SPL−/− mice with several other GPCR ligands (GLP-1, ADP, and AVP), using ligand concentrations known to enhance GSIS in mouse pancreatic islets (glucose concentration: 16.7 mM glucose; ref. 27). Compared with OXO-M, these 3 ligands showed less pronounced stimulatory effects on GSIS in WT islets (Supplemental Fig. S2). Moreover, GLP-1, ADP, and AVP responses were not significantly greater in SPL−/− than in WT islets (Supplemental Fig. S2). In contrast, under the same experimental conditions, treatment with OXO-M led to a pronounced augmentation in GSIS in SPL−/− islets (Supplemental Fig. S2; also see Fig. 8).
In vivo metabolic studies with SPL−/− mice and WT littermates
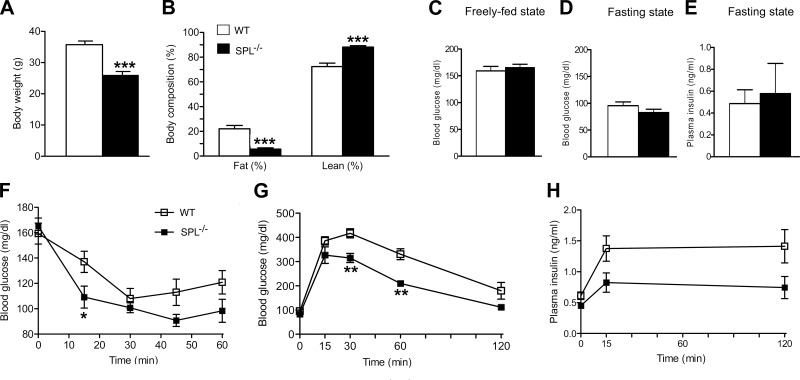
We next subjected SPL−/− mice and WT control littermates to a series of in vivo metabolic tests. SPL−/− mice and WT control littermates were obtained by intermating SPL+/− mice. We noted that SPL−/− mice showed a ∼30% reduction in body weight, associated with a dramatic decrease in percentage body fat mass (Fig. 9A, B). Blood glucose and plasma insulin levels did not differ significantly between SPL−/− mice and WT control littermates (Fig. 9C–E). An insulin tolerance test showed that SPL−/− mice displayed increased sensitivity toward exogenously administered insulin (Fig. 9F), most likely due to reduced adiposity. Moreover, compared with their WT control littermates, SPL−/− mice showed improved glucose tolerance in an intraperitoneal glucose (2 mg/g) tolerance test (Fig. 9G), consistent with the increase in insulin sensitivity associated with SPL deficiency. During the glucose tolerance test, SPL−/− mice secreted less insulin than their WT littermates (Fig. 9H), most likely as a result of increased insulin sensitivity; however, this difference failed to reach statistical significance (P>0.05).
Figure 9.
In vivo metabolic studies with SPL−/− mice and WT littermates. A) Body weight. B) Total body composition (% fat mass and % lean mass). C) Freely-fed blood glucose levels. D) Fasting blood glucose levels. E) Fasting plasma insulin levels. F) Insulin tolerance test. G) Intravenous glucose tolerance test (IGTT). H) In vivo insulin release during the IGTT. All studies were carried out with 16- to 18-wk-old male littermates (n=5–7/group; for experimental details, see Supplemental Data). Data are expressed as means ± se. ***P < 0.001, **P < 0.01, *P < 0.05 vs. corresponding WT values.
SPL is widely expressed in the brain and has been reported to modulate the activity of various GPCRs (see introduction for details). It therefore remains unclear by which mechanism SPL deficiency leads to reduced adiposity (leanness is usually associated with enhanced insulin sensitivity and improved glucose tolerance) and to which extent the lack of SPL/M3R interactions in β cells plays a role in modulating this phenotype.
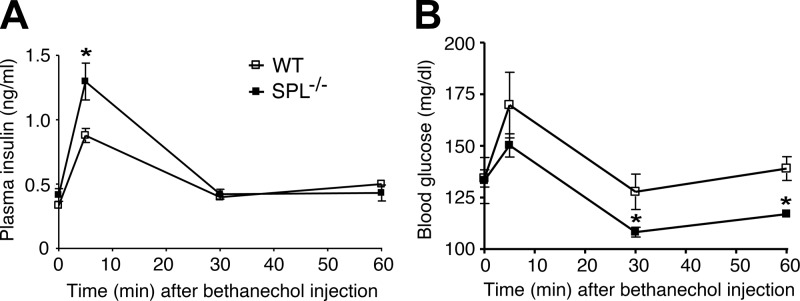
It is well known that treatment of WT mice with bethanechol, a peripherally acting muscarinic agonist, induces increases in plasma insulin concentrations and transient reductions in blood glucose levels (37). These bethanechol responses are absent in mutant mice selectively lacking M3Rs in pancreatic β cells (27), indicating that they are mediated by activation of β-cell M3Rs. Thus, to examine whether SPL was also able to interfere with M3R-mediated insulin release in vivo, we measured plasma insulin and blood glucose levels in WT and SPL−/− mice following bethanechol treatment (2 μg/g s.c.). We found that the insulin release-promoting and blood glucose-lowering effects of bethanechol were significantly more pronounced in SPL−/− mice than in WT control littermates (Fig. 10), indicating that SPL also acts as negative regulator of M3R-mediated insulin release in vivo.
Figure 10.
Bethanechol treatment of SPL−/− mice leads to enhanced insulin secretion and reduced blood glucose levels. SPL−/− mice and WT littermates (freely fed 14-wk-old female mice) received a single dose of the muscarinic agonist, bethanechol (2 μg/g s.c.). Plasma insulin (A) and blood glucose (B) levels were measured at the indicated time points. Data are shown as means ± se (n=4/group). *P < 0.05 vs. corresponding WT values.
For control purposes, we also injected WT and SPL−/− mice with the GLP-1 receptor agonist exendin-4 (12 nmol/kg i.p.; ref. 38). The acute effects of exendin-4 on plasma insulin and blood glucose levels are thought to be mediated by the activity of β-cell GLP-1 receptors that are coupled to the stimulatory G protein, Gs (39). In contrast to bethanechol, which was significantly more efficacious in SPL−/− mice than in WT littermates, exendin-4 had similar effects on plasma insulin and blood glucose levels in WT and SPL−/− mice (Supplemental Fig. S3).
DISCUSSION
Recent studies with mutant mice have shown that β-cell M3Rs play a central role in maintaining proper insulin release and normal whole-body glucose homeostasis (16, 17), suggesting that strategies aimed at enhancing signaling through β-cell M3Rs may prove beneficial in the treatment of T2D. At present, ligands that can selectively activate the M3R subtype are not available (40). Thus, one of our current research goals is to identify M3R-associated proteins or signaling pathways that can modulate the activity of β-cell M3Rs. Such studies may lead to the identification of novel protein targets for modulating β-cell M3R activity for therapeutic purposes.
In the present study, we examined whether SPL, a scaffolding protein known to regulate various functions of the CNS, was able to modulate signaling through β-cell M3Rs. This investigation was prompted by previous reports demonstrating that SPL can regulate the activity of various GPCRs (for a review, see ref. 2).
Initially, we used MIN6 cultured mouse insulinoma cells as an in vitro model system. Treatment of these cells with a muscarinic agonist (OXO-M) results in the activation of M3Rs, triggering a robust increase in [Ca2+]i and insulin secretion. Strikingly, siRNA-mediated knockdown of SPL expression led to a pronounced augmentation of these functional responses (Figs. 2 and 3), indicating that SPL is a potent negative regulator of M3R function in MIN6 cells.
To elucidate the molecular mechanisms underlying the ability of SPL to suppress M3R function in MIN6 cells, we carried out a series of biochemical and biophysical studies. Previous studies have shown that SPL can modulate the cell surface expression levels of certain GPCRs (6, 12) and can interfere with GPCR internalization (7, 12). However, we found that knockdown of SPL expression in MIN6 cells had no significant effect on cell surface M3R expression levels and agonist-induced M3R internalization (Fig. 7). Several years ago, Wang et al. (7) reported that SPL blocked the interaction of the α2A-adrenergic receptor with proteins of the arrestin family, thus interfering with multiple arrestin actions including receptor endocytosis. However, in the present study, we demonstrated, by using a BRET approach, that SPL overexpression did not interfere with the ability of the agonist-activated M3R to interact with arrestin-2, a ubiquitously expressed member of the arrestin family (Fig. 6C).
Biochemical and electrophysiological studies (11) also suggest that SPL can act as an adaptor molecule to recruit members of the RGS protein family to ligand-activated GPCR/G protein signaling complexes. RGS proteins act as GTPase-activating proteins to promote the hydrolysis of the active form of G-protein α-subunits (Gα-GTP), thus limiting the lifetime of the G-protein response (41, 42). Interestingly, we recently demonstrated that RGS4 is a potent negative regulator of M3R function in pancreatic β cells, both in vitro and in vivo (27). We therefore tested the hypothesis that the inhibitory effect of SPL on M3R signaling might be mediated by SPL-dependent recruitment of RGS4 to the active M3R/G-protein signaling complex. We found that siRNA-mediated RGS4 knockdown mimicked the stimulatory effects of siRNA-mediated SPL knockdown on M3R-mediated increases in [Ca2+]i and insulin secretion. Importantly, simultaneous knockdown of both SPL and RGS4 expression did not to further enhance the magnitude of these responses (Figs. 4 and 5). These observations are consistent with a model where SPL and RGS4 are components of the same cellular pathway that inhibits M3R activity in insulinoma cells.
To further explore M3R/SPL/RGS4 interactions in live cells, we carried out BRET studies using intact, transfected COS-7 cells. Initially, we performed saturation BRET studies using cells coexpressing an M3R-luciferase fusion construct (M3R-Luc; BRET donor) and a modified version of SPL fused to eGFP (SPL-eGFP; BRET acceptor). The resulting BRET data indicated that the M3R can interact with SPL-eGFP even in the absence of ligands. However, agonist (OXO-M) treatment of cells coexpressing M3R-Luc and SPL-eGFP resulted in a ∼4-fold shift to the left of BRET saturation curves (Fig. 6A), strongly suggesting that M3R activation enhances the affinity of SPL for the receptor. In agreement with these findings, a recent study demonstrated that the third intracellular loop of the M3R (fused to maltose binding protein) was able to interact with SPL in a pull-down assay using extracts from transfected HEK cells (3).
Additional BRET studies showed that OXO-M treatment of COS-7 cells coexpressing M3R-Luc and a Venus-tagged version of RGS4 (RGS4-V; BRET acceptor) did not lead to enhanced BRET signals. However, when M3R-Luc and RGS4-V were coexpressed with SPL, OXO-M caused concentration-dependent increases in BRET signals (Fig. 6B). Taken together, these findings are all consistent with a model in which SPL acts as an adaptor protein that is able to recruit RGS4 to the ligand-activated M3R/G protein complex, thus limiting the lifetime of M3R-activated G protein αq/11 subunits. This model also explains why SPL and RGS4 deficiency promote M3R signaling to a similar extent.
As is the case with other biophysical or biochemical techniques, the use of BRET technology is not without pitfalls. For example, we cannot completely exclude the possibility that some of the BRET signals were affected by changes in the relative orientation between BRET donor and acceptor (this caveat applies to BRET and FRET studies in general). However, the complete set of BRET data that we obtained, together with the functional data as well as published work, are consistent with a model in which SPL enhances RGS4 activity, probably by promoting the interaction between RGS4 and the receptor/G protein complex. Although the BRET data do not provide definite proof for such a mechanism, they are consistent with its likely existence.
To investigate whether SPL also inhibited M3R function in native β cells, we carried out insulin release studies using isolated pancreatic islets from SPL−/− mice and their WT littermates (Fig. 8). Consistent with previous studies (26, 27), OXO-M treatment of WT islets resulted in robust increases in GSIS (this response is mediated by β-cell M3Rs; ref. 26). Strikingly, the magnitude of these responses was greatly enhanced in SPL−/− islets. Interestingly, a low concentration of OXO-M (0.1 μM) that had no effect on GSIS in WT islets led to a pronounced augmentation of GSIS in SPL−/− islets. These data clearly indicate that SPL is a potent negative regulator of M3R function in native β cells.
Studies with MIN6 cells indicated that SPL did not only interfere with M3R signaling but also impaired signaling via other Gq-coupled receptors (P2Y1/6 and V1b vasopressin receptors; Supplemental Fig. S1). However, when we studied insulin release in isolated pancreatic islets from WT and SPL−/− mice, only OXO-M, but not ADP and AVP (these ligands act on β-cell P2Y1/6 and V1b vasopressin receptors, respectively; refs. 35, 36), led to enhanced GSIS in SPL-deficient islets (Supplemental Fig. S2). One possibility is that the very high sensitivity of pancreatic islets to treatment with muscarinic agonist is responsible for the observed differences between the in vitro and in vivo assays. However, the precise mechanisms underlying this phenomenon remain to be elucidated.
In general, SPL knockdown led to increases in agonist-induced maximal responses but had no significant effect on agonist EC50 values (in MIN6 cells). This observation is consistent with the absence of spare receptors, at least under the experimental conditions used in this study. However, we cannot exclude the possibility that more complex scenarios account for the selective increase in Emax values, involving, for example, differential expression levels and allosteric interactions between various receptor-associated proteins, including SPL and RGS4, and downstream signaling molecules.
To investigate whether SPL also interfered with the activity of β-cell M3Rs in vivo, we injected WT and SPL−/− mice with the muscarinic agonist, bethanechol (2 μg/g sc). Bethanechol treatment of WT mice is known to lead to increased plasma insulin and reduced blood glucose levels (37). Since these responses are not observed in mice that selectively lack M3Rs in pancreatic β cells, they are mediated by activation of β-cell M3Rs (27). Treatment of SPL−/− mice with bethanechol led to enhanced insulin release responses and blood glucose-lowering effects, as compared with WT control mice (Fig. 10). This observation clearly indicates that SPL also represents a potent negative regulator of β-cell M3R signaling in vivo.
Metabolic phenotyping studies showed that SPL-deficient mice had very little body fat (Fig. 9B). This lean phenotype was associated with improved insulin sensitivity and glucose tolerance (Fig. 9F, G). Since SPL is widely expressed throughput the CNS and is also present in many peripheral tissues, it remains unclear at present which specific signaling pathways are involved in the beneficial metabolic effects associated with SPL deficiency and whether the lack of SPL expression in pancreatic β cells contributes to the observed phenotype. Future studies with mutant mice that lack SPL only in certain tissues or cell types should help shed light on this issue.
In summary, our results demonstrate that SPL is an important negative regulator of β-cell M3R function. Given the important role of β-cell M3Rs in maintaining normal whole-body glucose homeostasis (16, 17), our findings suggest that strategies aimed at inhibiting SPL activity in pancreatic β cells might improve β-cell function and glucose tolerance in patients with T2D.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The authors thank Dr. Sara McMillin (NIDDK) and Drs. Diaz Gimenez and Vsevolod Gurevich (Vanderbilt University, Nashville, TN, USA) for their help setting up the BRET experiments. Dr. Paul Greengard (Rockefeller University, New York, NY, USA) generously provided the SPL−/− mice. Finally, the authors thank Drs. Patrick B. Allen (Yale University, New Haven, CT, USA) and Diaz Gimenez and Vsevolod Gurevich (Vanderbilt University), and Qin Wang (University of Alabama, Birmingham, AL, USA) for providing important reagents and plasmids used in this study. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AVP
- arginine vasopressin
- BRET
- bioluminescence resonance energy transfer
- CNS
- central nervous system
- DAG
- diacylglycerol
- GLP-1
- glucagon-like peptide 1
- GPCR
- G-protein-coupled receptor
- GSIS
- glucose-stimulated insulin secretion
- IP3
- inositol trisphosphate
- M3R
- M3 muscarinic acetylcholine receptor
- NMS
- N-methylscopolamine
- OXO-M
- oxotremorine-M
- RGS
- regulator of G-protein signaling
- SPL
- spinophilin
- T2D
- type 2 diabetes
- WT
- wild-type
REFERENCES
- 1. Allen P. B., Ouimet C. C., Greengard P. (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. U. S. A. 94, 9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarrouilhe D., di Tommaso A., Metaye T., Ladeveze V. (2006) Spinophilin: from partners to functions. Biochimie (Paris) 88, 1099–1113 [DOI] [PubMed] [Google Scholar]
- 3. Wang X., Zeng W., Kim M. S., Allen P. B., Greengard P., Muallem S. (2007) Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J. 26, 2768–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurogi M., Nagatomo K., Kubo Y., Saitoh O. (2009) Effects of spinophilin on the function of RGS8 regulating signals from M2 and M3-mAChRs. Neuroreport 20, 1134–1139 [DOI] [PubMed] [Google Scholar]
- 5. Richman J. G., Brady A. E., Wang Q., Hensel J. L., Colbran R. J., Limbird L. E. (2001) Agonist-regulated interaction between α2-adrenergic receptors and spinophilin. J. Biol. Chem. 276, 15003–15008 [DOI] [PubMed] [Google Scholar]
- 6. Brady A. E., Wang Q., Colbran R. J., Allen P. B., Greengard P., Limbird L. E. (2003) Spinophilin stabilizes cell surface expression of α2B-adrenergic receptors. J. Biol. Chem. 278, 32405–32412 [DOI] [PubMed] [Google Scholar]
- 7. Wang Q., Zhao J., Brady A. E., Feng J., Allen P. B., Lefkowitz R. J., Greengard P., Limbird L. E. (2004) Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science 304, 1940–1944 [DOI] [PubMed] [Google Scholar]
- 8. Wang Q., Limbird L. E. (2002) Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. J. Biol. Chem. 277, 50589–50596 [DOI] [PubMed] [Google Scholar]
- 9. Lefkowitz R. J., Rajagopal K., Whalen E. J. (2006) New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell 24, 643–652 [DOI] [PubMed] [Google Scholar]
- 10. Luttrell L. M., Gesty-Palmer D. (2010) Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 62, 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X., Zeng W., Soyombo A. A., Tang W., Ross E. M., Barnes A. P., Milgram S. L., Penninger J. M., Allen P. B., Greengard P., Muallem S. (2005) Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat. Cell Biol. 7, 405–411 [DOI] [PubMed] [Google Scholar]
- 12. Charlton J. J., Allen P. B., Psifogeorgou K., Chakravarty S., Gomes I., Neve R. L., Devi L. A., Greengard P., Nestler E. J., Zachariou V. (2008) Multiple actions of spinophilin regulate mu opioid receptor function. Neuron 58, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nag S., Wang Q., Limbird L. E., Mokha S. S. (2009) Knockout of spinophilin, an endogenous antagonist of arrestin-dependent α2-adrenoceptor functions, enhances receptor-mediated antinociception yet does not eliminate sex-related differences. Behav. Brain Res. 197, 457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wess J. (1996) Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 10, 69–99 [DOI] [PubMed] [Google Scholar]
- 15. Gilon P., Henquin J. C. (2001) Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 22, 565–604 [DOI] [PubMed] [Google Scholar]
- 16. Gautam D., Han S. J., Hamdan F. F., Jeon J., Li B., Li J. H., Cui Y., Mears D., Lu H., Deng C., Heard T., Wess J. (2006) A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 3, 449–461 [DOI] [PubMed] [Google Scholar]
- 17. Gautam D., Ruiz de Azua I., Li J. H., Guettier J. M., Heard T., Cui Y., Lu H., Jou W., Gavrilova O., Zawalich W. S., Wess J. (2010) Beneficial metabolic effects caused by persistent activation of beta-cell M3 muscarinic acetylcholine receptors in transgenic mice. Endocrinology 151, 5185–5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brady A. E., Wang Q., Allen P. B., Rizzo M., Greengard P., Limbird L. E. (2005) α2-adrenergic agonist enrichment of spinophilin at the cell surface involves βγ subunits of Gi proteins and is preferentially induced by the α2A-subtype. Mol. Pharmacol. 67, 1690–1696 [DOI] [PubMed] [Google Scholar]
- 19. Ishii M., Ikushima M., Kurachi Y. (2005) In vivo interaction between RGS4 and calmodulin visualized with FRET techniques: possible involvement of lipid raft. Biochem. Biophys. Res. Commun. 338, 839–846 [DOI] [PubMed] [Google Scholar]
- 20. Vishnivetskiy S. A., Gimenez L. E., Francis D. J., Hanson S. M., Hubbell W. L., Klug C. S., Gurevich V. V. (2011) Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J. Biol. Chem. 286, 24288–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng J., Yan Z., Ferreira A., Tomizawa K., Liauw J. A., Zhuo M., Allen P. B., Ouimet C. C., Greengard P. (2000) Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. U. S. A. 97, 9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishihara H., Asano T., Tsukuda K., Katagiri H., Inukai K., Anai M., Kikuchi M., Yazaki Y., Miyazaki J. I., Oka Y. (1993) Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 23. Scarselli M., Li B., Kim S. K., Wess J. (2007) Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J. Biol. Chem. 282, 7385–7396 [DOI] [PubMed] [Google Scholar]
- 24. Krumins A. M., Barker S. A., Huang C., Sunahara R. K., Yu K., Wilkie T. M., Gold S. J., Mumby S. M. (2004) Differentially regulated expression of endogenous RGS4 and RGS7. J. Biol. Chem. 279, 2593–2599 [DOI] [PubMed] [Google Scholar]
- 25. McMillin S. M., Heusel M., Liu T., Costanzi S., Wess J. (2011) Structural basis of M3 muscarinic receptor dimer/oligomer formation. J. Biol. Chem. 286, 28584–28598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duttaroy A., Zimliki C. L., Gautam D., Cui Y., Mears D., Wess J. (2004) Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in M3 muscarinic acetylcholine receptor-deficient mice. Diabetes 53, 1714–1720 [DOI] [PubMed] [Google Scholar]
- 27. Ruiz de Azua I., Scarselli M., Rosemond E., Gautam D., Jou W., Gavrilova O., Ebert P. J., Levitt P., Wess J. (2010) RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 107, 7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfleger K. D., Eidne K. A. (2006) Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Meth. 3, 165–174 [DOI] [PubMed] [Google Scholar]
- 29. Mercier J. F., Salahpour A., Angers S., Breit A., Bouvier M. (2002) Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J. Biol. Chem. 277, 44925–44931 [DOI] [PubMed] [Google Scholar]
- 30. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kocan M., Pfleger K. D. (2011) Study of GPCR-protein interactions by BRET. Methods Mol. Biol. 746, 357–371 [DOI] [PubMed] [Google Scholar]
- 32. Klewe I. V., Nielsen S. M., Tarpo L., Urizar E., Dipace C., Javitch J. A., Gether U., Egebjerg J., Christensen K. V. (2008) Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology 54, 1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamdan F. F., Audet M., Garneau P., Pelletier J., Bouvier M. (2005) High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin2 recruitment assay. J. Biomol. Screen. 10, 463–475 [DOI] [PubMed] [Google Scholar]
- 34. Sonoda N., Imamura T., Yoshizaki T., Babendure J. L., Lu J. C., Olefsky J. M. (2008) Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. U. S. A. 105, 6614–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balasubramanian R., Ruiz de Azua I., Wess J., Jacobson K. A. (2010) Activation of distinct P2Y receptor subtypes stimulates insulin secretion in MIN6 mouse pancreatic beta cells. Biochem. Pharmacol. 79, 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winzell M. S., Ahrén B. (2007) G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol. Ther. 116, 437–448 [DOI] [PubMed] [Google Scholar]
- 37. Fukudo S., Virnelli S., Kuhn C. M., Cochrane C., Feinglos M. N., Surwit R. S. (1989) Muscarinic stimulation and antagonism and glucoregulation in nondiabetic and obese hyperglycemic mice. Diabetes 38, 1433–1438 [DOI] [PubMed] [Google Scholar]
- 38. Arakawa M., Ebato C., Mita T., Hirose T., Kawamori R., Fujitani Y., Watada H. (2009) Effects of exendin-4 on glucose tolerance, insulin secretion, and beta-cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem. Biophys. Res. Commun. 390, 809–814 [DOI] [PubMed] [Google Scholar]
- 39. Doyle M. E., Egan J. M. (2007) Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 113, 546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wess J., Eglen R. M., Gautam D. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 6, 721–733 [DOI] [PubMed] [Google Scholar]
- 41. Hollinger S., Hepler J. R. (2002) Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol. Rev. 54, 527–559 [DOI] [PubMed] [Google Scholar]
- 42. Ross E. M., Wilkie T. M. (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.