Abstract
Normal pregnancy involves dramatic remodeling of the uterine vasculature, with abnormal vascular adaptations contributing to pregnancy diseases such as preeclampsia. The peptide hormone relaxin is important for the renal and systemic hemodynamic adaptations to pregnancy, and has been shown to increase arterial compliance and outward hypertrophic remodeling. Therefore, we investigated the possibility that relaxin acts on its receptor, RXFP1, to mediate uterine artery compliance in late pregnancy and increase uterine blood flow velocity in rats. RXFP1 was predominantly localized to the tunica media vascular smooth muscle cells in the uterine artery, although receptors were also detected in endothelial cells. Highest expression of Rxfp1 in the uterine artery occurred in estrus and early pregnancy. Isolated uterine arteries from late pregnant rats treated with a monoclonal antibody against circulating relaxin (MCA1) had significantly increased vessel wall stiffness compared with controls, with no reduction in wall thickness. Chronic infusion of relaxin (4 μg/h, osmotic minipump) for 5 d in nonpregnant rats significantly increased uterine artery blood flow velocity. Overall, these data demonstrate a functional role for relaxin in mediating uterine artery compliance in pregnant rats, which may be necessary to maintain adequate uterine blood flow to the uterus and placenta.—Vodstrcil, L. A., Tare, M., Novak, J., Dragomir, N., Ramirez, R. J., Wlodek, M. E., Conrad, K. P., Parry, L. J. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow.
Keywords: RXFP1, extracellular matrix, preeclampsia, vascular smooth muscle
In normal pregnancy, the uteroplacental vasculature undergoes dramatic structural and functional modifications to allow for a large increase in blood flow to the fetoplacental unit (1, 2). Failure of the maternal uterine vascular system to adapt to pregnancy, with reduced placental perfusion in early gestation, gives rise to serious complications, including gestational hypertension, preeclampsia, and intrauterine growth restriction (3–5). These pregnancy complications are a leading cause of maternal and fetal death and result in long-term health implications for the neonate.
The rapid and sustained increase in uterine blood flow during pregnancy is paralleled by outward hypertrophic remodeling of the uterine artery wall. Specifically, the lumen diameters of the main uterine and radial arteries increase 2- to 3- fold, with little thickening of the vessel wall in most species (2, 6, 7). In mice, there is an increase in uterine artery wall thickness in early pregnancy, associated with smooth muscle cell proliferation (8, 9). Uterine and radial arteries also become longer and more distensible in both axial and radial directions (10). In rats, maximum remodeling of the smaller distal arteries occurs in midgestation, whereas growth and remodeling of the main uterine artery is greatest in late pregnancy (11). These changes are attributed to an elongation of vascular smooth muscle cells (hypertrophy) and cell hyperplasia within the vessel wall, as indicated by increases in mitotic activity (11–13). Furthermore, changes in the composition of the vessel extracellular matrix (ECM) alter biomechanical properties, such as distensibility and compliance (1, 2). Distension of the uterine artery with advancing pregnancy is the result of a reduced collagen to elastin ratio due to decreased collagen in the ECM (8, 9, 14). The mechanisms underlying outward hypertrophic remodeling of the uterine vasculature during pregnancy, particularly of the main uterine artery, are influenced by a combination of both local and systemic pregnancy-dependent factors (11, 12, 15).
There is substantial evidence that the peptide hormone relaxin contributes to renal and systemic hemodynamic adaptations to pregnancy (16–18). Chronic administration of relaxin to conscious rats increases glomerular filtration rate and renal blood flow, attenuates the renal circulatory response to angiotensin II, reduces systemic vascular resistance, and increases global arterial compliance (19, 20). Myogenic reactivity is also reduced in small renal and mesenteric arteries isolated from rats treated with relaxin for 5 d (21). Conversely, treatment of pregnant rats with a relaxin-neutralizing antibody attenuates renal and systemic vasodilation (22, 23). Identification of gene transcripts for relaxin/insulin-like family peptide receptor 1 (Rxfp1) in mouse and pregnant rat renal arteries and aorta (24, 25) strengthens the hypothesis that relaxin plays a significant role in mediating renal and systemic vasodilation during pregnancy.
Relaxin administration in rats and mice also increases arterial compliance in small renal arteries, mesenteric arteries, brain parenchymal arteries, and carotid arteries (26–28). In relaxin-treated mice, the increase in small renal artery compliance is mediated by both geometric (outward) and compositional (decreased collagen) remodeling (26). Relaxin infusion in nonpregnant rats also increases wall thickness and inner diameter of brain parenchymal arterioles, effects mediated by peroxisome proliferator-activated receptor-γ (27). The small renal arteries of relaxin-deficient mice have decreased passive compliance relative to wild-type mice (24) and decreased unstressed wall areas (26). This is due to a decrease in wall-to-lumen area ratio, decreased smooth muscle cell density, and increased collagen to total protein ratio. Based on these effects of relaxin treatment and relaxin deficiency on arterial compliance and remodeling, we tested the hypothesis that relaxin mediates these parameters in the uterine vasculature during pregnancy and increases uterine artery blood flow. The main objectives of this study were to investigate whether relaxin receptor RXFP1 is expressed in the uterine artery, and if so, in which vascular compartments; whether neutralization of circulating relaxin in late pregnant rats compromises uterine artery remodeling and affects passive compliance; and whether relaxin administration to conscious, nonpregnant rats increases uterine artery flow velocity.
MATERIALS AND METHODS
The experimental protocols were approved either by the University of Melbourne Animal Experimentation Ethics Committee or the University of Akron Institutional Animal Care and Use Committee. Two groups of rats were used for different aspects of this study. For the studies in Australia, primigravid Wistar Kyoto rats (∼200 g body weight; Animal Resources Centre, Murdoch, WA, Australia) had access to standard rat cubes (Specialty Feed, Glenforrest, WA, Australia) and water ad libitum in the Biological Research Facility (Departments of Pharmacology and Physiology, University of Melbourne, Melbourne, VIC, Australia). The presence of sperm in the vaginal lavage was designated as d 1 of the 22-d gestation. For the uterine blood flow studies in nonpregnant conscious animals, Long-Evans rats were purchased from Harlan Sprague Dawley (Frederick, MD, USA) at 12–14 wk of age and housed in the University of Akron Research Vivarium (Akron, OH, USA). They were fed Prolab RMH2000 diet (PMI, Brentwood, MI, USA), with water available ad libitum.
Localization and expression of Rxfp1 in the uterine artery of pregnant rats
Wistar Kyoto rats were killed by an overdose of anesthetic (100 mg/kg ketamine and 30 mg/kg illium xylazil-20 i.p.) on d 8, 15, and 20 of gestation (n=6 rats/group). The main branch of the uterine artery was dissected from surrounding fatty tissue in ice-cold 0.1 M phosphate-buffered saline (PBS). Uterine arteries were also dissected from nonpregnant animals in estrus (n=8). Tissues were snap-frozen in liquid nitrogen and then stored at −80°C until RNA extraction. In addition, uterine arteries were fixed in paraformaldehyde-lysine-periodate (PLP) fixative (8% paraformaldehyde, 0.2 M lysine, and 0.01 M periodate in 0.1 M PBS) for 24 h and transferred to 0.1 M PBS for immunohistochemistry and double-labeling immunofluorescence.
Immunohistochemistry and immunofluorescence
Tissue samples fixed in PLP were dehydrated in a graded series of increasing ethanol concentrations, embedded in polyester wax (polyethylene glycol 400 distearate; Polysciences, Warrington, PA, USA), and 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Brightfield
Sections were incubated in 3% hydrogen peroxide for 90 min to block endogenous peroxidase and 2% normal goat serum (NGS) for 1 h to reduce nonspecific binding. Excess NGS was removed, and samples were incubated overnight at 4°C with either 3 μg/ml rat RXFP1 antiserum (#107, raised against amino acid residues 107–119 of the rat RXFP1 protein) or preimmune serum (rabbit IgG). These were kindly supplied by Prof. O. D. Sherwood (Department of Physiology and Biophysics, University of Illinois, Urbana, IL, USA) and purified on protein A agarose affinity columns. Immunoreactivity was detected using the MACH 2 system (Biocare Medical, Concord, CA, USA) and 3,3′-diaminobenzidine (DAB) as the Chromagen substrate (Vector Laboratories, Burlingame, CA, USA) as described previously (29). To enhance the signal, sections were incubated in 0.05 M sodium bicarbonate (pH 9.0) for 10 min and DAB Enhance (Vector Laboratories) for 20 s.
Fluorescence
All procedures were the same as described above, except sections were not incubated in hydrogen peroxidase and different secondary antibodies and detection systems were used. To identify RXFP1, goat anti-rabbit Dylight 549 (Biocare Medical) was applied for 1 h, followed by a 1:1000 dilution of mouse monoclonal antibody against rodent smooth muscle actin conjugated to fluorescein isothiocyanate (FITC; Sigma-Aldrich Pty. Ltd., Castle-Hill, NSW, Australia). Control samples were incubated with either 3 μg/ml preimmune serum (rabbit IgG) or a 1:1000 dilution of anti-mouse IgG conjugated to FITC. Sections were coverslipped using FluoroCare mounting medium (Biocare Medical) and imaged under 488 and 549 wavelengths on a Zeiss imager D1 with AxioCam mrC5 camera (Carl Zeiss Pty. Ltd., North Ryde, NSW, Australia).
Rxfp1 gene expression
Frozen arteries were placed in a prechilled Wig-L-Bug capsule with a metal silver ball and pulverized in a Digital Wig-L-Bug amalgamator (Dentsply Ltd, York, PA, USA). Total RNA was then extracted with Trizol (Invitrogen, Mulgrave, VIC, Australia) according to the manufacturer's instructions. The concentration of RNA was determined using a Bio-Rad Smart Spec 3000 (Bio-Rad Laboratories Pty. Ltd., Gladesville, NSW, Australia) with A260/A280 absorbance ratios > 1.9, indicating sufficiently pure RNA for PCR analysis. First-strand cDNA synthesis was performed using 1 μg total RNA in a 20-μl reaction with random hexamers (50 ng/μl) and 200 U SuperScript III (Invitrogen). Initially, the expression of Rxfp1 was examined by RT-PCR. One microliter of cDNA was used in a 25 μl reaction containing 5× Green Go Taq Flexi, 1.25 U Go TaqDNA Polymerase, 2 mM MgCl2, and 2 mM dNTPs (Promega, Annandale, SA, Australia), together with 50 ng of Rxfp1 forward and reverse oligonucleotide primers (GeneWorks, Hindmarsh, SA, Australia), which were designed to span an intron (30). The presence of a second relaxin-family peptide receptor, Rxfp2, was also assessed, using a similar protocol. Rat myometrium cDNA was used as a positive control for both Rxfp1 and Rxfp2. For negative controls, water replaced the cDNA template to verify that there was no contamination, and water replaced the reverse transcriptase in the cDNA synthesis to show that PCR products did not result from genomic DNA. The PCR reaction was performed in the GeneAmp PCR system 2700 (Applied Biosystems, Scoresby, VIC, Australia), with conditions as described previously (29). The PCR products were subjected to agarose gel electrophoresis (1.8% agarose in tris-borate-EDTA, with ethidium bromide) at 100 V for 40 min.
Quantification of Rxfp1 gene expression in the uterine artery was accomplished using the ΔΔCt method with ribosomal 18S (Rn18s) as the endogenous control, rat specific Rxfp1 forward/reverse primers and 6-carboxyl fluorescein (FAM)-labeled TaqMan probes (29). Optimal concentrations for all primers and probes were 300 and 100 nM, respectively. Quantitative PCR experiments were performed on the Rotor-Gene v6 (Corbett Life Science, Mortlake, VIC, Australia), in triplicate 20-μl volumes containing 1× SensiMix dT (Quantace Pty Ltd, Alexandria, NSW, Australia). The mean ribosomal Rn18s Ct value of each triplicate was subtracted from the corresponding mean gene of interest Ct triplicate value to normalize gene of interest expression to Rn18s. These normalized data were then presented as a value relative to nonpregnant rats, and plotted with the sd using the −2ΔΔCt method of analysis (29).
Effects of neutralizing endogenous relaxin on uterine arterial compliance
To test the hypothesis that relaxin contributes to uterine artery remodeling and increases distensibility in pregnancy, we neutralized circulating relaxin in pregnant rats with a monoclonal antibody against relaxin for 3 d. This duration was sufficient to decrease interpubic ligament length and myometrial VEGF expression, and to alter cervix morphology (31). On d 14 of gestation, rats were initially anesthetized with 4.2% fluorothane and then maintained at 2.9% (Univentor 400, Agnthos AB, Lidingö, Sweden). Once anesthetized, rats were chronically instrumented using sterile techniques with a Tygon catheter (PVC tubing, 1.50×1.00 mm; Microtube Extrusions, North Rocks, NSW, Australia) implanted in the right jugular vein with the tip located at the junction of the anterior vena cava and right atrium. From d 15 of gestation, the catheter was flushed twice daily with heparinized saline. Neutralizing monoclonal antibodies against rat relaxin (MCA1; n=8 pregnant rats) or control antibodies against fluorescein (MCAF; n=8 pregnant rats) were administered daily between 9 and 11 AM for 3 d beginning on d 17 of gestation. Each dose of 5 mg antibody in 0.5 ml 0.1 M PBS was infused over 5 min into the jugular vein catheter. Animals were killed on the morning of d 20 of gestation by an intraperitoneal overdose of an anesthetic (100 mg/kg ketamine and 30 mg/kg illium xylazil-20). The main right uterine artery was dissected from surrounding fat in ice-cold 0.1 M PBS for analysis of passive mechanical wall properties. The remaining portion of the right uterine artery was fixed in 10% neutral buffered formalin for histological analysis. The left uterine artery was carefully dissected and frozen in liquid nitrogen before storage at −80°C until RNA extraction.
Passive mechanical wall properties
Arteries were transferred to a Ca2+-free, EGTA (1 mM) containing physiological saline solution (PSS; 120 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 1 mM KH2PO4, 1.2 mM MgSO4, and 11 mM glucose) for 1 h. Leak-free segments (3–4 mm) were then mounted on a glass cannula (tip diameter ∼200 μm) filled with Ca2+-free PSS. The lumen was gently flushed to remove any remaining blood, and then the distal end was tied off to prevent flow (Living Systems Instrumentation, Burlington, VT, USA). Segments were superfused at ∼15 ml/min with Ca2+-free EGTA-PSS prebubbled with carbogen (95% O2, 5% CO2) at 37°C. After a 20-min equilibration period, intraluminal pressure was increased from 0 to 120 mmHg, in 10-mmHg increments. The vessel length, outside diameter (OD), and wall thickness (WT) were measured at each pressure step. Internal diameter (ID) was calculated by subtracting the WT from the OD. Wall stress and strain were derived from the following calculations: wall stress (kPa) = (intraluminal pressure × ID)/(2 × WT); wall strain = (ID − ID extrapolated to 5 mmHg pressure)/(ID extrapolated to 5 mmHg pressure), as described previously (32). For normalization of ID and OD, values were expressed as a percentage of diameter at 5 mmHg.
Analysis of the ECM
To analyze structural components of the uterine artery, rat specific forward/reverse primers and TaqMan probes for matrix metalloproteinase 2 and 9 (Mmp2 and Mmp9), tissue inhibitor of MMP 1 and 2 (Timp1 and Timp2), collagen type I α 1 (col1a1), collagen type III α 1 (col3a1), collagen type IV α 1 (col4a1), estrogen receptor 1 (Esr1), and vascular endothelial growth factor (Vegf) were designed and purchased from Biosearch Technologies (Novato, CA, USA) and used in quantitative PCR as described above. Histological analysis of collagen and elastin used fixed uterine artery segments embedded in paraffin. Transverse serial sections (5 μm) were stained with picrosirius red (0.1% sirius red in saturated picric acid) or Gomori aldehyde fuchsin to examine collagen and elastin, respectively, using the bright field microscopy and circular cross-polarized light (CCPL) techniques described in Mazzuca et al. (33). Details of the methods are provided in Supplemental Data. An equal number of uterine artery sections from MCAF- and MCA1-treated animals were stained at the same time under identical conditions and photographed using the same magnification and light intensity. Measurements were made only on sections consisting of complete cross sections of artery. Images were analyzed by 2 independent examiners blinded to the study group.
Effects of relaxin on uterine artery blood flow in conscious nonpregnant rats
All surgeries were performed under 2% isofluorane anesthesia. Female Long-Evans rats (8–10 wk) were ovariectomized to eliminate the effects of endogenous hormones, which fluctuate during the estrus cycle. To measure uterine artery flow velocity, a miniature Doppler probe (Crystal Biotech, Inc., Northborough, MA, USA) encased in a silastic cuff with a lumen diameter of 0.4 mm was placed on a segment of the left main uterine artery near its confluence with the left common iliac artery. The flow probe wires were then tunneled under the skin and exteriorized at the back of the neck. All animals were allowed a 7-d recovery period. Following the recovery period, uterine artery flow velocities were measured for 1 h at the same time each day (between 10 and 11 AM) for 3 d. A 7-d Alzet osmotic minipump (Model 2001; Durect Corp, Cupertino, CA, USA) was then inserted subcutaneously in the back of the animal under isofluorane anesthesia. Recombinant human relaxin (rhRLX; n=8) kindly provided by Corthera Inc. (San Mateo, CA, USA) or vehicle (20 mM sodium acetate, pH 5; n=3) was chronically infused at a dose of 4 μg/h for 5 d, which yields concentrations of circulating relaxin similar to those measured on gestational d 12–14 in pregnant rats (20–40 ng/ml; ref. 34). Daily 1-h measurements of uterine artery flow velocity were taken at the same time every day (10–11 AM) for the duration of the infusion. After the rhRLX-infusing minipump expired, uterine artery flow velocity was measured for 1 h daily for another 3 d. During each hour of recording, the data were collected in real time using a computer data acquisition program (Dataq, Akron, OH, USA). The data from each day's recording was averaged to give a mean uterine artery flow velocity reading measured in kilohertz.
Statistical analysis
One-way ANOVA was used to assess the differences in Rxfp1 gene expression across the pregnant and nonpregnant groups, with Tukey post hoc comparisons of individual group means (SPSS 17; SPSS Inc., Chicago, Illinois, USA). The stress-strain data were analyzed with repeated-measures 2-way ANOVA (treatment vs. strain; GraphPad Software Inc., San Diego, CA, USA). Independent t tests assessed statistical differences in arterial gene expression between MCA1- and MCAF-treated rats. Significant differences in the number of birefringent collagen fibers represented by each color (red, orange, yellow, and green) and treatment group were analyzed by 2-way ANOVA, with Tukey post hoc comparisons. A general linear model repeated-measures analysis determined differences in uterine artery flow velocity between days of treatment and in both rhRLX- and vehicle-treated rats (SPSS). Independent t tests were used to determine differences between rhRLX- and vehicle-infused rats on different days of treatment. A value of P < 0.05 was considered statistically significant.
RESULTS
Localization and expression of RXFP1 in the uterine artery
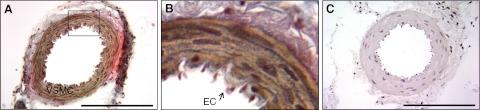
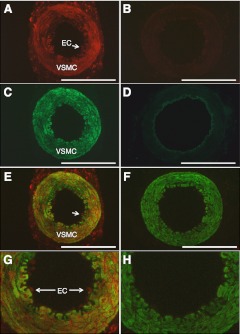
Immunoreactive RXFP1 was localized predominantly to the tunica media of the main branch of the uterine artery obtained on d 15 of gestation (Fig. 1A, B). There was no RXFP1 staining in the preimmune serum negative control (Fig. 1C). At higher magnification, immunoreactive RXFP1 appeared to be localized to endothelial cells lining the lumen of the artery (Fig. 1B). Double-labeling immunofluorescence clarified the cellular localization of RXFP1 in the uterine artery (Fig. 2). RXFP1 and α-smooth muscle actin (α-SMA) were detected in the vascular smooth muscle cells within the tunica media (Fig. 2A, C), with the overlay demonstrating colocalization (Fig. 2E, G). The negative controls with preimmune rabbit serum or mouse IgG revealed little if any background fluorescence (Fig. 2B, D), confirming the specificity of the two antibodies used. Similarly, when the primary RXFP1 antibody was replaced with preimmune serum in the overlay, there was only positive staining for α-SMA (Fig. 2F, H). Higher magnification revealed immunoreactive RXFP1 in endothelial cells that did not stain positive for α-SMA (Fig. 2G). In this method of analysis, immunoreactive RXFP1 was detected in the adventitia as well as the vascular smooth muscle (Fig. 2A).
Figure 1.
A) Localization of RXFP1 protein in the rat uterine artery on d 15 of gestation using immunohistochemistry. B) Enlarged view of boxed area in panel A. C) Preimmune rabbit serum was used as a negative control. VSMC, vascular smooth muscle cells; EC, endothelial cell. Scale bar = 500 μm.
Figure 2.
Colocalization of RXFP1 and α-SMA in the rat uterine artery on d 15 of gestation using immunofluorescence. A) Antiserum 107 for RXFP1 (3 μg/ml). B) Preimmune rabbit serum (3 μg/ml). C) α-SMA antiserum, 1:1000. D) α-SMA negative control, 1:1000. E) Fusion of images A, C. F) Image B fused with the same sample probed with α-SMA antiserum. G) Zoomed image of E. H) Zoomed image of F. VSMC, vascular smooth muscle cells; EC, endothelial cells (also indicated by white arrow). Scale bars = 500 μm.
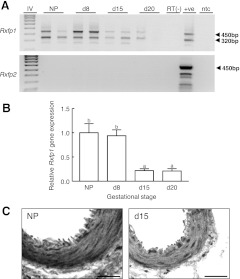
Two Rxfp1 gene transcripts were present in the rat uterine artery at all stages of gestation analyzed and in nonpregnant rats (Fig. 3A). The 450-bp product represents the full-length variant of Rxfp1, whereas the 320-bp product is likely to be the Rxfp1 splice variant reported in previous studies (30, 35). These two products were also present in the myometrium positive control, but were absent from the two negative controls, confirming that the PCR products did not originate from genomic DNA. Interestingly, the intensity of the bands was more prominent in uterine arteries from nonpregnant and gestation d 8 rats. There were no Rxfp2 transcripts detected in the uterine artery (Fig. 3A). Quantitative PCR confirmed that uterine artery Rxfp1 gene expression was significantly (F3,27=5.55, P<0.01) higher in nonpregnant rats and on d 8 of gestation compared with d 15 and 20 of gestation (Fig. 3B). The intensity of RXFP1 immunostaining in the vascular smooth muscle of the uterine artery was also higher in nonpregnant rats compared to d 15 of gestation (Fig. 3C).
Figure 3.
A) RT-PCR of Rxfp1 and Rxfp2 mRNA expression in the uterine artery of pregnant rats at different stages of gestation (d 8, 15, and 20) and nonpregnant (NP) rats. The two Rxfp1 bands represent the full-length (450 bp) and truncated (320 bp) variants. There was no Rxfp2 gene expression in uterine arteries. IV, Hyperladder IV DNA ladder; RT(−), reverse-transcriptase negative control; +ve, myometrium sample positive control; ntc, no-template control. B) Quantitative Rxfp1 expression in the uterine artery of pregnant rats at different stages of gestation relative to NP levels (n=6/d). Rxfp1 expression is standardized to the reference gene Rn18S and presented relative to NP uterine artery. Data labeled with different letters are significantly (P<0.05) different from each other. Bars represent means ± sd as calculated using the −2ΔΔCt method (n=6/group). C) Immunohistochemistry showing less intense immunoreactive RXFP1 in uterine arteries from d 15 pregnant rats compared with uterine arteries from nonpregnant animals. Scale bars = 100 μm.
Effects of neutralizing relaxin on uterine artery structure and compliance
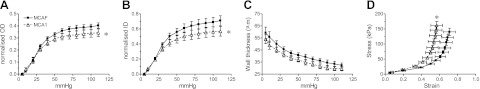
In uterine arteries (at 5 mmHg), OD (515±16 μm; mean±se) and ID (407±21 μm) were significantly (P=0.03) increased in MCA1-treated rats compared with MCAF controls (OD 470±12 μm ; ID 352±18 μm). WT, however, was marginally reduced in MCA1 rats (54±3 μm) compared with MCAF controls (59±4 μm). Over the pressurization range, OD and ID (normalized to diameters at 5 mmHg) of pregnant MCA1-treated rats were significantly reduced compared with MCAF-treated controls (both P<0.01; Fig. 4A, B). There was a small reduction in WT, but it was not statistically significant (P=0.16; Fig. 4C). The stress–strain curve for uterine arteries from the MCA1-treated rats was shifted to the left compared to MCAF controls (F1,91=16.26, P<0.01, Fig. 4D), indicating an increase in wall stiffness.
Figure 4.
Passive mechanical wall properties of the uterine artery. A–C) OD (A), ID (B), and WT (C) of uterine arteries isolated from rats treated with MCAF-treated (solid squares) and MCA1-treated rats (open triangles) on d 20 of gestation (n=8/treatment group). Data were normalized to diameters at 5 mmHg. D) Stress-strain relationship of uterine arteries of MCAF- and MCA1-treated rats. *P < 0.01 vs. MCAF-treated controls.
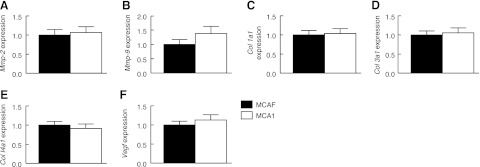
Despite the increase in uterine artery stiffness in the MCA1-treated rats, there were no significant differences in uterine artery Mmp2, Mmp9, col1a1, col3a1, col4a1, and Vegf gene expression between treatment groups on d 20 of gestation (Fig. 5). There was also no effect of MCA1 treatment on uterine artery Timp1, Timp2, Esr1, or Rxfp1 expression (data not shown).
Figure 5.
Quantitative mRNA expression of Mmp2 (A), Mmp9 (B), col1a1 (C), col3a1 (D), col4a1 (E), and Vegf (F) in the uterine arteries of rats treated with either MCAF or MCA1. Expression is standardized to the reference gene Rn18s and presented relative to MCAF. Bars represent means ± sd as calculated using the −2ΔΔCt method (n=6/group).
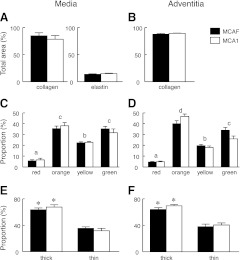
We then assessed the relative contribution of collagen and elastin fibers because they are associated with vascular wall stiffness. Collagen fibers were identified in the tunica media and adventitia of the uterine artery by bright field microscopy. There was no effect of MCA1 treatment on the amount of collagen in either the tunica media or adventitia of uterine arteries compared with MCAF-treated controls (Fig. 6A, B). Similarly, there was no effect of MCA1 treatment on the amount of elastin in the tunica media (Fig. 6A, B). Elastin in the adventitia could not be accurately quantified due to high background after staining. Picrosirius red-stained sections were then visualized under CCPL to reveal birefringent collagen. Differences in the proportion of red, orange, yellow, and green birefringent collagen fibers were observed in the tunica media and adventitia (Fig. 6C, D). In the tunica media, there were more orange (35.4–38.1% intermediate to thick) and green (31.6–35.2% thin) birefringent fibers compared with red (5.7–6.6% thick) and yellow (22.3% thick) fibers. A similar profile between the fiber types was seen in the tunica adventitia. However, there was no significant effect of MCA1 treatment on the proportion of each birefringent collagen fiber type within each region. A comparison of thick (red, orange, yellow) and thin (green) collagen fibers within the adventitia and media revealed a significantly (P<0.001) greater number of thick birefringent collagen fibers than thin fibers in both media and adventitia but no differences between the treatment groups (Fig. 6E, F).
Figure 6.
Histological analysis of uterine arteries from MCAF-treated (solid bars; n=8) and MCA1-treated rats (open bars; n=8) rats on d 20 of gestation. Sections were visualized under bright light (A, B) or CCPL (C–F). A) Collagen and elastin fibers as a percentage of the total tunica media area of the artery. B) Collagen fibers as a proportion (%) of the adventitia area of the artery. C, D) Birefringent collagen fibers separated for color and presented as a percentage of total birefringent collagen for media (C) or adventitia (D) area. E, F) Thick (red, orange, and yellow) and thin (green) collagen fibers as a percentage of total birefringent collagen for media (E) or adventitia (F) area. Data are presented as means ± se. Birefringent collagen fibers labeled with different letters are statistically (P<0.05) different from each other but there was no effect of MCA1 treatment. *P < 0.05 vs. thin collagen fibers.
Effects of relaxin on uterine artery flow velocity in conscious rats
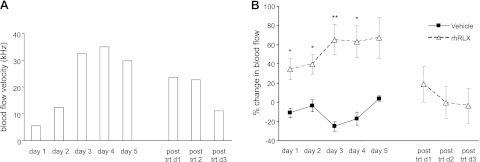
Figure 7A is a representative recording from one animal to show increased uterine artery blood flow velocity in response to chronic infusion of rhRLX for 5 d. On average, flow velocity increased from 24.0 ± 11.7 kHz on d 1 to a maximum of 39.6 ± 11.4 kHz on d 3 and 4 (data not shown). The percentage change in uterine artery flow velocity was significantly (P<0.01) higher compared with vehicle-infused controls from d 1–4 of the treatment period (Fig. 7B). Uterine artery flow velocity decreased by ∼40% in the posttreatment period and was similar to values measured in the vehicle-treated rats.
Figure 7.
Effects of chronic relaxin infusion on uterine artery flow velocity. A) Representation of uterine artery flow velocity (kHz) in one nonpregnant rat infused with rhRLX for 5 d. After the minipump infusing rhRLX expired, uterine artery flow velocity was measured for a further 3 d (posttreatment d 1–3). B) Average percentage change in uterine artery flow velocity compared to baseline in nonpregnant rats infused with either vehicle (solid squares; n=3) or rhRLX (open triangles; n=8). *P < 0.05; **P < 0.01 vs. vehicle-infused rats on same day of treatment.
DISCUSSION
The major findings of this study were that RXFP1 mRNA and protein are present in the uterine artery and that highest Rxfp1 expression occurred in nonpregnant rats in estrus and in early pregnancy. RXFP1 protein was localized predominantly to the vascular smooth muscle in the tunica media, although receptors were also detected in endothelial cells and tunica adventitia. Rats treated with MCA1 had significantly increased passive arterial wall stiffness. However, MCA1 treatment did not affect ECM gene expression, elastin, or the distribution of thick and thin collagen fibers in this artery. Chronic infusion of relaxin for 5 d in nonpregnant rats, which produced plasma relaxin levels similar to midpregnant rats (34), increased uterine artery flow velocity. Overall, these data demonstrate a functional role for relaxin in mediating uterine artery compliance in pregnant rats, which may be important to promote vasodilation and enhance blood flow to the placenta.
Previous studies have shown Rxfp1 expression in small renal and mesenteric arteries, as well as thoracic aorta isolated from mice and rats (24, 25). However, the current study is the first to document Rxfp1 expression in the uterine artery and describe its varying expression during gestation. Highest receptor expression in nonpregnant rats in estrus and on d 8 of gestation suggests that relaxin acting through Rxfp1 may be important in early pregnancy to increase uterine artery remodeling. Although relaxin is not detected in the rat circulation until d 10 of gestation (34), there is evidence of relaxin gene expression in the rat ovary from d 5 pregnancy (36) and uterus (37). This could provide sufficient relaxin locally to stimulate uterine artery remodeling in a paracrine manner. Furthermore, immunoreactive relaxin has been identified in both endothelial and vascular smooth muscle cells of small renal and mesenteric arteries, and thoracic aorta (24). The hypothesis of a local source of relaxin in the uterine artery was not tested in this study because our primary objective was to eliminate circulating relaxin. The decrease in Rxfp1 in late gestation was unexpected but could be explained by the high circulating levels of relaxin which act to suppress RXFP1. We have shown a similar decrease in Rxfp1 in the myometrium and cervix in late pregnant rats and mice (29, 38). Alternatively, because the artery has already undergone extensive remodeling by late gestation (2, 8, 11, 12), this action of relaxin may be negated through a reduction in uterine artery Rxfp1 expression. Interestingly, Longo et al. (39) reported a decrease in the vasodilatory response to relaxin in uterine arteries preconstricted with phenylephrine in late pregnant rats compared with midpregnant rats. The decrease in uterine artery RXFP1 in late pregnancy would explain the decreased sensitivity to relaxin.
Another important finding was the localization of RXFP1 to the tunica media and adventitia within the uterine artery. In double-labeling immunofluorescence, RXFP1 colocalized with α-SMA positive cells in the tunica media, confirming its localization to vascular smooth muscle. In the adventitia, there were no cells positive for α-SMA; we therefore suggest that RXFP1 is localized to fibroblasts. Studies in bovine aortae reported a direct action of relaxin on vascular smooth muscle cells to stimulate nitric oxide production (40). However, immunoreactive RXFP1 was apparent in endothelial cells lining the artery lumen that did not express α-SMA. This is an important finding because functional studies in the small renal artery and cultured human endothelial cells strongly suggest a direct effect of relaxin on endothelial cells to cause the release of vasodilators (41, 42). Preliminary data also demonstrated Rxfp1 transcripts and immunoreactive RXFP1 in cultured human aorta and coronary endothelial cells but at substantially lower levels compared with their respective cultured smooth muscle cells (unpublished results). In summary, the specific localization of RXFP1 in both endothelial and vascular smooth muscle cells in the uterine artery is consistent with the hypothesis that relaxin can act directly on blood vessels to cause vasodilation and influence arterial ECM and remodeling.
To examine the functional significance of RXFP1 in the uterine artery, we used a well-published approach to neutralize circulating relaxin in late pregnant rats with a monoclonal antibody MCA1 (22, 23, 43). Three days of MCA1 treatment in late gestation resulted in a leftward shift of the stress-strain relationship compared with controls, indicating increased stiffness of the uterine artery. Interestingly, previous studies in midterm pregnant rats showed no effect of MCA1 treatment on passive mechanical wall properties in small renal and first-order mesenteric arteries compared with controls or virgin rats (23). However, relaxin mutant male mice had decreased small renal arterial compliance (24, 26). The discrepancy between the two MCA1 studies could be related to differences in experimental design. Most notable is that the gestational period of study was different (mid vs. late gestation), and the artery type was also different (conduit vs. resistance). It is possible that relaxin only influences passive mechanical wall properties in late gestation, or that during pregnancy, the hormone only affects compliance of conduit vessels, such as the larger uterine artery. Neutralizing relaxin with MCA1 in midterm pregnant rats prevented the pregnancy-associated decrease in myogenic reactivity in small renal arteries (23), demonstrating a different role for relaxin as a vasodilator during pregnancy in a resistance artery.
The compliance of the vascular wall is dependent on the relative contribution and organization of collagen and elastin, in addition to geometric remodeling (44, 45). Several studies have reported increased passive arterial compliance in small renal arteries, mesenteric arteries, brain parenchymal arteries, and the carotid arteries dissected from rats and mice administered relaxin for 3–5 d (26–28). In mice administered relaxin, the relative increase in small renal artery compliance was mediated by both geometric (outward) and compositional (decreased collagen) remodeling (26). Specifically, there was an increase in unpressurized wall area and wall-to-lumen area ratio, increased smooth muscle cell density, and a decrease in collagen to total protein. Relaxin infusion in nonpregnant rats also increased wall thickness and inner diameter of brain parenchymal arterioles, effects mediated by peroxisome proliferator-activated receptor-γ (27). In contrast, Debrah et al. (26) showed that the small renal arteries of Rln−/− mice have decreased unpressurized wall area and wall-to-lumen area ratio, decreased smooth muscle cell density, and increased collagen to total protein ratio. In our study, the uterine arteries of MCA1-treated rats had increased ID and OD when unpressurized, with a small reduction in wall thickness. As luminal pressure was incrementally increased, both ID and OD of pregnant MCA1-treated rats were significantly reduced compared with controls and there was a small reduction in wall thickness. Our interpretation is that despite having smaller resting ODs, uterine arteries from MCAF controls were able to distend to a significantly larger diameter with increasing luminal pressure compared with arteries from the MCA1 rats. As there was no significant effect of MCA1 treatment on WT or wall-to-lumen ratio, it is unlikely that MCA1 affects geometric remodeling. It is important to consider that our pregnant rats only received MCA1 for 3 d, whereas the Rln−/− mice were 3 mo old and therefore subjected to a longer period of relaxin deficiency. This longer period of time may be necessary to reveal changes in geometric remodeling in the absence of relaxin.
We further investigated mechanisms of increased arterial wall stiffness in the uterine artery of MCA1-treated rats by examining ECM components through qPCR and polarized light microscopy. There was no effect of MCA1 on collagen gene expression. Nor was there any change in the mRNA expression of gelatinases (Mmp2 or Mmp9) or their inhibitors (Timp1 or Timp2), which mediate matrix turnover in the uterine artery wall during pregnancy (45). These specific MMPs were assessed because their expression in the uterine artery is markedly increased in late pregnant rats (46) and they are known regulators of relaxin-mediated vasodilation in small renal and mesenteric arteries (47, 48). In contrast, Debrah et al. (26) reported that relaxin infusion in nonpregnant mice did not increase MMP2 or MMP9 activity in small renal arteries. It is possible that mRNA expression does not accurately reflect potential differences in gelatinase protein or activity but it could be that the effects of MCA1 on the uterine artery are not mediated through MMPs. We also assessed the proportion of collagen and elastin as well as birefringent collagen fibers in the uterine artery. The prediction was that the collagen fibers would be more densely organized and that the proportion of thick fibers would be increased following MCA1 treatment. However, there was no effect of MCA1 treatment on any of these parameters, suggesting that the decrease in arterial compliance is not explained simply by an increase in fibrillar collagen. It should be noted that our method of collagen analysis may not be sensitive enough to measure differences in birefringent collagen in the uterine artery given the relatively low amount present in the tunica media. It is also possible that our MCA1 infusion time of 3 d was not sufficient to produce changes in fibrillar collagens or elastin. Vodstrcil et al. (31) showed that 3 d of MCA1 infusion in late pregnant rats did not decrease cervical wet weight or alter collagen fiber density, but it did result in a shorter pubic symphysis and changes in the epithelial lining of the cervical lumen. We conclude that relaxin-deficient late pregnant rats have compromised uterine artery remodeling, but this is unlikely due to an increase in collagen. The apparent dissociation from increased collagen may be due to insufficient sensitivity of our measurements. Alternatively, the impact of relaxin deficiency on vessel wall collagen may be more pronounced in the context of disease rather than healthy physiological states (26, 28).
It was important to investigate a potential functional significance of relaxin's action on the uterine artery. Five days of chronic relaxin infusion in ovariectomized virgin rats resulted in significant increases in uterine artery flow velocity compared to both baseline measurements and vehicle control animals. Notably, uterine artery flow velocity decreased after relaxin treatment stopped (after the minipump expired). These functional data support a role for relaxin in facilitating increases in uterine artery luminal diameter and arterial compliance, important changes in the uterine vascular system necessary to accommodate the increases in blood flow required during pregnancy. Although our analysis of vessel wall stiffness was in late-pregnant rats, we predict that these effects of relaxin on uterine artery remodeling occur much earlier in pregnancy, as indicated by the higher expression of RXFP1 in this artery. Indeed, the action of relaxin in modulating the structure and function of the uterine artery may be greatest in early pregnancy, the time when marked vascular adaptation is occurring (2). This may be relevant in humans when circulating relaxin levels are highest in the first trimester (49). It remains to be established whether relaxin deficiency in early pregnancy compromises uterine artery structure or function. Interestingly, serum relaxin levels are decreased throughout pregnancy in women with a history of recurrent miscarriage (50). This study also showed a positive correlation of serum relaxin with uterine artery resistance index at 10–12 wk, suggesting that relaxin plays a role in regulating the uteroplacental vasculature.
In summary, we provide the first evidence that relaxin receptors are predominantly localized in vascular smooth muscle cells, but are also likely to be present in endothelial cells. Importantly, neutralizing circulating relaxin in late pregnant rats increased uterine arterial stiffness without affecting geometric remodeling. Infusion of relaxin in conscious nonpregnant rats increased uterine artery flow velocity and supports our hypothesis that relaxin mediates important changes in the uterine vascular system necessary to promote adequate uteroplacental perfusion to allow for normal fetal and placental growth.
Supplementary Material
Acknowledgments
The authors thank Prof. O. David Sherwood (University of Illinois, Urbana, IL, USA) for providing the MCA1, MCAF, and rat RXFP1 antibodies, and for helpful advice with the experimental design. The authors also thank Dr. Elaine Unemori and Dr. Dennis Stewart (Corthera, Inc., San Mateo, CA, USA) for providing the recombinant human relaxin. The authors thank Dr. Jonathan McGuane, Emma Simpson, Kerryn Westcott, Dr. Jill Verlander, and John J. Reho for their valuable contributions to this work.
The research was funded by the National Health and Medical Research Council of Australia (NH&MRC; M.E.W., L.J.P., M.T.), and The U.S. National Institutes of Health (HL067937; K.P.C.). L.A.V. received a NH&MRC Dora Lush Postgraduate Scholarship, the John and Allan Gilmour Research Award, and an Albert Shimmin Award (Faculty of Science, University of Melbourne).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- α-SMA
- α-mooth muscle actin
- CCPL
- circular cross-polarized light
- col1a1
- collagen type I α 1
- col3a1
- collagen type III α 1
- col4a1
- collagen type IV α 1
- ECM
- extracellular matrix
- Esr
- estrogen receptor
- ID
- inner diameter
- MCA1
- monoclonal antibody against rat relaxin
- MCAF
- monoclonal antibody against fluorescein
- Mmp
- matrix metalloproteinase
- OD
- outer diameter
- PBS
- phosphate buffered saline
- PLP
- paraformaldehyde-lysine-periodate
- PSS
- physiological saline solution
- rhRLX
- recombinant human relaxin
- Rn18S
- ribosomal 18S
- Rxfp
- relaxin/insulin-like family peptide receptor
- Timp
- tissue inhibitor of MMP
- Vegf
- vascular endothelial growth factor
- WT
- wall thickness
REFERENCES
- 1. Kelly B., Stone S., Poston L. (1999) Cardiovascular adaptation to pregnancy: the role of altered vascular structure. Fetal Matern. Med. Rev. 11, 105–116 [Google Scholar]
- 2. Osol G., Mandala M. (2009) Maternal uterine vascular remodeling during pregnancy. Physiology 24, 58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gagnon R. (2003) Placental insufficiency and its consequences. Eur. J. Obstet. Gyn. Reprod. Biol. 110, S99–S107 [DOI] [PubMed] [Google Scholar]
- 4. Granger J. P., Alexander B. T., Llinas M. T., Bennett W. A., Khalil R. A. (2002) Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9, 147–160 [DOI] [PubMed] [Google Scholar]
- 5. Roberts J. M., Gammill H. S. (2005) Preeclampsia: recent insights. Hypertension 46, 1243–1249 [DOI] [PubMed] [Google Scholar]
- 6. Griendling K. K., Fuller E. O., Cox R. H. (1985) Pregnancy-induced changes in sheep uterine and carotid arteries. Am. J. Physiol. 248, H658–H665 [DOI] [PubMed] [Google Scholar]
- 7. Bird I. M., Zhang L., Magness R. R. (2003) Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am. J. Physiol. 284, R245–R258 [DOI] [PubMed] [Google Scholar]
- 8. Van der Heijden O. W., Essers Y. P., Fazzi G., Peeters L. L., De Mey J. G., van Eys G. J. (2005) Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol. Reprod. 72, 1161–1168 [DOI] [PubMed] [Google Scholar]
- 9. Van der Heijden O. W., Essers Y. P., Spaanderman M. E., De Mey J. G., van Eys G. J., Peeters L. L. (2005) Uterine artery remodeling in pseudopregnancy is comparable to that in early pregnancy. Biol. Reprod. 73, 1289–1293 [DOI] [PubMed] [Google Scholar]
- 10. Osol G., Cipolla M. (1993) Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am. J. Obstet. Gynecol. 168, 697–705 [DOI] [PubMed] [Google Scholar]
- 11. Cipolla M., Osol G. (1994) Hypertrophic and hyperplastic effects of pregnancy on the rat uterine arterial wall. Am. J. Obstet. Gynecol. 171, 805–811 [DOI] [PubMed] [Google Scholar]
- 12. Cipolla M. J., Binder N. D., Osol G. (1997) Myoendometrial versus placental uterine arteries: structural, mechanical, and functional differences in late-pregnant rabbits. Am. J. Obstet. Gynecol. 177, 215–221 [DOI] [PubMed] [Google Scholar]
- 13. Keyes L. E., Moore L. G., Walchak S. J., Dempsey E. C. (1996) Pregnancy-stimulated growth of vascular smooth muscle cells: importance of protein kinase C-dependent synergy between estrogen and platelet-derived growth factor. J. Cell. Physiol. 166, 22–32 [DOI] [PubMed] [Google Scholar]
- 14. Guenther A. E., Conley A. J., Van Orden D. E., Farley D. B., Ford S. P. (1988) Structural and mechanical changes of uterine arteries during pregnancy in the pig. J. Anim. Sci. 66, 3144–3152 [DOI] [PubMed] [Google Scholar]
- 15. Fuller R., Colton I., Gokina N., Mandala M., Osol G. (2011) Local versus systemic influences on uterine vascular reactivity during pregnancy in the single-horn gravid rat. Reprod. Sci. 18, 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conrad K. P. (2011) Maternal vasodilation in pregnancy: the emerging role of relaxin. Am. J. Physiol. 301, R267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conrad K. P. (2011) Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Semin. Nephrol. 31, 15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeyabalan A., Conrad K. P. (2007) Renal function during normal pregnancy and preeclampsia. Front. Biosci. 12, 2425–2437 [DOI] [PubMed] [Google Scholar]
- 19. Danielson L. A., Sherwood O. D., Conrad K. P. (1999) Relaxin is a potent renal vasodilator in conscious rats. J. Clin. Invest. 103, 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conrad K. P., Debrah D. O., Novak J., Danielson L. A., Shroff S. G. (2004) Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 145, 3289–3296 [DOI] [PubMed] [Google Scholar]
- 21. Novak J., Ramirez R. J. J., Gandley R. E., Sherwood O. D., Conrad K. P. (2002) Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am. J. Physiol. 283, R349–R355 [DOI] [PubMed] [Google Scholar]
- 22. Debrah D. O., Novak J., Matthews J. E., Ramirez R. J., Shroff S. G., Conrad K. P. (2006) Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147, 5126–5131 [DOI] [PubMed] [Google Scholar]
- 23. Novak J., Danielson L. A., Kerchner L. J., Sherwood O. D., Ramirez R. J., Moalli P. A., Conrad K. P. (2001) Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J. Clin. Invest. 107, 1469–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Novak J., Parry L. J., Matthews J. E., Kerchner L. J., Indovina K., Hanley-Yanez K., Doty K. D., Debrah D. O., Shroff S. G., Conrad K. P. (2006) Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 20, 2352–2362 [DOI] [PubMed] [Google Scholar]
- 25. Ferreira V. M., Gomes T. S., Reis L. A., Ferreira A. T., Razvickas C. V., Schor N., Boim M. A. (2009) Receptor-induced dilatation in the systemic and intrarenal adaptation to pregnancy in rats. PLoS One 4, e4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Debrah D. O., Debrah J. E., Haney J. L., McGuane J. T., Sacks M. S., Conrad K. P., Shroff S. G. (2011) Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J. Appl. Physiol. 111, 260–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan S. L., Cipolla M. J. (2011) Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-gamma. FASEB J. 25, 3229–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Q., Chakravorty A., Bathgate R. A., Dart A. M., Du X. J. (2010) Relaxin therapy reverses large artery remodeling and improves arterial compliance in senescent spontaneously hypertensive rats. Hypertension 55, 1260–1266 [DOI] [PubMed] [Google Scholar]
- 29. Vodstrcil L. A., Shynlova O., Verlander J. W., Wlodek M. E., Parry L. J. (2010) Decreased expression of the rat myometrial relaxin receptor (RXFP1) in late pregnancy is partially mediated by the presence of the conceptus. Biol. Reprod. 83, 818–824 [DOI] [PubMed] [Google Scholar]
- 30. Vodstrcil L. A., Wlodek M. E., Parry L. J. (2007) Effects of uteroplacental restriction on the relaxin-family receptors, Lgr7 and Lgr8, in the uterus of late pregnant rats. Reprod. Fertil. Dev. 19, 530–538 [DOI] [PubMed] [Google Scholar]
- 31. Vodstrcil L. A., Shynlova O., Westcott K. T., Laker R., Simpson E., Wlodek M. E., Parry L. J. (2010) Progesterone withdrawal, and not increased circulating relaxin, mediates the decrease in myometrial relaxin receptor (RXFP1) expression in late gestation in rats. Biol. Reprod. 83, 825–832 [DOI] [PubMed] [Google Scholar]
- 32. Wigg S. J., Tare M., Forbes J., Cooper M. E., Thomas M. C., Coleman H. A., Parkington H. C., O'Brien R. C. (2004) Early vitamin E supplementation attenuates diabetes-associated vascular dysfunction and the rise in protein kinase C-beta in mesenteric artery and ameliorates wall stiffness in femoral artery of Wistar rats. Diabetologia 47, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 33. Mazzuca M. Q., Wlodek M. E., Dragomir N. M., Parkington H. C., Tare M. (2010) Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J. Physiol. 588, 1997–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sherwood O. D., Crnekovic V. E., Gordon W. L., Rutherford J. E. (1980) Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology 107, 691–698 [DOI] [PubMed] [Google Scholar]
- 35. Scott D. J., Layfield S., Yan Y., Sudo S., Hsueh A. J., Tregear G. W., Bathgate R. A. (2006) Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J. Biol. Chem. 281, 34942–34954 [DOI] [PubMed] [Google Scholar]
- 36. Crish J. F., Soloff M. S., Shaw A. R. (1986) Changes in relaxin precursor mRNA levels in the rat ovary during pregnancy. J. Biol. Chem. 261, 1909–1913 [PubMed] [Google Scholar]
- 37. Gunnersen J. M., Crawford R. J., Tregear G. W. (1995) Expression of the relaxin gene in rat tissues. Mol. Cell. Endocrinol. 110, 55–64 [DOI] [PubMed] [Google Scholar]
- 38. Siebel A. L., Gehring H. M., Reytomas I. G. T., Parry L. J. (2003) Inhibition of oxytocin receptor and estrogen receptor-α, but not relaxin receptors (LGR7), in the myometrium of late pregnant relaxin gene knockout mice. Endocrinology 144, 4272–4275 [DOI] [PubMed] [Google Scholar]
- 39. Longo M., Jain V., Vedernikov Y. P., Garfield R. E., Saade G. R. (2003) Effects of recombinant human relaxin on pregnant rat uterine artery and myometrium in vitro. Am. J. Obstet. Gynecol. 188, 1468–1476 [DOI] [PubMed] [Google Scholar]
- 40. Bani D., Failli P., Bello M. G., Thiemermann C., Bani Sacchi T., Bigazzi M., Masini E. (1998) Relaxin activates the L-arginine-nitric oxide pathway in vascular smooth muscle cells in culture. Hypertension 31, 1240–1247 [DOI] [PubMed] [Google Scholar]
- 41. Novak J. (2002) Relaxin increases uterine blood flow in concious nonpregnant rats and decreases myogenic reactivity in isolated uterine arteries. FASEB J. 16, A824 [Google Scholar]
- 42. McGuane J. T., Debrah J. E., Sautina L., Jarajapu Y. P., Novak J., Rubin J. P., Grant M. B., Segal M., Conrad K. P. (2011) Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide. Endocrinology 152, 2786–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guico-Lamm M. L., Voss E. W. J., Sherwood O. D. (1988) Monoclonal antibodies specific for rat relaxin. I. Production and characterization of monoclonal antibodies that neutralize rat relaxin's bioactivity in vivo. Endocrinology 123, 2472–2478 [DOI] [PubMed] [Google Scholar]
- 44. Conrad K. P., Shroff S. G. (2011) Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr. Hypertens. Rep. 13, 409–420 [DOI] [PubMed] [Google Scholar]
- 45. Jacob M. P. (2003) Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed. Pharmacother. 57, 195–202 [DOI] [PubMed] [Google Scholar]
- 46. Kelly B. A., Bond B. C., Poston L. (2003) Gestational profile of matrix metalloproteinases in rat uterine artery. Mol. Hum. Reprod. 9, 351–358 [DOI] [PubMed] [Google Scholar]
- 47. Jeyabalan A., Kerchner L. J., Fisher M. C., McGuane J. T., Doty K. D., Conrad K. P. (2006) Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J. Appl. Physiol. 100, 1955–1963 [DOI] [PubMed] [Google Scholar]
- 48. Jeyabalan A., Novak J., Doty K. D., Matthews J., Fisher M. C., Kerchner L. J., Conrad K. P. (2007) Vascular matrix metalloproteinase-9 mediates the inhibition of myogenic reactivity in small arteries isolated from rats after short-term administration of relaxin. Endocrinology 148, 189–197 [DOI] [PubMed] [Google Scholar]
- 49. Stewart D. R., Celniker A. C., Taylor C. A., Jr., Cragun J. R., Overstreet J. W., Lasley B. L. (1990) Relaxin in the peri-implantation period. J. Clin. Endocrinol. Metab. 70, 1771–1773 [DOI] [PubMed] [Google Scholar]
- 50. Anumba D. O., El Gelany S., Elliott S. L., Li T. C. (2009) Serum relaxin levels are reduced in pregnant women with a history of recurrent miscarriage, and correlate with maternal uterine artery Doppler indices in first trimester. Eur. J. Obstet. Gyn. Reprod. Biol. 147, 41–45 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.