Abstract
Background
Allogeneic hematopoietic cell transplantation provides a reliable method for inducing tolerance towards solid organ grafts. However, this procedure can result in graft-versus-host disease (GVHD) thereby limiting its application. Here we test the hypothesis that mixed chimerism can be intentionally reverted to host hematopoiesis without rejection of a kidney graft.
Methods
Recipient dogs were given 2 Gy total body irradiation (TBI) before and a short course of immunosuppression after marrow infusion from dog leukocyte antigen-identical littermates. All dogs achieved stable mixed chimerism. After a mean of 20 weeks, one cohort of dogs received kidney transplants from their respective marrow donors. Subsequently, recipients were reconditioned with 2 Gy TBI and given autologous granulocyte-colony stimulating factor-mobilized leukocytes (recipient leukocyte infusion) that had been collected before marrow transplant.
Results
Dogs receiving a second TBI and recipient leukocyte infusion without a kidney transplant rejected their donor hematopoietic graft within 3 weeks. Dogs that received kidney grafts, followed by a second TBI and recipient leukocyte infusion, rejected their marrow graft without rejecting their transplanted kidneys for periods greater than one year.
Conclusion
Mixed chimerism may be clinically reverted to 100% recipient without rejection of a kidney allograft. This finding may have application towards minimizing the risk of GVHD in solid organ transplant patients given hematopoietic cell transplantation from HLA-identical donors.
Keywords: dog, nonmyeloablative conditioning regimen, mixed hematopoietic chimerism, kidney allograft, recipient lymphocyte infusion, tolerance
INTRODUCTION
Previous studies demonstrated that long-term immune tolerance to kidney grafts could be established in dog leukocyte antigen (DLA)-identical mixed donor/host hematopoietic chimeras (1–3). In this setting, hematopoietic and nonhematopoietic tolerance was established following a nonmyeloablative conditioning regimen of either 1 or 2 Gy total body irradiation (TBI) before and a short course (five weeks) of immunosuppression after marrow or peripheral blood stem cell transplantation. This simple and effective means of establishing tolerance led to 5 out of 5 dogs accepting their respective marrow and kidney grafts without additional immunosuppression for periods up to 5 years after transplantation (1,2). While this protocol eliminates the complications of long-term global immunosuppression, such as opportunistic infections and malignancies (4), allogeneic hematopoietic cell transplantation (HCT) introduces potential complications of graft-versus-host disease (GVHD) (5–7). Recent clinical combined kidney and marrow transplantation studies indicate that long-term-tolerance to kidney grafts from human leukocyte antigen HLA-mismatched donors can be maintained in some patients despite eventual loss in donor hematopoietic chimerism (8–10). This peculiarity of long-term immune tolerance to kidney transplantation with transient tolerance to a marrow transplant suggests that the threat of GVHD can be averted once donor chimerism is eliminated. Therefore, a safe and effective therapy needs to be defined that can eliminate donor hematopoietic chimerism without endangering the kidney allograft.
Here we tested the hypothesis that hematopoietic chimerism can be intentionally eliminated without affecting long-term engraftment of the kidney graft in the canine DLA-identical marrow transplant model. Our approach was based on preliminary studies (by G.G. & R.S.) that showed a nonmyeloablative dose of 2 Gy TBI followed by an infusion of recipient cryopreserved granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood mononuclear cells (intended as a recipient leukocyte infusion or RLI) could convert stable mixed hematopoietic chimeras to 100% recipient hematopoiesis. Using this protocol in our mixed chimeric recipients, we observed split tolerance characterized by rejection of donor hematopoiesis but not of the kidney graft.
RESULTS
Effect of TBI and Recipient Leukocyte Infusion on Hematopoietic Chimerism
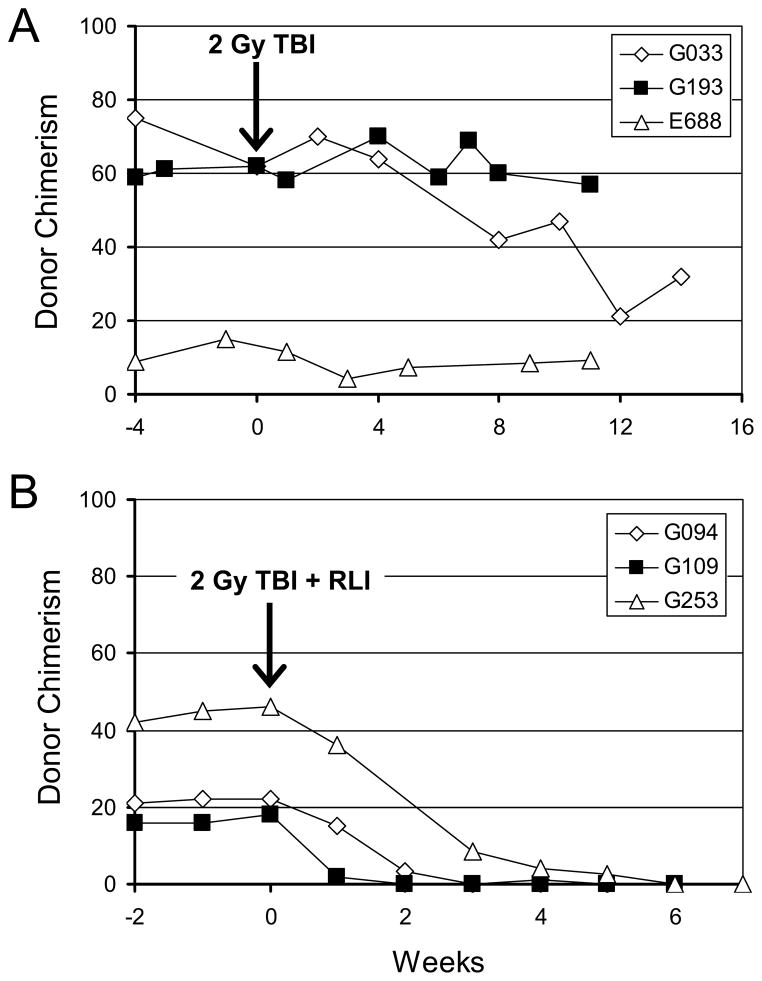
Preliminary studies tested the hypothesis that the tolerance mechanism that maintains stable mixed stable mixed chimerism is an active, potentially radiation-sensitive, suppressor cell mechanism. To this end, stable mixed hematopoietic chimeras, established using nonmyeloablative conditioning of 2 Gy TBI followed by a short course of postgrafting immunosuppression of CSP and MMF, were reconditioned with 2 Gy TBI after HCT with and without infusion of RLI. As shown in Figure 1A, mixed chimeras failed to reject donor grafts following reconditioning with 2 Gy TBI alone. However, when chimeric recipients were given 2 Gy TBI and RLI, all three dogs reverted from mixed hematopoietic chimerism, ranging from 17 to 47 (median, 21) percent donor to 100% host hematopoiesis within 2 to 4 weeks (Figure 1B). The median RLI CD3+ cell dose was 2.3×108/kg. To confirm that immunocompetence was restored after conversion to host hematopoiesis, each recipient received full thickness skin grafts from their respective donors, and autologous and unrelated 3rd party donors. Both, the skin grafts from the marrow donors and 3rd party donors were promptly rejected within 14 days, while the autologous grafts were maintained (data not shown). RLI alone (without reconditioning) was not tested, as previous studies revealed no measurable change in chimerism when nonsensitized donor lymphocyte infusions were given to mixed chimeric recipients (11).
Figure 1. Summary of results from mixed chimeras given radiation with or without recipient leukocyte infusion (RLI).
(A) The percentage of donor chimerism in the peripheral blood mononuclear cell fraction shown from 4 weeks before to 14 weeks after reconditioning with 2 Gy TBI. (B) The percentage of donor chimerism of peripheral blood mononuclear cells shown 2 weeks before and up to 6 weeks after reconditioning with 2 Gy TBI and RLI.
Marrow Grafting Prior to Kidney Transplantation
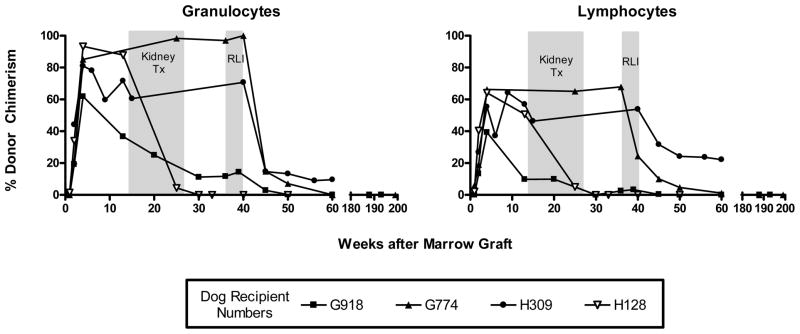
Following marrow infusion and postgrafting immunosuppression, five dogs successfully engrafted and were mixed donor/host hematopoietic chimeras as indicated by the percent donor chimerism at the time of kidney transplantation (Table 1, Figure 2). At the time of kidney transplantation the dogs’ complete blood counts were within normal range and donor cell contributions ranged from 3.1% to 58.2% of peripheral blood lymphocytes and from 13.8% to 100% of granulocytes (Table 1, Figure 2).
Table 1.
Donor chimerism after marrow and subsequent renal grafts followed by 2 Gy TBI and recipient lymphocyteinfusion
| Dog | Donor Marrow Cells × 108/kg | Weeks Between Marrow and Kidney Transplant | % Donor Chimerism at Kidney Transplant
|
RLI | Recipient G-Mobilized Leukocytes × 108/kg | % Donor Chimerism at End of Study (weeks)
|
Renal Graft Rejection (Weeks) | ||
|---|---|---|---|---|---|---|---|---|---|
| Grans | Lymphs | Grans | Lymphs | ||||||
| G774 | 4 | 27 | 100 | 58.2 | Yes | 2.8 | 0 (204) | 0 (204) | No (204) |
| G918 | 7.2 | 20 | 15 | 3.1 | Yes | 2.2 | 0 (193) | 0 (193) | No (193) |
| H309 | 5.4 | 12 | 76.8 | 47.8 | Yes | 13 | 7.1 (70) | 20.1(70) | No (70) |
| H128 | 3.5 | 15 | 87.3 | 36.8 | No | n/a | 0 (33) | 0 (33) | No (33) |
Dog H206 was mixed chimeric and received a transplanted kidney but had poor circulation 24 hours after surgery determined by Doppler ultrasound. The incision was reopened, the kidney repositioned, and circulation restored; however, after a double nephrectomy of the native kidneys serum creatinine levels indicated a nonfunctional transplanted kidney.
Figure 2. Percent donor chimerism in kidney recipient dogs before and after recipient leukocyte infusion.
Percent donor granulocyte and lymphocyte chimerism for five dogs that received kidney transplants after hematopoietic chimerism was determined. Dogs G918, G774, and H309 received 2 Gy TBI and recipient leukocyte infusion at an average of week 40 after marrow transplant. Data points were determined by VNTR-PCR analysis.
Kidney Graft
With the exception of one dog, H206 (excluded from the study due to inadequate blood flow to the transplanted kidney noted within 24 hours after surgery), kidneys were successfully transplanted from each marrow donor into their respective chimeric recipients. General anesthesia was well tolerated and dogs generally recovered within 24 hours. There were no significant changes in the lymphocyte and granulocyte chimerism after kidney transplantation with the exception of H128 in which a significant decline in donor hematopoietic chimerism was seen after kidney transplantation at week 14 after HCT (Figure 2). Physical exam and laboratory monitoring of complete blood counts, serum electrolytes, blood urea nitrogen, and creatinine revealed no abnormalities in any of the dogs up to time of bilateral native nephrectomy. There were no signs of GVHD in any of the recipients.
Recipient Leukocyte Infusion after Kidney Transplantation
Among the kidney transplant recipients, one dog (H128) was not reconditioned with 2 Gy TBI and did not receive RLI since, within a few weeks after kidney transplantation, this dog initiated spontaneous rejection of donor marrow graft (Figure 2). By week 27 after HCT, dog H128 had completely rejected donor hematopoiesis with no sign of rejection of the kidney graft as indicated by the absence of graft swelling and normal kidney biopsy 22 weeks after rejection of donor hematopoiesis. As described previously, after initial donor engraftment, this nonmyeloablative transplant regimen results in subsequent rejection of donor chimerism in about 10% of the cases (12).
In order to intentionally eliminate stable donor hematopoietic cell chimerism, three of the four kidney transplant dogs in the study (G774, G918 and H309) received cryopreserved RLI after reconditioning with 2 Gy TBI at the time points indicated (Figure 2). Two of the three dogs (G918 and G774) completely rejected donor hematopoiesis over a period of 12 and 18 weeks after RLI, respectively, while H309 showed a significant decrease in donor hematopoietic chimerism to 7% and 20% donor chimerism for granulocytes and lymphocytes, respectively, at the time of follow-up (Table 1, Figure 2). H309 received the largest cell dose of RLI indicating cell dose was not the primary cause for incomplete elimination of donor cell chimerism. The decrease in donor hematopoietic chimerism also did not result in clinical or histopathological signs of kidney rejection in any of the dogs.
Removal of Native Kidneys
Verification of the functional capacity of the transplanted kidneys was an important component of this study. Prior to performing a double nephrectomy of the recipients’ native kidneys, an intravenous pyelogram (IVP) was performed. The objective was to confirm accumulation of contrast dye in the renal pelvis and ureter of the transplanted kidney. As shown in supplemental digital content (SDC) figure S1, the outline of the transplanted kidney can be seen at the proximal end of the femur. In addition, contrast dye could be seen in all dogs accumulating in the renal pelvis of the transplanted kidney and bladder. In a majority of the dogs, it was not possible to visualize the ureter as it was located over the femur and pelvis.
Long-Term Assessment
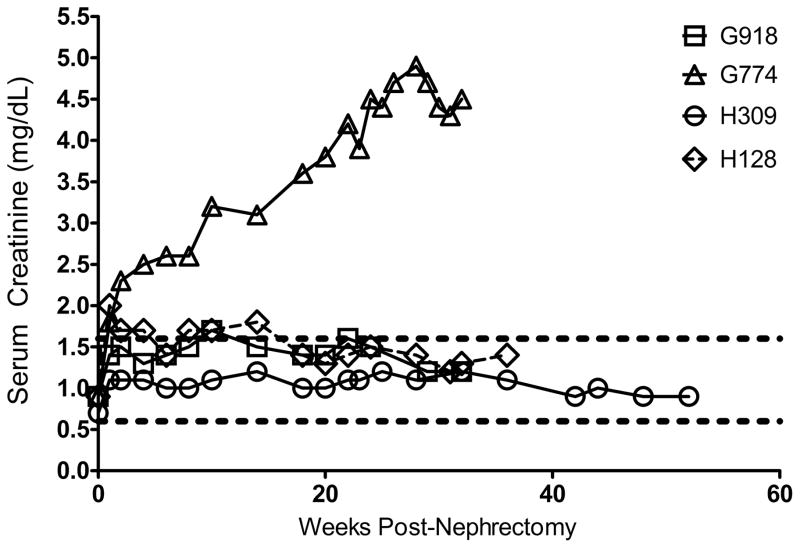
After double nephrectomy of native kidneys, renal function of the transplanted kidney was determined by analysis of serum creatinine levels. As shown in Figure 3, three of the 4 kidney transplant dogs (G918, H309, and H128) had normal or slightly elevated levels of serum creatinine. Following the bilateral nephrectomy of the native kidneys from G774, the dog contracted a severe systemic bacterial infection and was administered meropenem and gentamycin, the dosing of which when administered to normal dogs is well-tolerated but was apparently nephrotoxic in a dog relying on a single kidney. The outcome was reflected in the elevated levels of serum creatinine seen in this dog (Figure 3). Additionally, the dog required monthly transfusion support to counter anemia presumably due to the inability of the transplanted kidney to produce adequate amounts of erythropoietin. One week before euthanasia, total white blood cell and platelet counts were 5.37×106/mL and 290×106/mL respectively, indicating these hematopoietic lineages were within normal limits.
Figure 3. Renal function in dogs with mixed chimerism and rejected donor chimerism after bilateral nephrectomy.
Serum creatinine levels were monitored for all dogs from peripheral blood draws. Immediate elevations were seen after nephrectomy with stabilization following within a few weeks. Upper and lower limits of the normal range for serum creatinine shown by the broken lines.
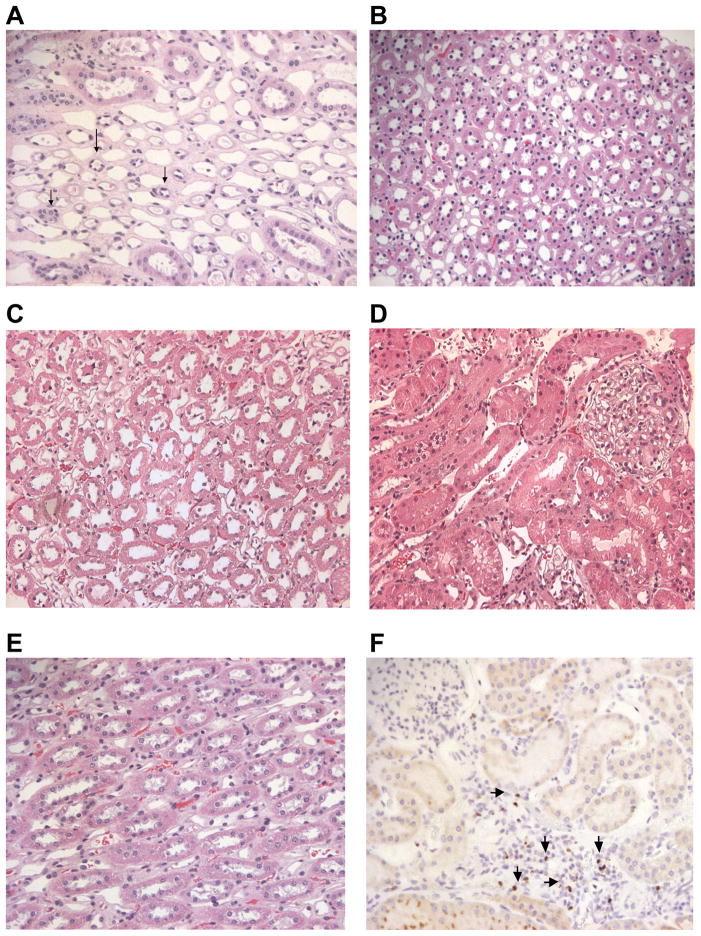
At the end of study, fine needle biopsies of the transplanted kidneys were collected, fixed, and stained with hematoxylin and eosin. The histopathological results for G774, H309, H128, and G918 all showed no evidence of significant lymphocytic infiltration (Figure 4A–E). The kidney biopsy for G774 (Figure 4A) showed significant atrophy of the tubules suggesting renal damage not associated with tubulitis or graft rejection. At no point during the study was tubulitis observed in any of the transplanted kidneys in the other three dogs. A biopsy obtained from G918 was stained for the intranuclear protein, FoxP3 (Figure 4F). Cells staining positive for FoxP3 were localized to limited areas of higher cellularity in the kidney biopsy. These results suggest T-regulatory cells are present within the kidney allograft and may assist in tolerance of the transplanted organ.
Figure 4. Biopsies of kidney allografts.
Biopsy of G774 (A) demonstrating significant atrophic tubules (arrows) among normal appearing distal tubules but no evidence of tubulitis, changes likely due to drug toxicity rather than acute rejection. Biopsy of H309 (B) demonstrating normal appearing tubules without evidence of tubulitis. Biopsy of H128 (C and D) demonstrating normal appearing tubules, collecting ducts and glomeruli without evidence of tubulitis. Biopsy of G918 showing normal distal tubules and no tubulitis (E) and stained for FoxP3 showing positively stained cells (arrows) proximal to tubules (F). Photomicrographs were taken at 200x magnification.
DISCUSSION
Our study was undertaken to test whether tolerance of host immune cells toward a kidney allograft in stable mixed hematopoietic chimeras would be disrupted coincident with intentional rejection of donor hematopoiesis. To accomplish this goal, we reconditioned mixed chimeric dogs with 2 Gy TBI followed by an infusion of recipient G-CSF mobilized peripheral blood cells (RLI). Preliminary studies showed that reconditioning with 2 Gy TBI alone was insufficient to accomplish rejection of donor chimerism, but when 2 Gy TBI was combined with a RLI, all three dogs quickly rejected their allogeneic marrow grafts. These results indicated that 2 Gy TBI together with RLI could break the tolerance mechanism established in canine mixed hematopoietic chimeras. Two of three dogs, transplanted with a kidney from the marrow donor and subsequently given RLI after 2 Gy TBI, rejected donor hematopoiesis yet remained tolerant to their respective donor kidneys. A third dog treated similarly maintained tolerance to the transplanted kidney but only partially rejected donor chimerism. In a fourth dog, rejection of donor hematopoietic chimerism occurred spontaneously, and immune tolerance to the donor kidney was also maintained long-term. One caveat to these studies is that we can not rule out the possibility of donor microchimerism as the sensitivity of the VNTR-PCR assay is ≥ 1%. However, the rapid shift of chimerism after RLI suggested that all donor hematopoiesis was lost.
Despite the loss of donor hematopoietic chimerism in our study, the induction of tolerance through marrow transplantation was undoubtedly required for the long-term acceptance of the donor kidney graft. Studies by Kuhr et al. (1) showed that after hematopoietic cell transplantation, kidney grafts were accepted long-term in all five mixed chimeric recipients, but in all cases, kidneys transplanted from the recipients to their respective HCT donor showed histopathological evidence of severe cellular rejection at a mean of 13 days.
Evidence of sustained renal allograft tolerance after transient engraftment of a donor marrow graft has been reported in nonhuman primates. Kawai et al. (13) showed that fully MHC-mismatched cynomolgus monkeys retained a kidney allograft for the relatively brief follow-up periods of >40 to 198 days after receiving a nonmyeloablative conditioning regimen of 3 Gy TBI, 7 Gy thymic irradiation, and anti-thymocyte globulin before marrow and kidney transplant, followed by cyclosporine for 4 weeks after transplantation. The maximum duration of granulocyte and lymphocyte chimerism after marrow transplant was 37 and 68 days, respectively. When chimerism was not established under less effective protocols, kidney graft survival was limited to <58 days, indicating the importance of at least the establishment of transient marrow engraftment for kidney tolerance to be established insofar as the given follow-up periods (13). Maintenance of kidney allograft tolerance in the setting of donor hematopoietic rejection has also been observed clinically. Spontaneous elimination of donor hematopoietic cell chimerism without rejection of the transplanted kidney was observed in 4 out of 5 patients with end stage renal failure who received a combined nonmyeloablative marrow and kidney transplant from HLA-haploidentical related donors (14).
Sustained renal engraftment has been reported in patients with end stage renal disease and multiple myeloma who received HLA-matched sibling bone marrow and kidney transplants after a nonmyeloablative conditioning regimen (15). In this setting, 3 of the 7 patients developed GVHD and chimerism ranged from 63 days to greater than 9.2 years for all 7 patients. Thus, our present study showed that reconditioning with 2 Gy TBI and RLI may be a clinically relevant application in the HLA-matched setting. HLA-identical kidney transplants represent a small but significant percentage of transplants performed. Data collected by the UNOS Registry between 1987 and 1996 indicated 4.3% of kidney transplants performed at 250 centers in the U.S were from HLA-identical sibling donors (16)
Currently there is no satisfactory explanation as to why induced or spontaneous rejection of hematopoietic chimerism does not also result in rejection of a kidney graft from the respective hematopoietic cell donor. It has been suggested that transient hematopoietic cell chimerism may result from engraftment of transplanted hematopoietic progenitor cells and mature lymphocytes but not hematopoietic stem cells, and that loss of chimerism would not be due to marrow rejection but rather to lack of marrow space for engraftment of the relatively rare transplanted donor stem cells (9). This explanation is not supported by our data since the kidneys were transplanted and the RLI was given well after multi-lineage hematopoietic chimerism was firmly established. Another explanation is that peripheral tolerance of a transplanted organ may be due to immune suppressive mechanisms, specifically regulatory T cells (17,18). We observed FoxP3 + cells in histopathological sections of biopsies from transplanted kidneys following 2 Gy TBI + recipient leukocyte infusion, and this marker for regulatory T cells has been observed in biopsies obtained from orthotopically transplanted lungs in a similar chimeric dog model (19). The loss of donor hematopoiesis after 2 Gy TBI and RLI suggests that 2 Gy TBI disrupts an active immune tolerance mechanism for hematopoiesis but does not inhibit the tolerance mechanism for kidney grafts. If the same cellular mechanism maintained both hematopoietic and kidney tolerance, then one would have expected kidney rejection coincident with loss or rejection of donor hematopoiesis. Apparently, the immune mechanisms for hematopoietic and solid organ tolerance are more complex. For example, the donor hematopoietic system is potentially a better antigenic target than the kidney, since in addition to MHC class I antigen presentation, minor antigens presented on MHC class II under noninflammatory conditions are primarily expressed on hematopoietic cells and may be targeted by CD4+ T cells (20). These CD4+ T-cells can provide antigen-specific helper function for maturation of antigen presenting cells and expansion of CD8+ minor antigen-specific cells further upregulating the immune response.
It is interesting to speculate whether, following rejection of donor hematopoiesis, tolerance would extend to other tissues following kidney transplantation. However, we opted not to pursue this investigation as studies by Kuhr et al. (2) found that dogs tolerant to kidney grafts from DLA-identical marrow donors rejected skin grafts in 2 out of 4 cases. Thus, in the absence of reconditioning and RLI, tolerance to other tissues in this model is evidently not universal.
The approach of using low dose TBI plus RLI to return hematopoiesis to 100% host has potential application for the prevention of GVHD in HLA-matched patients following the induction of mixed hematopoietic chimerism. Our results demonstrate that in the canine model only transient hematopoietic chimerism is required for establishing and maintaining DLA-identical kidney allografts and suggests that lower initial conditioning doses of irradiation at 0.5 or 1 Gy TBI may be possible to achieve kidney allograft tolerance. These lower doses of radiation are inadequate for sustained hematopoietic cell engraftment and nearly always lead to eventual marrow graft rejection (21,22). However, it is unknown whether the short duration of hematopoietic chimerism seen with low-dose TBI is sufficient to establish tolerance towards a kidney graft. Future studies will determine whether this less toxic, nonmyeloablative protocol can be applied to the DLA-haploidentical transplant setting or to transplantation of other organs with greater likelihood of rejection such as the lung, or vascularized composite allografts containing skin.
MATERIALS AND METHODS
Experimental Animals
Random-bred litters of beagle and mini-mongrel cross-breeds were either raised at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA, or purchased commercially. The dogs weighed from 7.0 to 13.3 (median, 9.6) kg and were 7 to 27 (median, 8) months old. They were observed for disease for at least 60 days before study. The Institutional Animal Care and Use Committee of the FHCRC approved the research protocols, and the American Association for Accreditation of Laboratory Animal Care certified the kennels. Five littermate donor/recipient pairs were DLA-identical on the basis of matching for highly polymorphic major histocompatibility complex (MHC) class I and class II micro-satellite markers (23). In addition, specific DLA DRB1 allelic identity was confirmed by direct sequencing (24).
Nonmyeloablative Conditioning and HCT
Conditioning for HCT consisted of a single dose of 2 Gy TBI delivered at a rate of 7 cGy/min from a high-energy linear accelerator (Varian Clinac 6, Palo Alto, CA), followed by transplantation of marrow from a respective DLA-identical littermate. Marrow was infused intravenously (i.v.) within 4 hours of TBI at doses of 3.5 to 7.2 (median, 3.9) × 108 nucleated cells/kg. The day of HCT infusion was designated as day 0 for establishing mixed chimeric dogs (22). Postgrafting immunosuppression consisted of mycophenolate mofetil (MMF) (10 mg/kg b.i.d., s.c., from day 0 to day 28) and cyclosporine (CSP) (15 mg/kg b.i.d. orally from day -1 to 35). All dogs were given standard postgrafting care (25). The dogs’ clinical conditions were assessed twice daily. White blood cell counts with differentials, platelet counts, and hematocrits, were performed daily through day 21 and twice weekly thereafter.
Assessment of Hematopoietic Cell Engraftment
Percent donor chimerism was determined as previously described (26–28). Briefly, a polymerase chain reaction (PCR)-based assay was performed using specific primers for informative microsatellite markers. Primers amplified regions throughout the genome possessing tandem repeats in which the transplant recipient and donor were non-identical. PCR products were separated by capillary electrophoresis on an ABI Prism 310 Genetic Analyzer (Life Technologies, Carlsbad, CA).
Kidney Transplantation
The surgical procedures for kidney transplantation were done as previously described (1). Kidneys were transplanted from the marrow donors into the respective mixed hematopoietic chimeric recipients within 12–27 (median, 20) weeks after marrow transplant. In brief, the marrow donor was anesthetized, a midline laparotomy was performed, and the left kidney exposed. The ureter, renal vein and renal artery were mobilized, secured, and transected. The kidney was removed from the peritoneal cavity and perfused with 4°C saline containing heparin (10 U/mL). The kidney was maintained in cold heparinized saline while the recipient was prepared for surgery. The kidney was transplanted into the recipient’s right anterior thigh; the renal artery and vein anastamosed to the femoral artery and vein, respectively; the ureter was passed through the inguinal canal and implanted into the bladder. Kidney graft recipients received no immunosuppressive therapy. Vascularity of the transplanted kidney was confirmed by an ultrasound Doppler flow detector (Parks Medical Electronics, Aloha, OR). Biopsies were done using a true-cut biopsy needle. The tissue specimens were fixed in 10% buffered formalin and stained using hematoxylin and eosin for evaluation by microscopy. In order to show that transplanted kidneys were functional, resection of the recipients native kidneys were done at week 28 for dogs H128 and H309 and at weeks 160 and 169 for dogs G918 and G774 respectively. All dogs successfully received kidney transplants with the exception of one dog (H206) in which the transplanted kidney was ischemic as determined 24 hours after surgery by Doppler ultrasound. Biopsy of the transplanted kidney revealed no lymphocytic infiltration, but upon resection of the native kidneys, H206 serum creatinine levels were sharply elevated and the dog was in anuric failure. At this point, dog H206 was removed from study.
Intravenous Pyelogram (IVP)
Before bilateral native nephrectomies were performed in the recipient animals, renal function of the transplanted kidney was assessed by intravenous pyelogram after injection of the contrast agent diatrizoate meglumine and diatrizoate sodium (Tyco Healthcare, Mallinckrodt, St Louis, MO) diluted 1:1 with dextrose solution (Baxter Healthcare, Deerfield, Ill). Infusion was done over a 10-minute period. Images were taken at 0, 10, and 15 minutes after completion of infusion.
Leukopheresis and Recipient Leukocyte Infusion
One month prior to allogeneic HCT, G-CSF-mobilized peripheral blood leukocytes were collected and cryopreserved for future use as RLI. To that end, recipients were treated with recombinant canine G-CSF at 5 μg/kg twice daily for five days prior to and on the day of leukopheresis (29,30). Leukopheresis was performed using a central venous catheter in the external jugular vein and a continuous-flow centrifuge (COBE 2997; Blood Component Technology, Lakewood, CO). The RLI leukopheresis product was cryopreserved for later use.
At 100 days after allogeneic HCT, 6control dogs not given a kidney transplant were reconditioned with 2 Gy TBI and received either no cells or RLI at an average dose of 7.4 × 108 mononuclear cells/kg. These dogs were monitored for chimerism and tolerance to full thickness skin grafts in accord with previously reported methods (31).
Dogs, given kidney allografts and, except for one dog which spontaneously rejected the donor marrow, were reconditioned with 2 Gy TBI and given thawed RLI between 37 and 40 weeks after DLA-identical marrow transplant consisting of 1.6 to 12 (median, 2.8) × 108 mononuclear cells/kg. No additional immunosuppression was given. Dogs were followed for 6 to 14 weeks after RLI for assessment of hematopoietic chimerism.
Supplementary Material
Acknowledgments
The authors thank the technicians of the Canine Shared Resources Core of the Fred Hutchinson Cancer Research Center for expert animal care. Recombinant canine G-CSF was generously supplied by Amgen Corporation. Dr. Michele Spector, DVM provided veterinary supervision. We are grateful to Deborah Gayle, Bonnie Larson, Helen Crawford and Sue Carbonneau for their outstanding administrative support, and Stacy Zellmer and Patrice Stroup for the DLA typing. We are grateful to Drs. Beard, Mielcarek, Parker, Venkataraman, Wang, Rezvani, Gyurkocza, Georges, Rosinski, Sato, Wurm, Kornblit, our program investigators who participated in weekend treatments.
Funding support information: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD, grants P01CA078902 and P30CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. In addition, David Mathes received support from the National Endowment for Plastic Surgery Grant from the Plastic Surgery Educational Foundation.
ABBREVIATIONS
- CSP
cyclosporine
- DLA
dog leukocyte antigen
- FHCRC
Fred Hutchinson Cancer Research Center
- G-CSF
granulocyte colony stimulating factor
- GVHD
graft-versus-host disease
- Gy
gray
- HCT
hematopoietic cell transplantation
- IVP
intravenous pyelogram
- MMF
mycophenolate mofetil
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- TBI
total body irradiation
- VNTR
variable number tandem repeat
Footnotes
Authors’ contributions:
Scott S. Graves: co-authored the manuscript, performed the pyelograms, and assisted in the surgeries
David W. Mathes: co-authored the manuscript and assisted in the surgeries
George E. Georges: supervised preliminary TBI and recipient leukocyte infusion studies and edited the manuscript
Christian S. Kuhr: chief surgeon and edited the manuscript
Jeffery Chang: assisted in surgeries, collected biopsies and performed histological analysis
Tiffany Butts: conducted chimerism analysis, aphaeresed G-CSF-mobilized leukocytes.
Rainer Storb: designed the study and edited the manuscript.
References
- 1.Kuhr CS, Allen MD, Junghanss C, et al. Tolerance to vascularized kidney grafts in canine mixed hematopoietic chimeras. Transplantation. 2002;73:1487–1493. doi: 10.1097/00007890-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 2.Kuhr CS, Yunusov M, Sale G, Loretz C, Storb R. Long-term tolerance to kidney allografts in a preclinical canine model. Transplantation. 2007;84:545–547. doi: 10.1097/01.tp.0000270325.84036.52. [DOI] [PubMed] [Google Scholar]
- 3.Niemeyer GP, Welch JA, Tillson M, et al. Renal allograft tolerance in DLA-identical and haploidentical dogs after nonmyeloablative conditioning and transient immunosuppression with cyclosporine and mycophenolate mofetil. Transplant Proc. 2005;37:4579–4586. doi: 10.1016/j.transproceed.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 5.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 6.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 7.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Sachs DH. Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism (Review) Current Opinion in Organ Transplantation. 2011;16:366–371. doi: 10.1097/MOT.0b013e3283484b2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LoCascio SA, Morokata T, Chittenden M, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90:1607–1615. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millan MT, Shizuru JA, Hoffmann P, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73:1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Georges GE, Storb R, Thompson JD, et al. Adoptive immunotherapy in canine mixed chimeras after nonmyeloablative hematopoietic cell transplantation. Blood. 2000;95:3262–3269. [PubMed] [Google Scholar]
- 12.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 13.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in Cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 14.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer TR, Sykes M, Tolkoff-Rubin N, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–676. doi: 10.1097/TP.0b013e31820a3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki MM, Cecka JM, Terasaki PI. Unrelated living donor kidney transplants (Review) Br Med Bull. 1997;53:854–859. doi: 10.1093/oxfordjournals.bmb.a011653. [DOI] [PubMed] [Google Scholar]
- 17.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection (Review) Nature Reviews Nephrology. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 18.Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23:165–173. doi: 10.1016/j.smim.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash RA, Yunusov M, Abrams K, et al. Immunomodulatory effects of mixed hematopoietic chimerism: immune tolerance in canine model of lung transplantation. Am J Transplant. 2009;9:1037–1047. doi: 10.1111/j.1600-6143.2009.02619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumpf AN, van der Meijden ED, van Bergen CA, Willemze R, Falkenburg JH, Griffioen M. Identification of 4 new HLA-DR-restricted minor histocompatibility antigens as hematopoietic targets in antitumor immunity. Blood. 2009;114:3684–3692. doi: 10.1182/blood-2009-03-208017. [DOI] [PubMed] [Google Scholar]
- 21.Mielcarek M, Torok-Storb B, Storb R. Pharmacological immunosuppression reduces but does not eliminate the need for total-body irradiation in nonmyeloablative conditioning regimens for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1255–1260. doi: 10.1016/j.bbmt.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Leisenring W, Mielcarek M, et al. Intensified postgrafting immunosuppression failed to assure long-term engraftment of dog leukocyte antigen-identical canine marrow grafts after 1 gray total body irradiation. Transplantation. 2008;85:1023–1029. doi: 10.1097/TP.0b013e318169be24. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JL, Burnett RC, Storb R. Molecular analysis of the DLA DR region. Tissue Antigens. 1996;48:549–553. doi: 10.1111/j.1399-0039.1996.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 24.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 25.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–15. [PubMed] [Google Scholar]
- 26.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 27.Hilgendorf I, Weirich V, Zeng L, et al. Canine haematopoietic chimerism analyses by semiquantitative fluorescence detection of variable number of tandem repeat polymorphism. Veterinary Research Communications. 2005;29:103–110. doi: 10.1023/b:verc.0000047486.01458.c5. [DOI] [PubMed] [Google Scholar]
- 28.Graves SS, Hogan W, Kuhr CS, et al. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandmaier BM, Storb R, Santos EB, et al. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508–3513. [PubMed] [Google Scholar]
- 30.Lupu M, Gooley T, Zellmer E, Graves SS, Storb R. Principles of peripheral blood mononuclear cell apheresis in a preclinical canine model of hematopoietic cell transplantation. J Vet Intern Med. 2008;22:74–82. doi: 10.1111/j.1939-1676.2007.0016.x. [DOI] [PubMed] [Google Scholar]
- 31.Yunusov MY, Kuhr CS, Georges GE, et al. Partial donor-specific tolerance to delayed skin grafts after rejection of hematopoietic cell graft. Transplantation. 2006;82:629–637. doi: 10.1097/01.tp.0000229449.09622.28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.