Abstract
Cyanovirin-N (CV-N) is a mannose-binding lectin that inhibits HIV-1 infection by blocking mannose-dependent target-cell entry via C-type lectins. Like HIV-1, Mycobacterium tuberculosis expresses mannosylated surface-structures and exploits C-type lectins to gain cell-access. Here we investigated whether CV-N, as for HIV-1, can inhibit M. tuberculosis infection. We found that CV-N specifically interacted with mycobacteria by binding to the mannose-capped lipoglycan lipoarabinomannan. Furthermore, CV-N competed with the C-type lectins DC-SIGN and mannose receptor for ligand binding and inhibited the binding of M. tuberculosis to dendritic cells but, unexpectedly, not to macrophages. Subsequent in vivo infection experiments in a mouse model demonstrated that CV-N, despite its activity, did not inhibit or delay M. tuberculosis infection. This outcome argues against a critical role for mannose-dependent C-type lectin interactions during initial stages of murine M. tuberculosis infection and suggests that, depending on the circumstances, M. tuberculosis can productively infect cells using different modes of entry.
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is the most deadly bacterial disease worldwide and kills ~1.7 million people each year (1). As the prevalence of drug-resistant M. tuberculosis is rising and the current vaccine for TB, i.e. Mycobacterium bovis BCG, shows a variable efficacy (2), new TB vaccines and drugs are urgently needed (1). M. tuberculosis uses a large repertoire of strategies to modulate the host immune response, several of which are associated with mannosylated cell-surface structures (3). Upon inhalation, M. tuberculosis enters the lungs and establishes an infection by invading alveolar macrophages (Mϕ) and dendritic cells (DCs). For this, M. tuberculosis may use different receptors, including the mannose-binding C-type lectins macrophage mannose receptor (MMR) (4) and the dendritic cell-specific ICAM-3 Grabbing Non-Integrin (DC-SIGN) (5). Both DC-SIGN and the MMR recognize mannosylated structures, including mycobacterial cell-surface glycolipids such as mannose-capped lipoarabinomannan (ManLAM) and phosphatidylinositol mannosides (PIMs) (6–9). Yet, the exact role of DC-SIGN and the MMR in establishing and maintaining M. tuberculosis infection remains unclear, as these receptors not only mediate mycobacterial phagocytosis, but also seem to have a role in modulating M. tuberculosis-related immune pathology (3,10,11).
Cyanovirin-N (CV-N) is a mannose-binding lectin derived from the cyanobacterium Nostoc ellipsosporum extensively studied for its ability to block infections with HIV-1 (12–14). It does so by interacting with mannosylated HIV-1 gp120 and block target-cell entry via C-type lectins (12,15). In addition, CV-N has been reported to reduce infectivity of the Ebola and Hepatitis C viruses, also by targeting surface-exposed mannosylated proteins (16,17). As mentioned above, M. tuberculosis expresses a large number of mannosylated cell-surface structures which it may exploit to gain access to host immune cells (3,4,6,7). This suggests that CV-N, in analogy with its ability to block viral infections, may also abrogate infection with M. tuberculosis. Here we report our study investigating the ability of CV-N to inhibit M. tuberculosis infection.
METHODS
Bacteria
M. tuberculosis strains H37Ra, H37Rv, CDC1551, HN878, and double auxotroph mc26020 (18), M. bovis BCG Copenhagen (19), M. marinum E11 (20), and M. smegmatis mc2155 were grown in Middlebrook 7H9 broth (Difco) with 10% Middlebrook albumin/dextrose/catalase enrichment (BBL) and 0.05% Tween-80 or on Middlebrook 7H10 agar (Difco) with 10% Middlebrook oleic acid/albumin/dextrose/catalase enrichment (BBL) at 37°C, or 30°C for M. marinum. Medium for M. tuberculosis mc26020 was supplemented with 100 μg mL−1 L-lysine and 25 μg mL−1 D-pantothenic acid (Sigma-Aldrich). Escherichia coli DH5α was grown on Luria Bertani (LB) agar or broth at 37°C.
Cyanovirin-N
Recombinant CV-N was expressed in E. coli BL21(DE3) cells as described previously (21,22). Cells were harvested by centrifugation (7500× g) and resuspended in 100 mL cold phosphate-buffered saline (PBS) (pH7.4) containing 10 mM benzamidine prior to lysis in a microfluidizer. The total cell lysate was centrifuged at 25,000× g, and the supernatant loaded directly onto a C18 cartridge from which CV-N was eluted with a step gradient from 0–80% CH3CN in H2O. Fractions containing CV-N (40–70% CH3CN) were combined, lyophilized, and purified by reverse-phase-HPLC yielding milligram quantities of CV-N with greater than 95% purity. These fractions were subjected to size-exclusion chromatography after reconstitution in PBS adjusted to pH7.4 with 0.5 M NaOH before loading onto a Superdex75 16/60 column. Fractions corresponding to homogeneous, monomeric CV-N were combined. The periodate method was used to conjugate horseradish peroxidase (HRP; Sigma-Aldrich) to CV-N (23,24). Before use, CV-N-HRP was dialyzed overnight (o/n) against H2O at 4°C.
Monoclonal antibodies
Murine monoclonal antibodies (MAb) of the IgM class were used. MAb F30-5 recognizes the hexa-arabinan domain of LAM and binds both ManLAM and AraLAM (25,26). MAb 55.92.1A1 binds the mannose cap on LAM (25). F183-24 recognizes mainly α(1→2)-linked mannosyl residues as present in PIM6 and with lower affinity the mannose cap on ManLAM as well (6,26). F30-5 and F183-24 were provided by Arend Kolk, Royal Tropical Institute, Amsterdam, the Netherlands. 55.92.1A1 was provided by Peter Willemsen, Central Institute for Animal Disease Control, Lelystad, the Netherlands.
SDS-PAGE/immunoblot
Bacterial cultures were grown until mid/late-log phase and disrupted with a Beadbeater (BioSpec). Supernatants were equalized for protein content using a BCA Protein Assay kit (Pierce). Cell lysates were run on a 12% SDS-PAGE gel in a tricine buffer (27), followed by transfer on a polyvinylidene fluoride membrane (Millipore) (28). Membranes were incubated in 1% bovine serum albumin (BSA) (Sigma-Aldrich) in PBS + 0.05% Tween-80 (PBST+BSA) followed by o/n incubations at room temperature (RT) with CV-N-HRP in PBST+BSA. For the immunoblots with MAbs: the membranes were incubated in 0.5% (w/v) blocking reagens (Boehringer) followed by o/n incubations at RT with the MAb in 1:1 v:v blocking reagens and PBST. As secondary antibody peroxidase-labeled goat-anti-mouse IgM (American Qualex) in 0.5% (v/v) normal goat serum was applied. After thorough washing, all membranes were developed using a mixture of 4-chloro-1-naphtol (Bio-Rad) and 3,3′-diaminobenzidine 4HCl (Sigma-Aldrich).
CV-N-HRP adhesion assay
Man- and AraLAM, polyacrylamide (PAA)-linked glycoconjugates (0.1 μg per well) (29), or bacterial suspensions (A600=1; heat-killed at 85°C for 45 min) in NaCl-buffer were coated onto 96-well polyvinyl chloride-plates (BD Falcon, Biosciences) o/n at RT. ELISA-plates were washed with 20 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM MgCl2, 2 mM CaCl2 (TSM) supplemented with 0.05% Tween-80 (TSMT). TSM + 1% BSA (Fluka) was used as blocking buffer. CV-N-HRP was titrated starting from 2.5 μg mL−1 in TSM + BSA and left to incubate for 2h at RT. Binding of CV-N-HRP was detected using o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich) as coloring reagent and measuring absorption at 490 nm.
CV-N inhibition assay
The same protocol was used as described above, but plates were probed with serial dilutions of MMR-Fc (30) or a DC-SIGN–Fc construct (31) in the absence or presence of 50 mg/ml CV-N for 2 h at RT. Goat anti-human IgG-peroxidase (Jackson Laboratories) in 0.5% normal goat serum was used as secondary antibody.
Cells
Human peripheral blood mononuclear cells were isolated from buffy coats (Sanquin Bloodbank, Amsterdam, NL) by centrifugation on a Ficoll-Paque gradient (GE Healthcare), followed by selection for CD14+ monocytes using anti-CD14 magnetic beads and the Midi-MACS system (Miltenyi Biotech). Isolated monocytes were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (Gibco) (37°C, 5% CO2)in presence of IL-4 (500 U mL−1) and GM-CSF (800 U mL−1) (PeproTech) to produce immature monocyte-derived dendritic cells (MoDC) (32) or in presence of 50 U mL−1 GM-CSF for MoMϕ. For MoMϕ, the culture medium was replenished with GM-CSF on day three after isolation (33). Cells were used for further experiments on day 6 after isolation and tested for expression of DC-SIGN, MMR, and CD14 [MoDCs: DC-SIGN(high), MMR(high), CD14(low); MoMϕ: DC-SIGN(low), MMR(high), CD14(high)] (Supplemental Fig. 3). RAW264.7 cells stably transfected or not with SIGNR1 or SIGNR3 were cultured in RPMI 1640 GlutaMAX (Invitrogen) supplemented with 10% FCS (Sigma-Aldrich) in the presence of 100 U mL−1 penicillin/streptomycin (Invitrogen) and 10μg mL−1 Blasticidin when appropriate (34).
MoDCs and MoMϕ binding assays
M. tuberculosis mc26020 cultures were grown until mid-log phase (A600=0.6–0.8), concentrated 10-fold in FITC-buffer (150 mM NaCl, 0.2 M Na2CO3 (pH9.2), 0.05% Tween-80), and incubated with 250 μg mL−1 fluorescein isothiocyanate (FITC; Sigma-Aldrich) for 20 min at RT, after which the suspensions were washed and diluted in TSMT depending on the desired final multiplicity-of-infection (MOI). CV-N (or PBS) was added to the mycobacterial suspensions at a concentration of 200 μg mL−1 and incubated for 1h at RT. After this, the bacteria were washed and diluted in RPMI 1640 medium containing 10% (v/v) FCS, and fluorescence of the different suspensions was quantified using the FLUOstar-Galaxy microplate reader (BMG). Mycobacteria were added to MoDCs and MoMϕ which were either 10 min pre-incubated with AZN-D1 (50 μg mL−1) (7), or mannan (2 mg mL−1), respectively. After incubation for 45 min at 37°C, the percentage of fluorescent cells was determined using a Flow Cytometer (C6, Accuri) and analyzed using manufacturer’s software (CFlow Plus version 1.0.208.2).
Phagocytotic uptake
M. tuberculosis mc26020 was pre-incubated with CV-N at a concentration of 200 μg mL−1 or PBS for 1h at RT. After this, the bacteria were washed and diluted in RPMI 1640 medium containing 10% (v/v) FCS. Human MoDCs, RAW264.7 cells, or RAW cells transfected with SIGNR1 or SIGNR3 were co-incubated for two hours in presence of serum with M. tuberculosis at a MOI of 5. After two hours, 200 ug mL−1 amikacin was added to kill extracellular bacteria and incubated for another two hours. Cells were washed three times, lysed with 1% Triton X-100 in PBS for 5 minutes at 37°C after which the mycobacteria were plated in dilution series onto 7H10 agar plates. CFUs were counted after 3 weeks incubation at 37°C.
Statistical analysis
In vitro cell binding studies: all values were expressed as means of at least three independent experiments (each carried out in triplicate) ± standard error of mean (n ≥ 3). The unpaired Student t test was used to identify significant differences between groups (p < 0.05).
In vivo experiments
M. tuberculosis H37Rv was grown to an A650 of 0.4. Cells were harvested, resuspended in PBST, and filtered through a 5μm-filter to obtain a single-cell suspension (A650=0.67). Of this, 80 μL was added to 320 μL PBS supplemented or not with CV-N (100 and 500 μg mL−1; CV-N:mycobacteria ratio similar to pretreatment of MOI5-suspensions in the in vitro assays and five-fold higher, respectively). After 2h pre-incubation at RT, cells were diluted with 10 mL of PBST and used for aerosol infection of mice. Eight-week-old C57Bl/6 mice were infected with ~100 M. tuberculosis colony forming units (CFUs) using a BioAerosol nebulizing generator (CH Technologies Inc., Westwood, NJ) for 10 min. Mice from separate groups were euthanized at days 0, 7, 14 and 28. Organs (lungs, spleens and livers) were homogenized in 7H9 Middlebrook supplemented medium and dilutions plated on 7H11 Middlebrook agar plates supplemented with 10% OADC (35).
The Animal Care and Use Committee (ACUC) of the National Institute of Allergy and Infectious Diseases, Division of Intramural Research, with permit number NIH IRP, PHS Assurance A4149-01, approved the animal study protocol LCID-3E under which all animal experiments were performed.
RESULTS
CV-N specifically interacts with M. tuberculosis
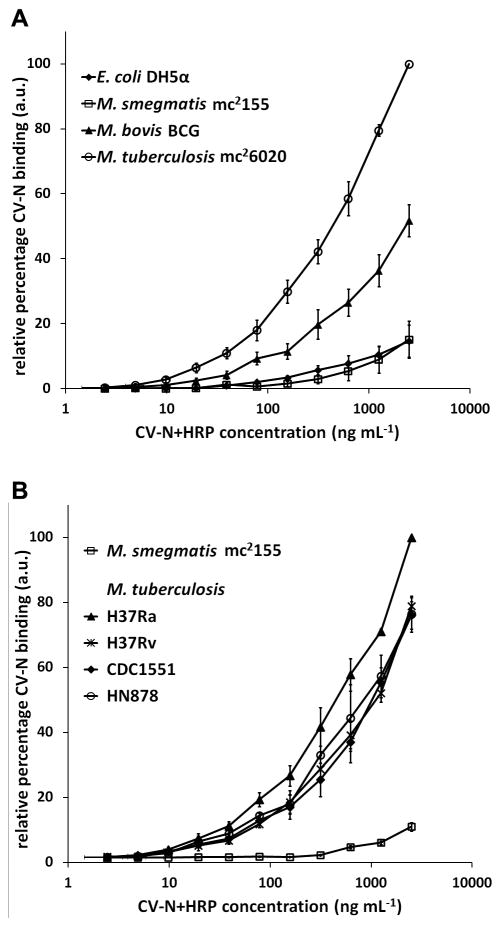
To examine the ability of CV-N to interact with mycobacteria, three different mycobacterial species were coated onto 96 well-plates and probed with serial dilutions of HRP-coupled CV-N (CV-N+HRP). Besides M. tuberculosis auxotrophic strain mc26020, the closely related vaccine strain M. bovis BCG strain Copenhagen and the non-pathogenic species M. smegmatis strain mc2155 were also tested. Escherichia coli strain DH5α was included as a negative control. As shown in Fig. 1A, CV-N strongly bound to M. tuberculosis and M. bovis BCG, whereas it showed only weak binding to M. smegmatis and E. coli (Fig. 1A). To investigate possible intra-species differences, CV-N-binding to a panel of different M. tuberculosis strains was also tested. As shown in Fig. 1B, CV-N bound equally well to all four isolates tested suggesting that binding of CV-N was species rather than strain specific. Overall, these data demonstrate that CV-N binding varies between different mycobacterial species and that CV-N strongly binds to species belonging to the M. tuberculosis-complex.
FIGURE 1.
CV-N differentially recognizes various mycobacterial species. A, M. tuberculosis strain mc26020, M. bovis BCG strain Copenhagen, M. smegmatis strain mc2155, and E. coli strain DH5α were coated on a 96-well plate and probed with a serial dilution of CV-N+HRP. CV-N binding was detected using OPD as coloring reagent and absorption was measured at 490 nm. B, In a similar assay, binding of CV-N to M. tuberculosis strains H37Rv, H37Ra, CDC1551, and HN878 was tested. In all cases, the data represent the average of three independent experiments plus the standard error of mean.
ManLAM is the primary CV-N ligand
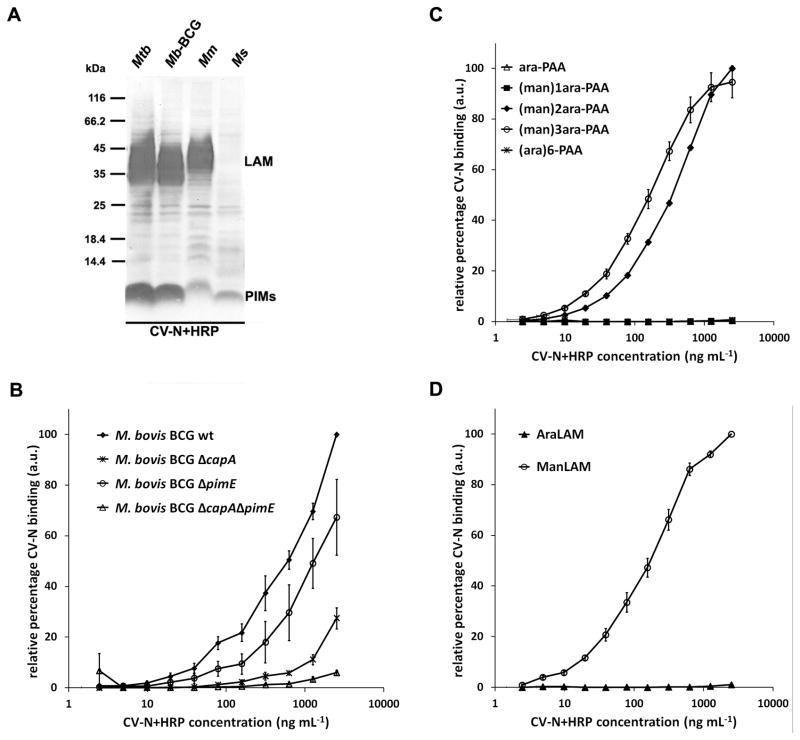
Next we investigated which mycobacterial structures were recognized by CV-N. It has been reported that CV-N recognizes α(1,2)-linked mannosyl residues (21,22), which are also present in the mannose cap of ManLAM, and in the phosphatidylinositol hexamannoside PIM6. First, total cell lysates of M. tuberculosis mc26020, M. bovis BCG Copenhagen, the fish-pathogen M. marinum strain E11, and M. smegmatis mc2155 were examined on SDS-PAGE/immunoblot with CV-N+HRP. Staining was observed at the position of ManLAM for M. tuberculosis, M. bovis BCG, and M. marinum, but not for M. smegmatis which is known to express a phosphatidylinositol-capped version of LAM (PILAM) (Fig. 2A and Supplemental Fig. 1A). In addition, CV-N staining at the position of PIM6 was observed in all four species although with varying intensity (Fig. 2A and Supplemental Fig. 1A).
FIGURE 2.
CV-N binds the α(1,2)-linked mannosyl residues present in the mannose caps of ManLAM and in PIM6. A, Cell lysates of M. tuberculosis mc26020 (Mtb), M. bovis BCG (Mb-BCG), M. marinum E11 (Mm), and M. smegmatis mc2155 (Ms) were examined on SDS-PAGE/immunoblot with CV-N+HRP. CV-N+HRP binds to ManLAM of M. tuberculosis, M. bovis BCG, and M. marinum, but not to PILAM of M. smegmatis. Staining with CV-N+HRP is also seen at the position of PIMs on the immunoblot. See Supplemental Fig. 1A for staining of the same cell lysates on immunoblot with (Man)LAM- and PIM6-specific antibodies. B, Whole cells of M. bovis BCG wild-type, ΔcapA (deficient for the mannose cap on LAM), ΔpimE (deficient in biosynthesis of PIM6), and double knockout ΔcapAΔpimE were examined for CV-N+HRP binding in whole-cell ELISA. The average of three independent experiments is shown. See Supplemental Fig. 1B for concomitant SDS-PAGE/immunoblot with M. bovis BCG cell lysates. C, PAA-coupled glycoconjugates and, D, lipoglycans ManLAM and AraLAM were coated on a 96-well plate and probed with a serial dilution of CV-N+HRP. The average of three independent experiments is shown plus the standard error of mean. Note, in C, binding of CV-N+HRP to glycoconjugates (ara)6-PAA, ara-PAA and (man)1ara-PAA, was below detection levels (i.e. 0.0 in figure) in all three cases.
To obtain further evidence that ManLAM and PIM6 were indeed CV-N ligands, lysates prepared from wild-type M. bovis BCG and isogenic mutant strains with specific defects in ManLAM and/or PIM6 biosynthesis were subjected to SDS-PAGE/immunoblot and probed with CV-N+HRP. As shown in Supplemental Fig. 1B, the mutant strains, as compared to wild-type M. bovis BCG, showed a different immunoblot profile. In case of the M. bovis BCG ΔcapA strain, which expresses LAM devoid of mannose caps (25), staining at the position of LAM was no longer visible (Supplemental Fig. 1B). On the other hand, probing a M. bovis BCG ΔpimE mutant, which synthesizes PIM2 and PIM4, but not PIM6 (6), demonstrated that the reactivity at the position of PIM6 was absent. Finally, with the ΔcapAΔpimE double mutant strain, devoid of both ManLAM and PIM6, staining for both structures was absent (Supplemental Fig. 1B). To gain insight into the relative contribution of ManLAM and PIM6 for binding of CV-N, the wild-type and mutant M. bovis BCG strains were analyzed for CV-N binding by whole-cell ELISA. As shown in Fig. 2B, mutation of either pimE or capA led to a reduction in CV-N binding whereas binding to the ΔcapAΔpimE double mutant was almost absent. However, when compared to each other, the abrogating effect of the capA mutation was much stronger than the mutation in pimE, suggesting that the mannose caps on ManLAM are the dominant CV-N ligands. This finding is consistent with our earlier observation that M. smegmatis, which is devoid of ManLAM but does express PIM6, shows minimal CV-N binding (Fig. 1A).
Binding of CV-N to ManLAM is dependent on the length of the mannose cap
The mannose caps of ManLAM contain one to three mannosyl residues. To investigate the influence of mannose cap length on CV-N binding, a range of polyacrylamide (PAA)-coupled glycoconjugates resembling the LAM-mannose cap structures were probed with CV-N+HRP. Glycoconjugates (man)1ara-PAA, (man)2ara-PAA and (man)3ara-PAA are equivalent for short to long mannose caps on ManLAM respectively, and the structure of (ara)6-PAA is equivalent to the uncapped Ara6 motif found in LAM (29). As shown in Fig. 2C, CV-N recognized both (man)2ara-PAA and (man)3ara-PAA, while the single mannose-structure (man)1ara-PAA and the hexa-arabinose motif were not bound (Fig. 2C). In a similar assay, ManLAM and non-capped LAM (AraLAM) were also tested. Consistent with the data for the glycoconjugates, CV-N recognized ManLAM but not AraLAM (Fig. 2D). Hence, the presence of at least a dimannoside cap is essential for recognition by CV-N.
CV-N inhibits the binding of MMR-Fc and DC-SIGN-Fc to ManLAM and M. tuberculosis
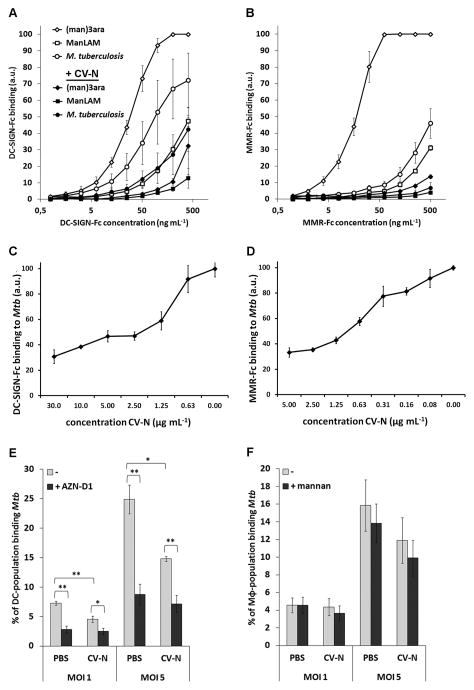
ManLAM is a ligand for the human C-type lectins DC-SIGN and the MMR (7,29,36). To test whether CV-N can compete with DC-SIGN and the MMR for ligand binding, glycoconjugate (man)3ara-PAA, purified ManLAM, or whole M. tuberculosis cells were coated onto a 96-well plate and probed with a DC-SIGN-Fc or MMR-Fc construct in the presence or absence of CV-N. Binding of both DC-SIGN-Fc and MMR-Fc to the coating was inhibited by CV-N (Fig. 3A and 3B). Binding of the highest concentration of DC-SIGN-Fc to (man)3ara-PAA, ManLAM, and M. tuberculosis, was reduced by CV-N by 68±17% (mean±SEM), 71±16%, and 44±6%, respectively (Fig. 3A). Similarly, binding of MMR-Fc to (man)3ara-PAA, ManLAM, and M. tuberculosis was reduced by CV-N by 86±1%, 87±3%, and 87±5%, respectively (Fig. 3B). Noteworthy, a clear difference in affinity for the three ligands between the lectin-Fc constructs could be observed, with MMR-Fc exhibiting a relatively high affinity for (man)3ara-PAA (Fig. 3B). Furthermore, CV-N inhibits binding of DC-SIGN-Fc and MMR-Fc to M. tuberculosis in a dose-dependent manner (Fig. 3C and D, respectively).
FIGURE 3.
CV-N inhibits binding of M. tuberculosis mc26020 or mycobacterial mannosylated structures to DC-SIGN-Fc, MMR-Fc, and to MoDCs, but not to MoMϕ. A–B, Whole M. tuberculosis mc26020 cells, (man)3ara-PAA, and ManLAM were coated on a 96-well plate and incubated with serial dilutions of either A, DC-SIGN-Fc (starting at 400 ng mL−1), or B, MMR-Fc (starting at 500 ng mL−1) in the presence or absence of 50 μg mL−1 CV-N. The average of three independent experiments is shown plus the standard error of mean. C–D, CV-N inhibits binding of M. tuberculosis mc26020 by C, DC-SIGN-Fc and D, MMR-Fc in a dose-dependent manner. Whole M. tuberculosis mc26020 cells were coated on a 96-well plate and incubated DC-SIGN-Fc (400 ng mL−1), or MMR-Fc (500 ng mL−1) in the absence or presence of a dilution series of CV-N. Shown is one representative experiment with average of triplicates plus standard deviation. E–F, M. tuberculosis cells were fluorescently labeled, diluted to the correct MOI, pre-treated with PBS or 200 μg mL−1 CV-N, washed, and added to E, MoDCs and, F, MoMϕ. MoDCs and MoMϕ were used untreated, or after pre-incubation with the DC-SIGN-specific inhibitory antibody AZN-D1 or with 2 mg mL−1 mannan, respectively. The percentage of the cell population binding M. tuberculosis was determined by flow cytometry. Shown is the average of results from five donors plus the standard error of mean. * p < 0.05; ** p < 0.005.
CV-N inhibits binding of M. tuberculosis to DCs but not to macrophages
As the MMR is reported to be an important receptor for mycobacteria on Mϕ (4), and DC-SIGN the major receptor for mycobacteria on DCs (5), our next goal was to investigate whether CV-N was also able to block binding of M. tuberculosis to intact Mϕ and DCs. Fluorescently labeled mycobacteria were pre-incubated with CV-N or PBS, after which the bacilli were washed to remove free CV-N and added to human monocyte-derived (Mo)Mϕ or MoDCs. Binding of M. tuberculosis was monitored by flow cytometry. While pre-treatment with CV-N reduced binding of M. tuberculosis to MoDCs by ~40% at both MOIs tested (Fig. 3E), no significant inhibition was observed for binding to the MoMϕ (Fig. 3F), although the MoMϕs expressed significant amounts of MMR (Supplemental Fig. 3). To determine whether the binding of M. tuberculosis to MoMϕ was indeed mannose independent, an excess of mannan was added extracellularly. As shown in Fig. 3F, addition of mannan did not reduce the binding of M. tuberculosis to the macrophages, indicating that the MMR, at least under these circumstances, was dispensable for M. tuberculosis binding. Conversely, although binding of CV-N pre-treated M. tuberculosis to MoDCs could still be further blocked by DC-SIGN-blocking antibody AZN-D1 (Fig. 3E), the level of inhibition never exceeded that of AZN-D1 combined with PBS-treated bacteria (~8%), suggesting that CV-N directly competed with DC-SIGN for binding to the same mannose-containing structures. Finally, to confirm that CV-N can also inhibit binding of M. tuberculosis to MoDCs for an extended period of time in the presence of serum (i.e. complement), we co-incubated MoDCs for up to four hours with M. tuberculosis pre-treated or not with CV-N in the presence of serum, after which the infection load was determined by counting CFUs. As shown in Supplemental Fig. 2A, CV-N pre-treatment of M. tuberculosis significantly reduced the amount of CFUs retrieved from the MoDCs suggesting that CV-N inhibits both DC-SIGN-dependent binding and subsequent phagocytotic uptake of M. tuberculosis by human MoDCs, also in the presence of serum.
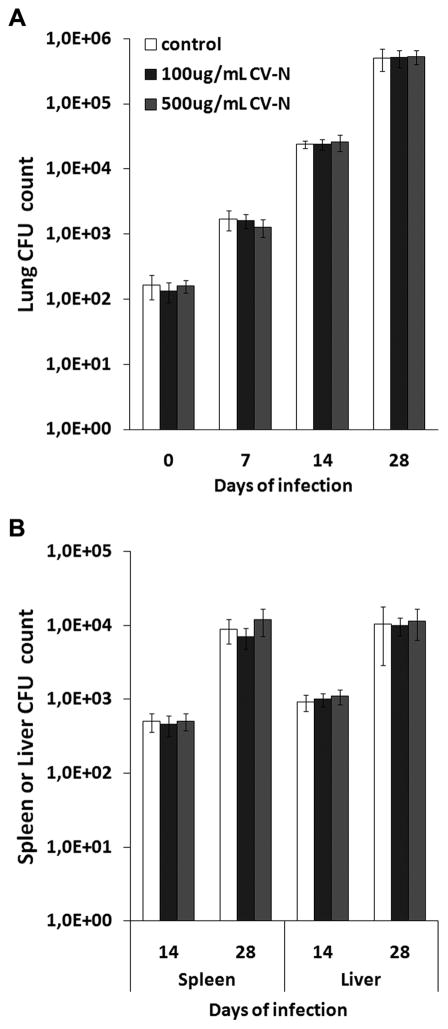
CV-N does not inhibit M. tuberculosis-infectionin a murine aerosol infection model
As CV-N is able to inhibit interaction of M. tuberculosis with DC-SIGN(−Fc) and MMR(−Fc), the effect of CV-N on M. tuberculosis infection was next examined in vivo. For this, mice were infected with ~100 CFUs of M. tuberculosis H37Rv pre-incubated or not with CV-N using a validated aerosol infection model (35). Mice were sacrificed at days 0, 7, 14 and 28 and CFUs were enumerated in the lungs, liver and spleen. As shown in Fig. 4, no differences in CFU counts were observed between mice infected with untreated or CV-N-pre-treated M. tuberculosis. Also when a higher infection dose was applied (~10-fold higher), no significant differences in infection with CV-N-treated or untreated bacteria were observed (Supplemental Fig. 4). To investigate whether the lack of an in vivo effect was due to the fact that mouse and human DC-SIGN/MMR do not behave in the same way or that inhibiting mannose-dependent interactions has no major impact on M. tuberculosis host-cell interaction, a phagocytotic uptake assay was performed using the murine alveolar macrophage-like cell line RAW264.7 stably transfected or not with the murine DC-SIGN homologs SIGNR1 or SIGNR3. As shown in Supplemental Fig. 2B, the contribution of SIGNR1 and SIGNR3 to the uptake of mycobacteria by the macrophages appeared minor under these conditions, and more importantly, CV-N only induced a small, statistically non-significant, reduction in phagocytosis in all three cell lines tested. This result is consistent with the in vivo data and suggests that mannose-dependent interactions are (at least partially) dispensable for M. tuberculosis infection of murine macrophages. Furthermore, it suggests that, unlike human DC-SIGN, expression of murine SIGNR1 or SIGNR3 does not necessarily enhance the rate of infection. Thus, in conclusion, at least in this mouse model system, CV-N pre-coating of M. tuberculosis did not delay or alter the outcome of infection most likely because mannose-dependent interactions do not play an important role during early M. tuberculosis infection in the murine infection model.
FIGURE 4.
Aerosolic infection of mice with untreated or CV-N-pre-treated M. tuberculosis H37Rv. Mycobacteria were pre-incubated with PBS (‘untreated’) or with CV-N at concentrations of either 100 μg mL−1 or 500 μg mL−1 (is comparable CV-N:bacteria ratio as used for pre-treatment of the MOI5-suspensions in the in vitro assays in Figure 3E–F, or five-fold higher, respectively). Mice were infected with the untreated or CV-N-treated M. tuberculosis via aerosolic solution on day 0. CFUs were counted in A, lung, and B, spleen and liver. The CFU count on day 0 and day 7 for spleen and liver was zero in all mice. Shown is median of five mice per group plus median absolute deviation.
DISCUSSION
Mannosylated mycobacterial cell-surface structures have been associated with various immunomodulating properties of mycobacteria (3,37). Binding of these structures to host C-type lectins, including DC-SIGN and MMR, has been suggested to modulate the host immune response and to assist in establishing M. tuberculosis infection (3-5,7,10,36,38,39). In this study, we demonstrate that CV-N, a mannose-binding lectin, specifically interacts with M. tuberculosis and reduces the interaction with host mannose-binding C-type lectins but that this inhibition does not alter the outcome of short-term M. tuberculosis infection in C57Bl/6 mice.
First, we tested the ability of CV-N to interact with different mycobacterial species. CV-N displayed enhanced binding to M. tuberculosis as compared to the vaccine strain M. bovis BCG, whereas it showed only marginal binding to non-pathogenic M. smegmatis (Fig. 1A). This indicates a possible relationship between the amount of surface-exposed mannosyl residues and the concomitant recognition by CV-N.
The observed binding pattern of CV-N shows similarity to that of host-cell receptors DC-SIGN and MMR (6,9,29,36): CV-N recognized the α(1,2)-linked mannosyl residues in PIM6 and in the mannose cap of ManLAM, but not theα(1,2)-linked mannosyl side branches present on the mannan core domain of LAM/lipomannan or the less mannosylated PIMs PIM4 and PIM2 in which α(1,2)-linked mannosyl residues are missing (Fig. 2 and Supplemental Fig. 1). However, some differences between the binding patterns of DC-SIGN/MMR and CV-N may also exist: the MMR has been suggested to be a phagocytic uptake receptor on MoMϕ for virulent M. tuberculosis strains Erdman and H37Rv, but not for strain H37Ra (4), while CV-N, at least in our assay system, did not discriminate between strains H37Rv and H37Ra (Fig. 1B). Furthermore, genetically uncapping LAM by mutating capA in combination with the absence of PIM6 (pimE-mutation) almost completely abrogated CV-N binding (Fig. 2B). In contrast, the same set of mutations was previously reported not to disturb the binding of DC-SIGN to M. bovis BCG (6,25). The reason for this discrepancy is unclear, but may be related to differences in specificity.
CV-N induced a significant reduction of binding of M. tuberculosis to MoDCs (Fig. 3E), but residual DC-SIGN-dependent binding was still present. One possible explanation is the presence of DC-SIGN-ligands that are not targeted by CV-N. One good candidate is capsular α-glucan. This extracellular glucose polymer was recently demonstrated to be a DC-SIGN ligand (40). Testing of CV-N for binding to α-glucan demonstrated that this structure does not bind CV-N (data not shown). Therefore,α-glucan could be responsible for the residual binding of M. tuberculosis to MoDCs in the presence of CV-N. In our assay system, binding of M. tuberculosis to MoMϕ was lower as compared to binding to MoDCs. Furthermore, neither CV-N nor mannan inhibited the binding of M. tuberculosis to MoMϕ (Fig. 3F). In contrast to DCs, for which DC-SIGN is the dominant receptor for binding of mycobacteria (5), Mϕ express several receptors all of which are reported to bind mycobacteria (41). Since CV-N did not block the M. tuberculosis-MoMϕ interaction, binding of M. tuberculosis in our assay system was most likely mediated by receptors that do not depend on the recognition of mannosyl residues, e.g. complement receptors (4). This is in sharp contrast with previous studies which suggested a major role for the MMR as uptake receptor on Mϕ for M. tuberculosis (3,4,36). We do not have a clear explanation for this discrepancy other than differences in the assay systems. Of note, as M. tuberculosis induces DC-SIGN expression in human alveolar Mϕ (42), uptake by these cells in humans may still be inhibited by CV-N.
The effect of CV-N surface-coating on M. tuberculosis infection in vivo was tested in a murine aerosol infection model. Results from two independent experiments (Fig. 4 and Supplemental Fig. 4), revealed that CV-N, although capable of inhibiting mannose-dependent M. tuberculosis-C-type lectin interactions in vitro, did not block or delay the infection. As CV-N is also known to be active in vivo in a vaginal gel used in macaques (13), this suggests that mannose-dependent interactions, in this model system, were (at least partially) dispensable during the early stages of infection. This finding is consistent with earlier reports in which it was demonstrated that mutants defective in producing mannose caps on LAM and/or unable to produce PIM6 behave similar to wild-type strains both in vitro and in vivo (6,25). Whether this finding is specific for the murine infection model or is also true in other models, most importantly humans, remains to be seen. Possibly, a protective effect by CV-N cannot be observed in this model system as the murine C-type lectins differ too much from the human versions, in particular DC-SIGN (43) (compare Fig. 3E with Supplemental Fig. 2B). Furthermore, CV-N did not fully block Mycobacterium-DC-SIGN interactions leaving open the possibility that the residual binding, possibly via capsular α-glucan, was enough to support cell-entry via mannose-binding C-type lectins.
In conclusion, we demonstrated here that coating mycobacterial mannosylated surface structures with an exogenous compound other than antibodies abrogates mannose-dependent Mycobacterium-C-type lectin interactions. This makes CV-N, and CV-N-like molecules, an interesting class of compounds. However, as demonstrated in the in vivo infection experiments, inhibition of these mannose-dependent interactions did not alter the outcome of infection. This finding suggests that mannose-dependent interactions are, at least partially, dispensable during the early phases of infection. Important remaining questions are then what are the preferred routes for M. tuberculosis host-cell entry in vivo and more importantly, does entry via different routes lead to different outcomes of infection? Further addressing these issues will not only answer the important question whether or not targeting mycobacterial cell-surface structures, for instance through carbohydrate-based antibody vaccines, and thereby altering the cellular uptake route can be used to combat M. tuberculosis, but may also provide further insight into the mechanisms explaining the success of M. tuberculosis as a very potent human adversary.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Jacqueline Gonzales for help with the mouse infections. We thank Drs. T.B.H. Geijtenbeek and S.I. Gringhuis (Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands) for providing DC-SIGN-Fc; Dr. L. Martinez-Pomares (University of Nottingham, Nottingham, United Kingdom) and Dr. R.J. Stillion (University of Oxford, Oxford, United Kingdom) for providing MMR-Fc; and Dr. O. Neyrolles (Centre National de la Recherche Scientifique, Universite de Toulouse, Toulouse, France) for providing (SIGNR1/SIGNR3-transfected) RAW264.7 cells. Glycolipids were a gift from Dr. J.T. Belisle, Colorado State University, Fort Collins, CO, and the National Institutes of Health, Bethesda, MD, USA (contract NO1 AI-75320).
Financial support
JG is financially supported by the Netherlands Organization for Scientific Research (NWO) through a VENI research grant (016.101.001). This work was supported by the NIH Intramural AIDS Targeted Antiviral Program, Office of the Directory (CAB), and the NIH Intramural Research Program (NIDDK and NIAID).
Nonstandard abbreviations
- ara
arabinosyl residue
- AraLAM
non-capped LAM
- CV-N
cyanovirin-N
- DCs
dendritic cells
- DC-SIGN
dendritic cell-specific ICAM3-grabbing non-integrin
- HIV
human immunodeficiency virus
- LAM
lipoarabinomannan
- man
mannosyl residue
- ManLAM
mannose-capped LAM
- MMR
macrophage mannose receptor
- Mϕ
macrophages
- MoMϕ/-DCs
monocyte-derived Mϕ/-DCs
- PAA
polyacrylamide
- PIMx
phosphatidylinositol mannosides (x= number of mannose residues)
- TB
tuberculosis
Footnotes
Conflict of interest
The authors declare that no conflict of interest exists related to this work.
References
- 1.World Health Organization. Global tuberculosis control: WHO report 2010. WHO Press; Switzerland: 2010. Report No.: WHO/HTM/TB/2010.7. [Google Scholar]
- 2.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 3.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 5.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driessen NN, Ummels R, Maaskant JJ, Gurcha SS, Besra GS, Ainge GD, Larsen DS, Painter GF, Vandenbroucke-Grauls CM, Geurtsen J, Appelmelk BJ. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geijtenbeek TB, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitarque S, Herrmann JL, Duteyrat JL, Jackson M, Stewart GR, Lecointe F, Payre B, Schwartz O, Young DB, Marchal G, Lagrange PH, Puzo G, Gicquel B, Nigou J, Neyrolles O. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem J. 2005;392:615–624. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. Eur J Cell Biol. 2010;89:95–101. doi: 10.1016/j.ejcb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Neyrolles O, Gicquel B, Quintana-Murci L. Towards a crucial role for DC-SIGN in tuberculosis and beyond. Trends Microbiol. 2006;14:383–387. doi: 10.1016/j.tim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 14.Xiong S, Fan J, Kitazato K. The antiviral protein cyanovirin-N: the current state of its production and applications. Appl Microbiol Biotechnol. 2010;86:805–812. doi: 10.1007/s00253-010-2470-1. [DOI] [PubMed] [Google Scholar]
- 15.Balzarini J, Van HY, Vermeire K, Vanham G, Schols D. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol Pharmacol. 2007;71:3–11. doi: 10.1124/mol.106.030155. [DOI] [PubMed] [Google Scholar]
- 16.Barrientos LG, Lasala F, Otero JR, Sanchez A, Delgado R. In vitro evaluation of cyanovirin-N antiviral activity, by use of lentiviral vectors pseudotyped with filovirus envelope glycoproteins. J Infect Dis. 2004;189:1440–1443. doi: 10.1086/382658. [DOI] [PubMed] [Google Scholar]
- 17.Helle F, Wychowski C, Vu-Dac N, Gustafson KR, Voisset C, Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 18.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, Jacobs WR., Jr Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun. 2005;73:1196–1203. doi: 10.1128/IAI.73.2.1196-1203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behr MA, Small PM. A historical and molecular phylogeny of BCG strains. Vaccine. 1999;17:915–922. doi: 10.1016/s0264-410x(98)00277-1. [DOI] [PubMed] [Google Scholar]
- 20.Puttinaowarat S, Thompson K, Lilley J, Adams A. Characterization of Mycobacterium spp. isolated from fish by pyrolysis mass spectrometry (PyMS) analysis. Inland Aquat Anim Health Res Institute Newslett. 1999;8:4–8. [Google Scholar]
- 21.Bewley CA. Solution structure of a cyanovirin-N:Manα1-2Manα complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure. 2001;9:931–940. doi: 10.1016/s0969-2126(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 22.Bewley CA, Otero-Quintero S. The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man8 D1D3 and Man9 with nanomolar affinity: implications for binding to the HIV envelope protein gp120. J Am Chem Soc. 2001;123:3892–3902. doi: 10.1021/ja004040e. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MB, Nakane PP. Recent developments in the periodate method of conjugating horse radish peroxidase (HRPO) to antibodies. In: Knapp W, Holubar K, Wick G, editors. Immunofluoresence and related staining techniques. Elsevier/North Holland Biomedical; Amsterdam: 1978. pp. 215–224. [Google Scholar]
- 24.Wisdom GB. Horseradish peroxidase labeling of antibody using periodate oxidation. In: Walker JM, editor. The protein handbook. 2. Humana Press Inc; Totowa, NJ: 1996. pp. 273–274. [Google Scholar]
- 25.Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, Larrouy-Maumus G, Gurcha SS, Movahedzadeh F, Geurtsen J, Brown EJ, Eysink Smeets MM, Besra GS, Willemsen PT, Lowary TL, van Kooyk Y, Maaskant JJ, Stoker NG, van der Ley P, Puzo G, Vandenbroucke-Grauls CM, Wieland CW, van der Poll T, Geijtenbeek TB, van der Sar AM, Bitter W. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 26.Kolk AH, Ho ML, Klatser PR, Eggelte TA, Kuijper S, de Jonge S, van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984;58:511–521. [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koppel EA, I, Ludwig S, Hernandez MS, Lowary TL, Gadikota RR, Tuzikov AB, Vandenbroucke-Grauls CM, van Kooyk Y, Appelmelk BJ, Geijtenbeek TB. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209:117–127. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Linehan SA, Martinez-Pomares L, da Silva RP, Gordon S. Endogenous ligands of carbohydrate recognition domains of the mannose receptor in murine macrophages, endothelial cells and secretory cells; potential relevance to inflammation and immunity. Eur J Immunol. 2001;31:1857–1866. doi: 10.1002/1521-4141(200106)31:6<1857::aid-immu1857>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Krieger E, Vriend G, Figdor CG, van Kooyk Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J Biol Chem. 2002;277:11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanne A, Ma B, Boudou F, Tailleux L, Botella H, Badell E, Levillain F, Taylor ME, Drickamer K, Nigou J, Dobos KM, Puzo G, Vestweber D, Wild MK, Marcinko M, Sobieszczuk P, Stewart L, Lebus D, Gicquel B, Neyrolles O. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med. 2009;206:2205–2220. doi: 10.1084/jem.20090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boshoff HI, Reed MB, Barry CE, III, Mizrahi V. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell. 2003;113:183–193. doi: 10.1016/s0092-8674(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 37.Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev. 2011;35:1126–1157. doi: 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geurtsen J, Chedammi S, Mesters J, Cot M, Driessen NN, Sambou T, Kakutani R, Ummels R, Maaskant J, Takata H, Baba O, Terashima T, Bovin N, Vandenbroucke-Grauls CM, Nigou J, Puzo G, Lemassu A, Daffe M, Appelmelk BJ. Identification of mycobacterial α-glucan as a novel ligand for DC-SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. J Immunol. 2009;183:5221–5231. doi: 10.4049/jimmunol.0900768. [DOI] [PubMed] [Google Scholar]
- 41.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P, Schwartz O, Scheinmann P, Lagrange PH, de Blic J, Tazi A, Gicquel B, Neyrolles O. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.