Abstract
The sterile inflammatory response to cell death and irritant crystals is medically important because it causes disease. Although these stimuli are structurally distinct, they cause inflammation through a common pathway that requires the cytokine IL-1. In vitro, the inflammasome, and in particular its generation of active caspase-1, is absolutely required to produce bioactive IL-1β. However, here we report that caspase-1 is not required in vivo for much of the IL-1β-dependent sterile inflammatory response. Furthermore, we find that cathepsin C, which controls the activity of a number of leukocyte serine proteases capable of processing IL-1β, plays a major role in this caspase-1-independent pathway. Mice that are deficient in cathepsin C have reduced inflammatory responses to dying cells and silica crystals. In the absence of cathepsin C, caspase-1 becomes rate-limiting such that mice doubly-deficient in both of these proteases make little IL-1β in vivo and have markedly attenuated inflammatory responses to the sterile stimuli. In contrast, these mutant mice generate normal inflammation in response to exogenous IL-1β, indicating that cathepsin C and caspase-1 function upstream of IL-1β, and in their absence, all components of the pathway downstream of mature IL-1β are intact.
Introduction
Cell death and a number of irritant particles, such as silicates, calcium pyrophosphate and urate, stimulate robust inflammatory responses in vivo and, as a result, can cause or exacerbate tissue damage and disease (1). Recently, it has been recognized that these diverse stimuli all elicit inflammation through a common pathway. These sterile inflammatory responses all require the IL-1 receptor (IL-1R) and its signaling adaptor molecules (2). Moreover, where examined, IL-1 blocking reagents also inhibit these responses(3, 4). Therefore, it is now established that the cytokine IL-1 plays an essential role in these sterile inflammatory responses. There are two distinct forms of IL-1, IL-1α and IL-1β, and both of these cytokines work through the same receptor, IL-1R1 (5). It has generally been assumed that proinflammatory particles cause inflammation by stimulating the production of IL-1β and, indeed, in vitro they stimulate macrophages to produce this cytokine; however, IL-1α is also produced and in most cases a key role for IL-1β in responses to these particles has not been formally shown in vivo (6).
IL-1β is initially transcribed and translated into a pro-form that is biologically inactive (5). The generation of mature and active IL-1β requires proteolytic removal of a pro-peptide sequence from the cytokine precursor. This proteolytic processing of pro-IL-1β in cells is controlled by a macromolecular complex, called the inflammasome (7-11). This structure forms upon the oligomerization of 3 distinct components. One subunit is a nucleotide-binding domain, leucine rich containing (NLR) family protein that is thought to control the activity of the complex; for many sterile particulates, the NLR protein is NLRP3. A second component is the Apoptosis-associated speck-like protein containing a CARD (ASC) subunit, which is thought to serve as a scaffold that allows the complex to oligomerize. The third component is pro-caspase-1, which is the inactive precursor of the protease caspase-1. When the NLRP3 inflammasome is stimulated, pro-caspase is cleaved to its active form and this in turn cleaves pro-IL-1β to mature IL-1β. The mature cytokine is then released from cells via a non-classical secretion pathway and this process may also be controlled by the inflammasome.
The inflammasome has been shown to be absolutely essential for the production of IL-1β in vitro (12, 13). Macrophages that genetically lack NLRP3, ASC or caspase-1 fail to produce any mature IL-1β in response to a number of sterile stimuli, including crystals of silica, urate or other particles (14, 15). Based on these results, it has generally been assumed that the inflammasome similarly controls the production of IL-1β in vivo and, indeed, genetic “knock outs” of inflammasome components do reduce responses in animals in which IL-1 participates (12, 16). However, where examined, at least some of these responses are not completely eliminated in inflammasome-deficient mice (14, 16, 17), although it has not been clear why. The inflammasome-independent portion of these responses could be due to IL-1α (that doesn't require caspase-1 for activity), other cytokines, or other ways of producing IL-1β.
In addition to the inflammasome pathway there are other potential machanisms by which pro-IL-1β could be cleaved into mature IL-1β. In vitro a number of cellular proteases, including neutrophil serine proteases (elastase, cathepsin G and protease 3), matrix metalloproteinases and mast cell chymase, can cleave purified pro-IL-1β to bioactive IL-1β (18-21). Whether and to what extent these mechanisms contribute to IL-1 production in various settings is unclear. There is limited data that chemical inhibitors of elastase and chymase could reduce the processing of pro-IL-1β by neutrophils or mast cells, respectively, in vitro and reduce IL-1β levels and inflammation in vivo (22). Another neutrophil serine protease, protease 3, has been implicated in IL-1β-dependent chronic arthritis in a streptococcal cell wall model (23). A limitation of studies using protease inhibitors is the potential that they may have pleotropic effects that can influence responses. Overall, the role of these alternate IL-1 processing mechanisms in most situations in vivo is poorly understood.
We previously found that the sterile inflammatory response to cell death required the IL-1 receptor but was substantially caspase-1-independent (3). We initially attributed this caspase-1-independence to a dominant role of IL-1α in these responses. However, subsequent experiments with mutant mice found that IL-1β was also critical for the inflammation (6). This led us in this report to examine the contribution of caspase-1 and other proteolytic mechanisms in the generation of IL-1-dependent inflammation to cell death and other sterile particulate stimuli.
Materials and methods
Reagent and antibodies
Antibodies against Ly-6G (clone 1A8) were obtained from BD Bioscience. Anti-7/4 antibody was purchased from Serotec. Antibody against IL-1β (24) was obtained from Leinco Technologies (St. Louis, MO). 7-AAD was obtained from Molecular probe. Recombinant MIP-2 and human IL-8 ELISA kit were purchased from PeproTech (Rocky Hill, NJ). IL-1β and TNF-α ELISA kits were purchased from BD Biosciences and eBioscience, respectively (San Diego, CA). Ultrapure LPS from S. minnesota was purchased from Invivogen (San Diego, CA). Poly(dA-dT) and Nigericin were purchased from Sigma-Aldrich (St. Louis, MO). Silica crystal (MIN-U-SIL 15) was obtained from U.S. Silica (Frederick, MD).
Animal and cell lines
Wild type C57BL/6 mice were purchased from Jackson Laboratories or Japan SLC, Inc. Caspase-1-deficient mice (25), cathepsin C-deficient mice (26), IL-1 deficient mice (27) and IL-1 receptor deficient mice (28) were previously described. Caspase-1-deficient mice also lack caspase-11 (29). All animal protocols were approved by the UMass and Teikyo University animal care and use committee. EL4 cells were maintained in RPMI-1640 with 10% fetal calf serum (FCS) and antibiotics.
Preparation of necrotic cells
Necrotic EL4 cells were prepared as described (6). EL4 cells were washed 5 times with phosphate buffered saline (PBS), resuspended in PBS at 10 million cells / 50 microL and then heat-shock at 45°C for 10 min followed by 37°C incubation for 5 hours; this resulted in necrosis (7-AAD/PI positive cells).
Neutrophil and monocyte recruitment to peritoneal cavity
Quantification of recruited neutrophils and monocytes to the peritoneal cavity was described before (6). Mice were injected i.p. with indicated amount of silica crystal or necrotic EL4 cells in 150 microL of PBS. After 4 or 16 hours of injection, the peritoneum was lavaged with 6 mL of PBS with 2%FCS, 3mM of EDTA and 10U/mL of heparin. The absolute number of neutrophil (Ly-6G+ 7/4+) and monocyte (Ly-6G– 7/4+) in 100 microL of lavage was counted using a flow cytometer equipped with a high throughput sampler (BD bioscience).
Measurement of the mature IL-1β of the peritoneal cavity
Mature IL-1β was measured using a previously described MRC-5 fibroblast bioassay (30). The peritoneal cavity of the silica-stimulated mouse was lavaged using 1mL of PBS. The supernatant of the lavage was treated with anti-IL-1β or control antibody (24). These specimens were added to the MRC-5 fibroblast, and the IL-8 level of the supernatant was determined by ELISA. The IL-1β bioactivity was calculated by subtracting the result of the anti-IL-1β antibody treated samples from the control antibody treated ones.
Production and measurement of IL-1β and TNF-α from in vitro cultures
Peritoneal exudate cells were elicited by i.p. injection of 3mL of 1% thioglycollate and collected after 72 hrs by peritoneal lavage. Bone marrow neutrophils were isolated from whole bone marrow, following RBC lysis, using the Anti-Ly-6G Microbead Kit from Miltenyi Biotec (Cambridge, MA). Purity was assessed to be >95-98% by flow cytometry. Murine bone marrow-derived mast cells were derived from whole bone marrow using recombinant murine IL-3 (PeproTech, Rocky Hill, NJ) and purity was assessed to be >95% by toluidine blue (31). In all cases, cells were primed in 96-well plates in RPMI (or MC/9 medium for Mast Cells; see reference) for 3h with LPS (200 ng/mL) prior to stimulation for an additional 6 h with either silica, nigericin or poly(dA-dT). Supernatants were collected and cytokine levels analyzed by ELISA.
Statistical analyses
Data are reported as means ± standard errors. Statistical analyses in each independent experiment was performed with an unpaired, two-tailed Student's t-test. One - way ANOVA and Dunnett's multiple comparison post- test were used to compare the means of multiple groups to the control group. P < 0.05 was considered statistically significant.
Results
Caspase-1-independent component of IL-1-driven sterile inflammation
We have previously reported that the sterile inflammatory response to dying cells was IL-1β-dependent but caspase-1-independent, at least at the peak of responses (3). In subsequent investigation we have confirmed this result, although in some experiments we have observed a partial reduction (0-50%) of these responses in caspase-1-deficient mice. The reason for this variable contribution of caspase-1 is not entirely clear; presumably at some times it is a rate limiting component for maximal responses and at others it is not. This led us to examine the contribution of caspase-1 at earlier time points in the response.
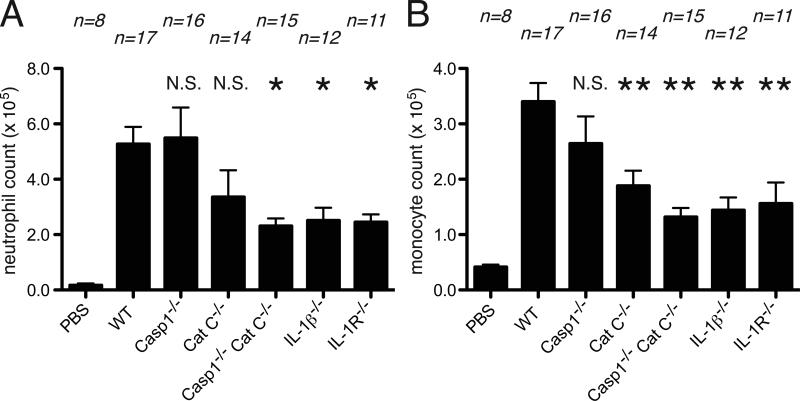
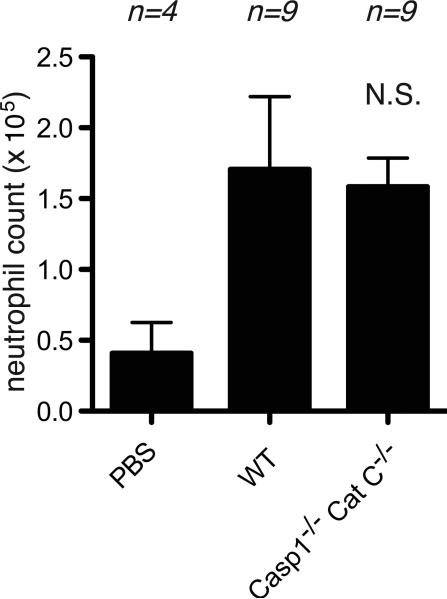
Cell death-induced inflammation can be detected within 4 hours of the injection of dying cells. To examine the role of caspase-1 in the initial phase of the inflammatory response, we injected sterile necrotic EL4 cells i.p. into mice and 4 hours later the cellular influx into the peritoneum was evaluated by immunofluorescent staining and flow cytometry (3, 6). We found that sterile dead cells elicited an equivalent influx of neutrophils and monocytes in caspase-1-deficient and wild type mice (Figure 1). In contrast, these sterile inflammatory responses were markedly attenuated in mice that lacked IL-1β and the magnitude of this reduction was similar to that observed in IL-1R-deficient animals. Therefore, in the total absence of caspase-1, IL-1β was contributing to this sterile inflammatory response.
Figure 1.
Inflammation to necrotic cells after 4 hour of injection to peritoneal cavity. The total neutrophil (A) and monocyte(B) numbers in peritoneal cavity of WT C57BL/6, caspase-1-deficient, cathepsin C-deficient, caspase-1 and cathepsin C double-deficient, IL-1β-deficient and IL-1R-deficient mice after 4 hours i.p. injection of 10 million of heat-shocked necrotic EL4 cells. The data are combined results of 4 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). * P<0.05, **P<0.01, NS, not significant versus WT groups using ANOVA with Dunnett's multiple camparison tests. PBS groups, WT mice received i.p. PBS.
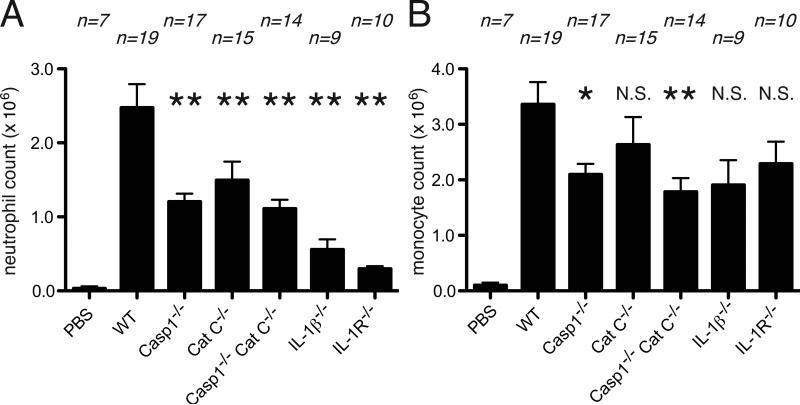
Given our findings of a caspase-1-independent pathway in cell death-induced inflammation, we sought to analyze another stimulus that also elicits an IL-1-dependent sterile inflammatory response. Sterile silica crystals, like many other particles, stimulate inflammatory responses in vivo that require the IL-1 receptor (32). The species of IL-1 that drives these responses in animals has not been elucidated. However, in vitro, these particles stimulate macrophages to produce IL-1β (32). Moreover, we have previously shown (32) and confirmed again here (described below) that the production of Il-1β in response to silica stimulation in vitro is absolutely dependent on the activation of caspase-1.
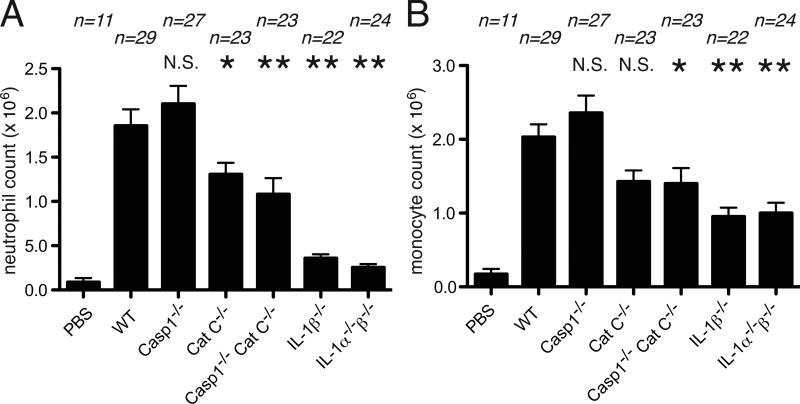
When silica is injected i.p. into mice it stimulates an inflammatory response. This response is markedly reduced in mice that lack the IL-1β gene (figure 2, 3). The loss of inflammatory responses in the IL-1β-deficient mice was similar in magnitude to what was observed with animals that lacked either the IL-1R or IL-1α+β (figure 2, 3). In contrast, at the peak of the response (16hrs), there was no reduction in inflammation in caspase-1-deficient mice (Figure 3). Similarly, at very early time points (4 hours), there was only a modest reduction (~25%) in inflammatory responses in mice that lacked caspase-1 (Figure 2). These results again demonstrated a contribution of IL-1β that could be partially or completely independent of caspase-1.
Figure 2.
Inflammation to silica crystal after 4 hour of injection to peritoneal cavity. The total neutrophil (A) and monocyte(B) numbers in peritoneal cavity of WT C57BL/6, caspase-1-deficient, cathepsin C-deficient, caspase-1 and cathepsin C double-deficient, IL-1β-deficient and IL-1R-deficient mice after 4 hours i.p. injection of 0.125 mg of silica crystal. The data are combined results of 3 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). *P<0.05, **P<0.01, NS, not significant versus WT groups using ANOVA with Dunnett's multiple camparison tests. PBS groups, WT mice received i.p. PBS.
Figure 3.
Inflammation to silica crystal after 16 hour of injection to peritoneal cavity. The total neutrophil (A) and monocyte(B) numbers in peritoneal cavity of WT C57BL/6, caspase-1-deficient, cathepsin C-deficient, caspase-1 and cathepsin C double-deficient, IL-1β-deficient and IL-1α and β-double-deficient mice after 16 hours i.p. injection of 0.5 mg of silica crystal. The data are combined results of 3 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). *P<0.05, **P<0.01, NS, not significant versus WT groups using ANOVA with Dunnett's multiple comparison tests. PBS groups, WT mice received i.p. PBS.
Role of cathepsin C in sterile inflammatory responses
In vitro, a number of serine proteases have been shown to process pro-IL-1β into mature IL-1 and very limited data suggest that these enzymes may function similarly in vivo (19-21, 33). We were interested in taking a genetic approach to evaluate the potential role of these proteases in the caspase-1-independent pathway of IL-1β production in vivo, but mutant animals lacking all of these proteases are not available. However, these proteases have a common mechanism that controls their activity and which was amenable to a genetic disruption.
The neutrophil serine proteases (cathepsin G, elastase and proteinase 3) contain a leader sequence that targets them into the exocytic pathway (34). After transport into the endoplasmic reticulum, the leader is removed by the signal peptidase, but two extra amino acids remain on the N-terminus of the proteases that keep them in an inactive state (35). These zymogens are transported into the endocytic compartments of cells. In this location the two N-terminal residues are removed by cathepsin C (dipeptidyl peptidase I), resulting in the proteases becoming catalytically active (36). Because of this mechanism, genetic inactivation of cathepsin C will result in the loss of activity of all of these serine proteases (26). Cathepsin C-deficient mice are viable, fail to activate their neutrophil serine proteases and were shown to have reduced inflammatory responses in some settings (in arthritis models, to microbial antigens and immune complexes) but not others (to thioglycollate injection) (37). We therefore examined the caspase-1-independent pathway of IL-1β-dependent inflammation in a cathepsin C-deficient mouse model.
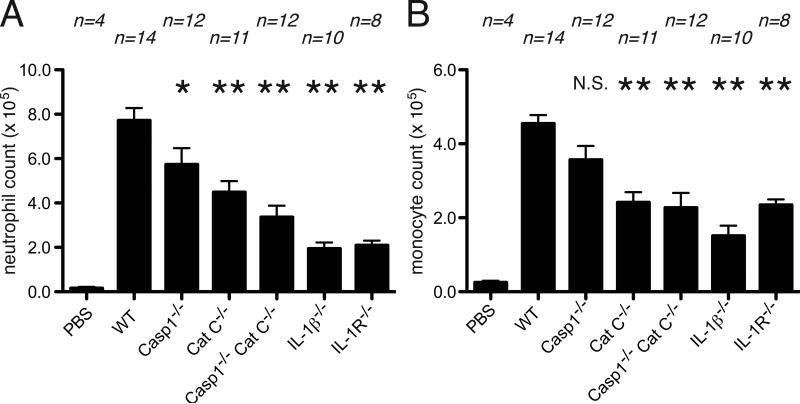
The inflammatory response to silica was reduced in cathepsin C-deficient mice at both initial and peak time points (Figure 2, 3). Similarly, inflammation to dead cells was also decreased in these mutant mice at early and later time points (Figure 1,4). These reductions in inflammation were partial and of lesser magnitude than was observed in mice lacking IL-1β (Figure 1,2,3,4).
Figure 4.
Inflammation to necrotic cells after 16 hour of injection to peritoneal cavity. The total neutrophil (A) and monocyte(B) numbers in peritoneal cavity of WT C57BL/6, caspase-1-deficient, cathepsin C-deficient, caspase-1 and cathepsin C double-deficient, IL-1β-deficient and IL-1R-deficient mice after 16 hours i.p. injection of 30 million of heat-shocked necrotic EL4 cells. The data are combined results of 4 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). *P<0.05, **P<0.01, NS, not significant versus WT groups using ANOVA with Dunnett's multiple comparison tests. PBS groups, WT mice received i.p. PBS.
Loss of cathepsin C reveals a contribution of caspase-1 to the sterile inflammatory response
Our finding that the loss of cathepsin C only partially inhibited the IL-1β-dependent inflammatory responses indicated that there must be other proteases contributing to the processing of pro-IL-1β in vivo. Since these animals still had caspase-1, we evaluated whether this protease might become rate-limiting in the absence of cathepsin C and be responsible for the cathepsin C-independent mechanism of IL-1 generation.
Indeed, the combined loss of cathepsin C and caspase-1 reduced the initial cell death-induced inflammatory response down to the level of inflammation observed in the IL-1β-deficient mice (Figure 1). This increased impairment of inflammation in the double-deficient mice was not evident at the peak of responses (Figure 4, N.S. by one-way ANOVA with a Bonferroni test, Casp1-/- vs Casp1-/-CatC-/-). The inflammatory response to silica was also further decreased in the double-deficient mice compared to single “knock outs” and this greater reduction was observed both at 4 and 16 hours (Figure 2, 3). The responses to silica in the double-deficient mice, while substantially attenuated, was not reduced down to the level seen in IL-1β-deficient mice, especially at the peak of the response (P<0.01 by one-way ANOVA with a Bonferroni test, Casp1-/-CatC-/- vs IL-1β-/-, Figure 3). These results demonstrate enzymes other than caspase-1, and those activated by cathepsin C, can also participate in the maturation of IL-1β in certain settings.
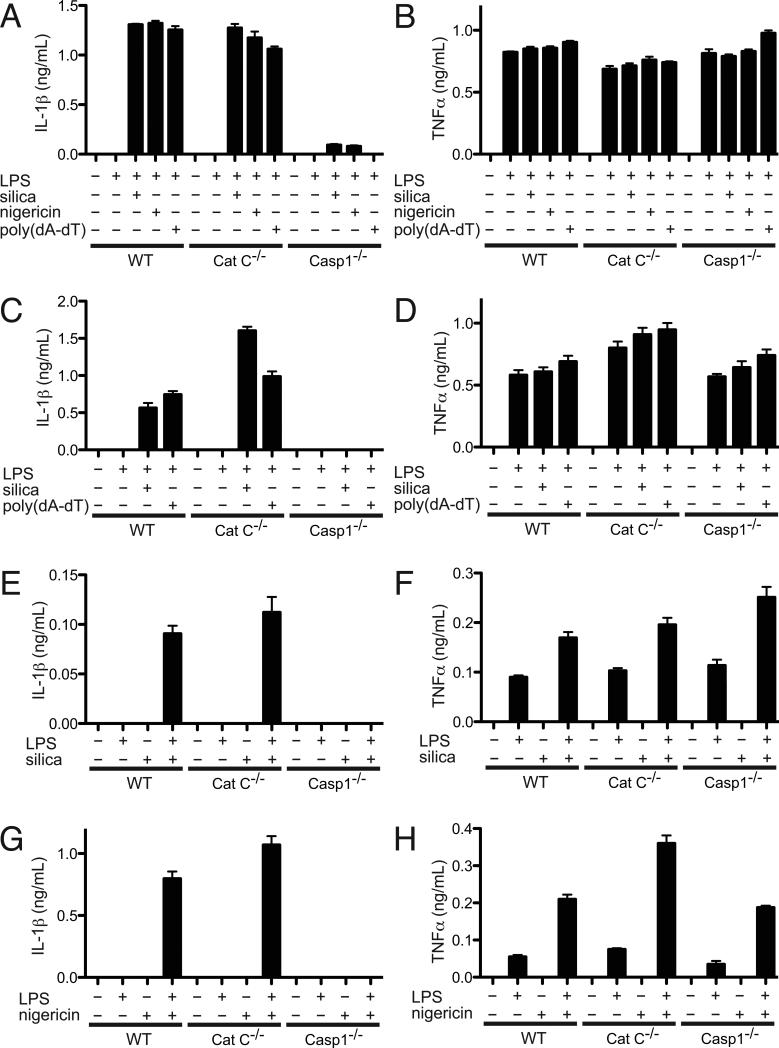
The production of IL-1β by macrophages, neutrophils and mast cells in vitro is dependent on caspase-1 but not cathepsin C
Given the contribution of cathepsin C to IL-1β production in vivo, we sought to determine whether it was involved in the generation of this cytokine by various leukocytes in in vitro assays. Silica stimulates LPS-primed cathepsin C-deficient macrophages, mast cells and neutrophils to produce IL-1β and the magnitude of these responses is similar to that of their wild type counterparts (Fig. 5). Similar results were obtained with other inflammasome stimulators, poly(dA-dT) or nigericin. In contrast, we had previously reported that the production of IL-1β by macrophages stimulated with silica or nigericin was absolutely dependent on caspase-1 and we again confirmed this finding (32). Similarly, we found that caspase-1-deficient mast cells and neutrophils also fail to produce IL-1β to inflammasome stimulators. Neutrophils secreted relatively low amounts of IL-1β in response to silica compared with macrophages and mast cells (Fig. 5E), but produced an equivalent amount of IL-1β in response to nigericin (Fig. 5G).
Figure 5.
Caspase-1 dependent, but not cathepsin C dependent secretion of IL-1β from macrophages (A, B), mast cells (C, D) and neutrophils (E-H) in vitro.
(A, B) IL-1β (A) and TNF-α (B) levels in the supernatants of murine peritoneal exudate macrophages from wild type, cathepsin C deficient, or caspase-1-deficient C57BL/6 mice. Cells were primed for 3h with LPS (200 ng/mL), and treated with silica (35 ug/mL), nigericin (1.5 uM) or poly(dA-dT) (0.5 ug/mL) for an additional 6 h.
(C, D) IL-1β (C) and TNF-α (D) levels in the supernatants of murine bone marrow-derived mast cells from wild type, cathepsin C deficient, or caspase-1-deficient C57BL/6 mice. Cells were primed for 3h with LPS (200 ng/mL), and treated with silica (40 ug/mL) or poly(dA-dT) (0.3 ug/mL) for an additional 6 h. (E-H) IL-1β (E, G) and TNF-α (F, H) levels in the supernatants of murine bone marrow neutrophils from wild type, cathepsin C deficient, or caspase-1 deficient C57BL/6 mice. Cells were primed for 3h with LPS (200 ng/mL), and treated with silica (80-100 ug/mL, E, F) or nigericin (1 uM, G, H) for an additional 6 h. In all experiments, cytokine levels in the supernatants were analyzed by ELISA and represented as means ± SEM. Data are representative of more than 3 experiments.
The requirement for cathepsin C is upstream of IL-1β
While our data showed a role for cathepsin C and caspase-1 in the IL-1β-dependent sterile inflammatory response in vivo, the results don't define how these proteases are participating in this response. To investigate whether cathepsin C and caspase-1 were required for aspects of inflammation aside from the production of IL-1β, we injected recombinant IL-1β into wild type versus the double-deficient mice and compared the resulting inflammatory responses (Figure 6). Injection of IL-1β is sufficient to stimulate a neutrophilic inflammatory response in wild type mice (monocyte recruitment was not seen in these experiments, at least at the time point analyzed). Importantly, this cytokine stimulated an equivalent inflammatory response when injected into cathepsin C + caspase-1 mutant mice. These results indicated that the double-deficient mice are fully capable of mounting IL-1β-dependent inflammatory responses. Moreover, they strongly suggest that the locus of action of cathepsin C and caspase-1 in vivo is upstream of IL-1β.
Figure 6.
Caspase-1 and cathepsin C are not required to induce inflammation to IL-1β The total neutrophil numbers in peritoneal cavity of WT C57BL/6, caspase-1 and cathepsin C double-deficient mice after 6 hours i.p. injection of 2 microgram of recombinant IL-1β. The data are combined results of 2 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). NS, not significant versus WT groups using t-test. PBS groups, WT mice received i.p. PBS.
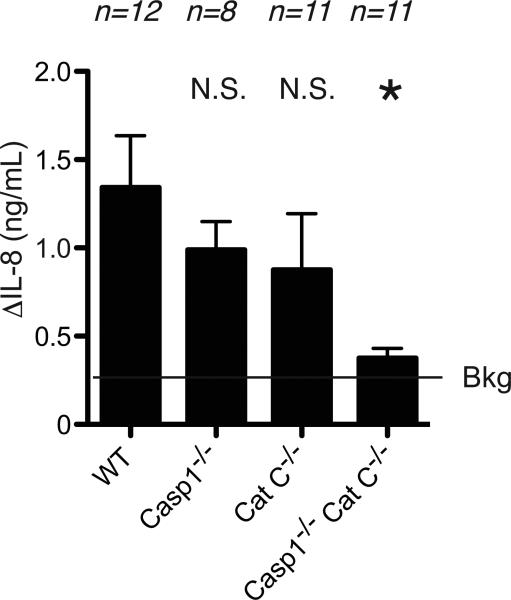
To further investigate this issue, we investigated whether mature IL-1β levels were altered in the sterile inflammatory responses. We focused on responses to silica at 4 hours because, technically, we were able to consistently measure mature IL-1β in the peritoneal lavage fluid from mice injected i.p. with these crystals using a bioassay. To eliminate the participation of IL-1α or other activities in the bioassay, the level of IL-1β was determined by subtracting the anti IL-1β antibody treated sample from the control IgG treated sample. IL-1β levels were significantly reduced in the peritoneal fluid of cathepsin C and caspase-1 double-deficient mice injected with silica i.p (Figure 7). Levels of this cytokine were also decreased in caspase-1 or cathepsin C-deficient mice compared to wild type mice, although these differences were not statistically significant.
Figure 7.
The production of bioactive IL-1β in vivo. The level of mature IL-1β in peritoneal cavity of WT C57BL/6, caspase-1-deficient, cathepsin C-deficient, caspase-1 and cathepsin C double-deficient mice after 4 hours i.p. injection of 0.5 mg of silica crystal. Functional IL-1β activities were measured using MRC-5 fibroblast bioassay, which the MRC-5 fibroblasts produce IL-8 in response to IL-1. The data are combined results of 3 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). *P<0.05, NS, not significant versus WT groups using ANOVA with Dunnett's multiple comparison tests. Bkg, background level in IL-1β deficient mice.
Discussion
In this report we demonstrate that there is a caspase-1-independent pathway that in vivo contributes to IL-1β-dependent sterile inflammatory responses to cell death and silica crystals. The extent to which this pathway contributed varied somewhat over the course of responses, but it was always quite substantial. This finding is surprising because there is an appreciable literature that the inflammasome complex, which controls caspase-1 activity, and caspase-1 itself, is required for the generation of IL-1β, including in response to stimuli like silica (25, 32). However, the studies showing that the inflammasome is required for IL-1β production are primarily ones performed in vitro (7-11, 32, 38). Our findings show that the situation in vivo is clearly more complex.
We show that the inflammasome-independent pathway of the inflammatory responses in vivo is dependent on cathepsin C. Our data strongly suggest that cathepsin C is acting in ways that control the generation of bioactive IL-1β. Specifically, bioactive IL-1β levels are reduced in sterile inflammation in cathepsin C-deficient mice and inflammatory responses to exogenous IL-1β are intact in these animals (Fig. 5). Presumably, cathepsin C is contributing to the caspase-1-independent pathway by controlling the activation of neutrophil serine proteases that can cleave pro-IL-1β into its mature and bioactive form (37). This is consistent with earlier work that showed that these proteases can process pro-IL-1β and that inhibiting of these enzymes reduced inflammatory responses in arthritis models (an acute one induced by K/BxN serum and a chronic one induced by streptococcal cell wall) and to MSU (22) (23). It should be noted that, while these enzymes are often collectively referred to as “neutrophil serine proteases,” this is a bit of a misnomer because they are expressed in a number of cell types including macrophages.
The difference in caspase-1's contribution to responses in vitro versus in vivo was surprising. It is possible that this dichotomy is due to some cell type whose production of IL-1β in vivo is inflammasome-independent. However, we examined macrophages, neutrophils and mast cells and all 3 cell types require caspase-1 to make IL-1β in vitro. Moreover, we have previously shown that dendritic cells are dispensable for cell death-induced, IL-1-dependent responses in vivo (6). Perhaps there is another cell involved or that macrophages or other leukocytes manifest a caspase-1-independent pathway in vivo but not in vitro. There is the opposite dichotomy with cathepsin C, wherein this protease is required for the inflammasome-independent pathway of IL-1 production in vivo, but not for the processing of IL-1β in vitro.
These dichotomies may actually make sense when the likely mechanisms of action are considered. Cathepsin C-dependent IL-1β processing is presumably mediated through cathepsin C-dependent proteases, like elastase, cathepsin G and protease 3, and in living cells this hydrolytic machinery is localized in vesicles and granules. In contrast, pro-IL-1β. Consequently, pro- IL-1β would be accessible to processing by activated caspase-1, but not the vavuolar proteases. Therefore, processing of IL-1β by cells in vitro would be caspase-1-dependent and cathepsin C-independent. On the other hand, pro-IL-1β can be released from dying cells undergoing primary or secondary necrosis and many leukocytes containing this cytokine precursor die at sites of inflammation in vivo. The released pro-IL-1β could then be cleaved into mature IL-1β by the cathepsin C-dependent proteases when these enzymes are released into the extracellular fluids or possibly in vesicular compartments, if cells internalize the cytokine precursor by endocytic mechanisms.
In some of our experiments we observed that while inflammation was markedly reduced in cathepsin C and caspase-1 double-deficient mice, it was still higher than in animals lacking IL-1β (Figs. 2,3,4). This finding suggests that there are yet other mechanisms in vivo that can generate mature IL-1, albeit more minor ones. Presumably, the IL-1 processing is mediated by other proteases whose activity is not dependent on cathepsin C. One such protease could be matrix metalloproteinase 9 (20). This protease can process pro-IL-1β into its mature form in vitro and metalloproteinase 9-deficient mice has been reported to reduce IL-1β-dependent neuropathic pain (39).
We believe that understanding how IL-1β is produced during sterile inflammation is important because this process underlies a number of diseases. The inflammation that occurs in response to cell death can cause tissue damage and disease. Sterile inflammation to crystals underlies a number of diseases including silicosis, gout, pseudogout, asbestosis and possibly also even atherosclerosis (14, 17, 32). IL-1β has also been implicated in metabolic syndrome and type II diabetes among other diseases (40). Our findings have implications for potential therapeutic targets to block IL-1 production and may help to explain why caspase-1 inhibitors were not effective in clinical trials (41).
Acknowledgements
The authors thank Sharlene Hubbard, Janice BelleIsle and Tamiko Yanagida for technical assistance.
HK contributed to the design, execution and analysis of all experiments and writing of the manuscript. GMO and ZP contributed to design, perform and analysis of experiments. KLR contributed to the design and analysis of all experiments and writing of the manuscript.
This work was supported by grants from the NIH to KLR and core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used. This work was also supported by a grant for Research on intractable diseases from the Ministry of Health, Labour and Welfare and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. HK is supported by Mochida Memorial Foundation for Medical and Pharmaceutical Research, Public Trust Cardiovascular Research Fund, Senshin Medical Research Foundation, Kowa Life Science Foundation, The Naito Foundation, Takeda Science Foundation and NOVARTIS Foundation (Japan) for the Promotion of Science. GMO is supported by Medical Scientist Training Program Training Grant T32 AI095213-01 from NIH.
Abbreviations
- IL-R
IL-1 receptor
- NLR
nucleotide-binding domain, leucine rich containing
- ASC
Apoptosis-associated speck-like protein containing a CARD
References
- 1.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shito M, Wakabayashi G, Ueda M, Shimazu M, Shirasugi N, Endo M, Mukai M, Kitajima M. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143–148. doi: 10.1097/00007890-199701150-00026. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 4.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 6.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 11.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 13.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 15.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990;265:6318–6322. [PubMed] [Google Scholar]
- 19.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 21.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers HW, Sheehan KC, Brunt LM, Dower SK, Unanue ER, Schreiber RD. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci U S A. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 26.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci U S A. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 29.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA, Muegge K, Durum SK. Measurement of Soluble and Membrane-Bound Interleukin 1 Using a Fibroblast Bioassay. Current Protocols in Immunology. 2000;37:6.2.1–6.2.7. doi: 10.1002/0471142735.im0602s37. [DOI] [PubMed] [Google Scholar]
- 31.Jensen BM, Swindle EJ, Iwaki S, Gilfillan AM. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr Protoc Immunol Chapter. 2006;3 doi: 10.1002/0471142735.im0323s74. Unit 3 23. [DOI] [PubMed] [Google Scholar]
- 32.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irmler M, Hertig S, MacDonald HR, Sadoul R, Becherer JD, Proudfoot A, Solari R, Tschopp J. Granzyme A is an interleukin 1 beta-converting enzyme. J Exp Med. 1995;181:1917–1922. doi: 10.1084/jem.181.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenne DE, Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 35.Caputo A, Garner RS, Winkler U, Hudig D, Bleackley RC. Activation of recombinant murine cytotoxic cell proteinase-1 requires deletion of an amino-terminal dipeptide. J Biol Chem. 1993;268:17672–17675. [PubMed] [Google Scholar]
- 36.McGuire MJ, Lipsky PE, Thiele DL. Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J Biol Chem. 1993;268:2458–2467. [PubMed] [Google Scholar]
- 37.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 41.Cornelis S, Kersse K, Festjens N, Lamkanfi M, Vandenabeele P. Inflammatory caspases: targets for novel therapies. Curr Pharm Des. 2007;13:367–385. doi: 10.2174/138161207780163006. [DOI] [PubMed] [Google Scholar]