Abstract
Background
The role of natural killer (NK) cells in organ transplantation is poorly understood as studies link these cells to both regulatory and inflammatory functions. NK cells exacerbate inflammation and adaptive immunity under conditions of allograft rejection, but little is known regarding their roles in allograft tolerance. We test the hypothesis that NK cells have regulatory function and promote tolerance induction to murine cardiac allografts.
Methods
Murine hearts were transplanted as fully vascularized heterotopic grafts from BALB/c donors into C57BL/6 recipients. Allograft tolerance was achieved using donor splenocyte transfusion (DST) + anti-CD40L mAb prior to transplantation. The requirement for NK cells in tolerance induction was tested by administering anti-NK1.1 depleting mAb or anti-NKG2D blocking mAb. Intragraft and peripheral immune cell populations were determined by flow cytometry and immunohistochemistry. CD4 T cell alloantigen-specific responses and donor specific alloantibody were also determined.
Results
NK cell depleted recipients acutely reject allografts despite anti-CD40L blockade, but rejecting recipients lacked alloantibody and alloantigen-specific CD4+ T cell responses. NK cell depletion resulted in elevated numbers of graft-infiltrating macrophages. NKG2D blockade in tolerized recipients did not cause acute rejection, but increased macrophage graft infiltration and increased the expression of NKG2D ligand Rae-1γ on these cells.
Conclusions
Our data show that NK cells are required for tolerance induction in recipients given DST + anti-CD40L mAb. Our data suggest NK cells regulate monocyte and/or macrophage activation and infiltration into allografts by a mechanism partially dependent on NKG2D receptor-ligand interactions between NK cells and monocytes/macrophages.
Keywords: NK cells, tolerance, graft-infiltrating cells
Introduction
Allograft tolerance remains the paramount goal for achieving long-term graft survival in organ transplant recipients. Transcriptional profiling of liver and renal transplant patients demonstrated a correlation between CD4+ regulatory T cells (Treg) activity and allograft tolerance, but also suggested that NK cell associated genes are up regulated in tolerant patients (1, 2). Treg are well characterized mediators in suppressing alloantigen specific immunity and have been shown to favor tolerance (3, 4). However, relatively few studies have addressed the relevance of NK cells in tolerance induction or maintenance. NK cells are a subset of lymphocytes with cytokine secreting and cytotoxic effector functions that respond to viral infection, cell stress, and tumors (5). NK cell specific activation and inhibitory receptors monitor the expression of self MHC I, while also sensing for the presence of stress ligands and pathogen-related molecules (6). In addition to mediating innate immunity, NK cell effector functions actively shape adaptive immunity. IFNγ secreted by NK cells enhances dendritic cell maturation (7, 8) while IL-10 and NK cell activating receptors have been implicated in regulating T cell responses (9–12).
Defining the role of NK cells in transplantation is less clear due to evidence suggesting their involvement both in rejection as well as in tolerance. During acute rejection, graft-infiltrating NK cells secrete CCL3/MIP-1α and CCL4/MIP-1β which amplify local inflammation (13). NK cell secreted IFNγ exacerbates allograft chronic rejection (14) and enhances T cell mediated immunity against alloantigen (15, 16). In contrast to these observations during rejection, transplantation models using co-stimulatory blockade such as anti-CD40L or anti-LFA1 mAb, have also demonstrated a requirement for NK cells in tolerance induction (17, 18). Skin transplant studies in RAG deficient or CD8 T cell deficient recipients showed that NK cells kill allogeneic donor dendritic cells and prevent the activation of alloreactive T cells (19, 20). However, precise mechanisms for how NK cells contribute to tolerance in wild-type transplant recipients remain poorly defined.
To further investigate the influence of NK cells in fully allogeneic organ transplantation, we administered anti-NK1.1 depleting mAb or anti-NKG2D blocking mAb in recipients receiving co-stimulatory blockade. Fully allogeneic vascularized cardiac grafts were transplanted to recipients conditioned with donor splenocyte transfusion (DST) and anti-CD40L mAb to induce allograft tolerance. Anti-NK1.1 mAb administration prior to transplantation depleted NK cells and caused acute rejection despite co-stimulatory blockade. Rejected grafts contained elevated levels of infiltrating macrophages, but recipients did not have alloantigen specific CD4+ T cell reactivity or detectable alloantibody.
Results
Anti-CD40L mAb induced tolerance requires NK cells
Anti-NK1.1 mAb effectively eliminated NK1.1-expressing cells following intravenous injection. 95% of splenic NK cells were depleted within 24 hours (Fig 1a). NK1.1-expressing cells normally found in the splenic red pulp as well as in the lymph node cortical ridge and medulla were absent (Fig. 1b). Equivalent depletion was also observed in the blood, liver, and lymph nodes (not shown). anti-NK1.1 treatment was specific to NK and NKT cell populations and did not deplete other lymphoid populations of CD4 T cells, CD8 T cells, or B cells. Populations of myeloid-derived cells including monocytes, macrophages, granulocytes, and dendritic cells were also unaffected (data not shown). Depletion persisted for at least 15 days. Staining with the additional discrete NK-specific markers NKp46 and DX5 both demonstrated equivalent amounts of NK cell depletion, showing that anti-NK1.1 mAb caused cellular depletion and did not cause shedding, down-modulation or masking of NK1.1. To evaluate the role of NK cells in co-stimulatory blockade induced tolerance, C57BL/6 (H-2b) recipients received DST plus anti-CD40L mAb and were transplanted with fully allogeneic BALB/c (H-2d) donor hearts. Co-stimulatory blockade with DST plus anti-CD40L mAb resulted in long-term allograft survival (Fig 1c). The administration of anti-NK1.1 mAb prior to transplantation, however, resulted in early acute rejection between 10 and 15 days (n= 10, MST = 13 days). Examination of H&E stained grafts revealed perivascular mononuclear cell infiltration of the allograft by day 10, with a dense mononuclear infiltrate of the myocardium by day 13 (Fig 1d). Syngeneic graft recipients receiving anti-NK1.1 accepted their grafts, showing that NK cell depletion did not cause a nonspecific inflammatory effect. Rejection of previously tolerated allografts in recipients could not be achieved by administration of anti-NK1.1 mAb on day 30 (not shown) suggesting that NK1.1+ cells are important in the induction of transplantation tolerance, but not maintenance. Because NK1.1 is expressed on both NK and NKT cells, transplants were performed in CD1d deficient recipients that lack mature NKT cells (21). Tolerance to allograft was reliably induced in CD1d −/− recipients, suggesting that NK1.1-expressing NK cells, but not NKT cells, regulated host responses to allograft. Our results contrast with work by others that demonstrate a requirement for NKT cells for prolonged cardiac graft survival (22, 23). Combination treatment using anti-CD40L with DST may favor NK cell activation and compensate for the requirement of NKT cells.
Figure 1.
Anti-NK1.1 mAb depletes NK cells and abrogates tolerance. (A) 100μg IgG2a isotype control (C1.18.4) mAb or anti-NK1.1 (PK136) was administered intravenously. CD3-NKp46+ NK cells were analyzed 24 hours post-injection. Data representative of four mice. (B) NKp46+ cells (red) and B220+ B cells (blue) in the splenic red pulp or lymph node cortical ridge 24 hours following anti-NK1.1 mAb injection compared to isotype control. Representative images from immunohistochemical analysis. Scale bar 50μm. (C) Balb/c to C57Bl/6 cardiac allografts in recipients receiving DST + anti-CD40L mAb tolerogen. Syngeneic + anti-NK1.1 treatment (inverted triangle) (n=4), allogeneic CD1d KO (triangle) (n=3), and allogeneic wild-type recipients receiving mIgG2a (circle) (n=12) accepted grafts. Allogeneic recipients (open square) (n=12) receiving anti-NK1.1 mAb rejected at MST=13, p<0.001. (D) H&E analysis of allograft myocardium in mIgG2a isotype treated and anti-NK1.1 mAb treated recipients. Perivascular and myocardial mononuclear cell infiltration observed in mIgG2a and anti-NK1.1 mAb treated mice at days 5, 8, 10 and 13 post- transplant. Histology representative of 3 independent experiments.
NK cell depletion does not influence T cell or alloantibody responses to alloantigen
Because NK cells have the potential to regulate T cell responses (24–26), we investigated the fate of alloantigen-specific T cell responses following NK cell depletion. CD4+ T cells were isolated from T cell receptor (TCR) transgenic TEa T mice. The TEa TCR transgene recognizes donor I-Ed peptide presented by recipient I-Ab. TEa T cells were labeled with carboxyfluoroscein succinimidyl ester (CFSE) and adoptively transferred to transplanted recipients receiving tolerogen plus anti-NK1.1. TEa cells from tolerogen treated recipients failed to proliferate after five days, in comparison to untreated rejecting controls in which TEa cells proliferated well (Fig. 2a). Surprisingly, TEa cells from tolerogen plus anti-NK1.1 mAb treated recipients also failed to proliferate after five days and retained the CD44-CD62+ naïve phenotype. TEa cells from recipients receiving tolerogen alone or tolerogen plus anti-NK1.1 mAb also failed to proliferate after ten days (not shown). No differences in TEa T cell location or position could be detected in the spleen or lymph nodes of anti-NK1.1 mAb versus isotype control treated recipients. Total numbers of graft-infiltrating CD4+, CD8+, and Foxp3+ T cells in the allografts were also not significantly altered in tolerized recipients receiving isotype control versus anti-NK1.1 mAb (Fig. 2b). Mixed lymphocyte reactions using BALB/c stimulator cells and responding CD4 or CD8 T cells isolated from day 5 tolerized recipients receiving mIgG2a or anti-NK1.1 showed no differences in T cell proliferation (Fig. 3b). Additionally, CD44, CD62L, and CD69 expression on T cells were not significantly different between NK cell sufficient or NK cell depleted recipients following transplantation (Fig. 3a and 3c).
Figure 2.
NK cell depletion does not alter CD4+ T cell or alloantibody responses. (A) CFSE labeled TEa T cells (2x107) adoptively transferred to fully allogeneic cardiac allografts on day of transplant were analyzed five days later by flow cytometry gated on CD4+CFSE+ cells. TEa cells from tolerized recipients receiving anti-NK1.1 or isotype control did not proliferate (n=2) compared to untreated rejecting control recipients (n=2). Representative of 2 independent experiments. (B) Quantitative immunofluorescence analysis of allografts stained for CD4, CD8, and Foxp3 in recipients receiving tolerogen plus isotype control (white bars) or anti-NK1.1 mAb (black bars). Cells were counted per 200X field of myocardium. Results are mean ± SEM (n = 3 grafts/group, 3 sections/graft, 5 fields/section).
Figure 3.
T cell and B cell activation is not affected by NK cell depletion post-transplant. (A) Graft-infiltrating T cells in NK cell sufficient and depleted recipients analyzed by flow cytometry on post-transplant day 10. CD4 and CD8 T cells stained for CD44 and CD62L. (B) Mixed lymphocyte reactions using Balb/c stimulator splenic CD11c+ dendritic cells and CFSE-labeled flow sorted CD4+ or CD8+ T cells from naïve, day 5 tolerized mIgG2a treated, or day 5 tolerized anti-NK1.1 treated C57Bl/6 recipients. (C) Peripheral CD4+ and CD8+ T cell expression of CD69 determined on post-transplant day 5. Results are mean ± SEM (n = 4) (D) Alloantibody detection in tolerogen treated transplant recipients. Serum from untreated rejecting recipients (rejecting), tolerogen treated recipients receiving isotype control (IgG2a) or anti-NK1.1 (anti-NK1.1) mAb was collected 9 days following transplantation and assayed for binding to donor alloantigen specific targets relative to background signal of unstained cells (bg) and alloantibody detection from non-transplanted naïve mice (naïve). Results are mean ± SEM (n = 2) (E) Graft-infiltrating and peripheral CD19+ B cell frequency was determined by flow cytometry on NK cell sufficient and depleted recipients on post-transplant day 5 Results are mean ± SEM (n = 4).
To determine if acute rejection resulting from NK cell depletion was due to B cell responses, serum levels of alloantibody were measured ten days following transplantation. P815 (H-2d) mastocytoma target cells ubiquitously express high levels of MHC I and were used to detect anti-H-2d immunoglobulin in recipient serum. Alloantibodies were detected in untreated rejecting control recipients, but not in tolerogen treated recipients given either isotype control mAb or anti-NK1.1 mAb (Fig. 3d). The absence of anti-donor MHC-II specific antibodies, however, could not be excluded from our assay since the P815 target cells used in our assay lack MHC-II. The number of graft infiltrating B cells and peripheral B cells were not significantly different between the two groups (Fig. 3e). These findings showed that NK cell depletion did not directly antagonize the tolerizing effect of anti-CD40L mAb treatment on responding B or T cells.
CD11bloCD27hi NK cells are enriched in tolerized versus untreated rejecting allografts
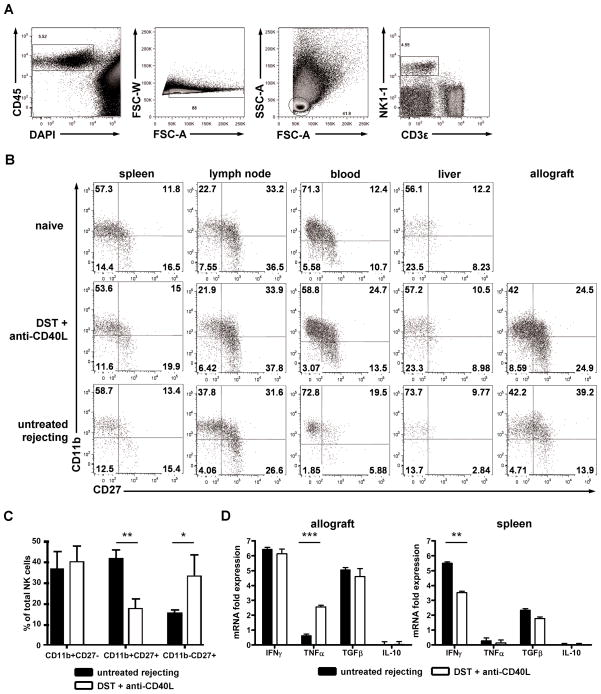
The absence of TEa T cell activation or enhanced alloantibody production in response to alloantigen led us to hypothesize that NK cells served a regulatory function that did not directly alter alloantigen specific lymphocyte responses. Thus, NK cells may favor tolerance by regulating the local inflammatory microenvironment or infiltrating cells in the allograft. Peripheral NK cell subsets and maturation states have been broadly characterized in mice using the markers CD11b and CD27 (27). CD11b and CD27 expression have also been linked to the cytokine-secreting potential of NK cells and their ability to become activated (28). It is not known which of these NK cell subsets migrate to allograft tissues under conditions of rejection or tolerance. Using multi-parameter flow cytometry, we found NK cells in the allograft during rejection as well as tolerization, with more NK cells in grafts from tolerized recipients (Fig. 4a). The distribution of CD11b and CD27 was similar among NK cells in naïve mice compared to transplanted recipients receiving DST plus anti-CD40L mAb (Fig. 4b). However, under conditions of untreated rejection, there was a lower frequency of CD11bloCD27hi NK cells and a higher frequency of CD11bhiCD27hi NK cells in the blood and allografts of rejecting compared to tolerant or naïve recipients (Fig. 4b and 4c). The frequency of CD11bhiCD27lo NK cells remained unchanged. To investigate if there were changes in cytokine production between NK cells from tolerized and untreated rejecting recipients, total CD3-NK1.1+ cells were sorted from the spleens and allografts of transplanted recipients five days following transplantation, and their cytokine expression measured by RT-PCR. However, no significant changes in IFNγ, TNFα, TGFβ, or IL-10 cytokine transcript profiles were observed (Fig 4d). Global cytokine levels of IL-1β, IL-4, IL-6, IL-10, IL-17, IFNγ, and TNFα determined by Luminex assay on sera showed no significant differences between tolerized recipients receiving anti-NK1.1 or mIgG2a isotype control at post-transplant day 10 (not shown). CD27lo and CD27hi NK cell subsets were further sorted from transplanted recipients five days following transplantation, but no significant differences in their cytokine profiles were observed in the spleen or allograft under untreated rejecting versus tolerized conditions (not shown). NK cells were FACS sorted on CD3-NK1.1+ cells based on the expression of CD11b and CD27. CD11bhiCD27lo and CD11bloCD27hi NK cells were tested for their ability to rescue graft survival following total NK cell depletion. Sorted NK cells were adoptively transferred to day 10 transplant recipients that were previously depleted with one dose of anti-NK1.1 at day −1. Both subsets were able to prolong graft rejection >60 days (n=4), suggesting that these markers do not uniquely identify regulatory NK cells.
Figure 4.
NK cell subsets, but not cytokine profiles, vary under tolerized versus rejecting conditions. (A) Flow cytometry gating scheme on collagenase digested allograft tissue. CD45+ leukocytes were gated on DAPI- and FSC-W to exclude dead cells and doublets; FSC-A and SSC-A to identify lymphocytes; and CD3ε-NK1.1+ used to identify NK cells. (B) Flow cytometric CD11b and CD27 expression profile of CD3ε-NK1.1+ NK cells in non-transplanted naïve mice compared to tolerized and untreated rejecting recipients five days following transplant. (C) NK cell subset frequency in the allograft from untreated rejecting (black bars) or tolerized (white bars) recipients based on CD11b and CD27 expression five days following transplant. CD11bhiCD27hi cells were more prevalent under untreated rejecting conditions (p<0.001) while CD11bloCD27hi cells were more prevalent under tolerized conditions (p<0.05). Results are mean ± SEM (n = 6 mice). P values determined by Student’s t test. (D) Sorted NK cells from untreated rejecting (black bars) or tolerized (white bars) allograft tissue (n = 4 mice) or splenocytes (n = 4 mice) were processed for quantitative RT-PCR analysis of IFNγ, TNFα, TGFβ, and IL-10.
NK cell depleted recipients have increased monocyte and macrophage infiltration
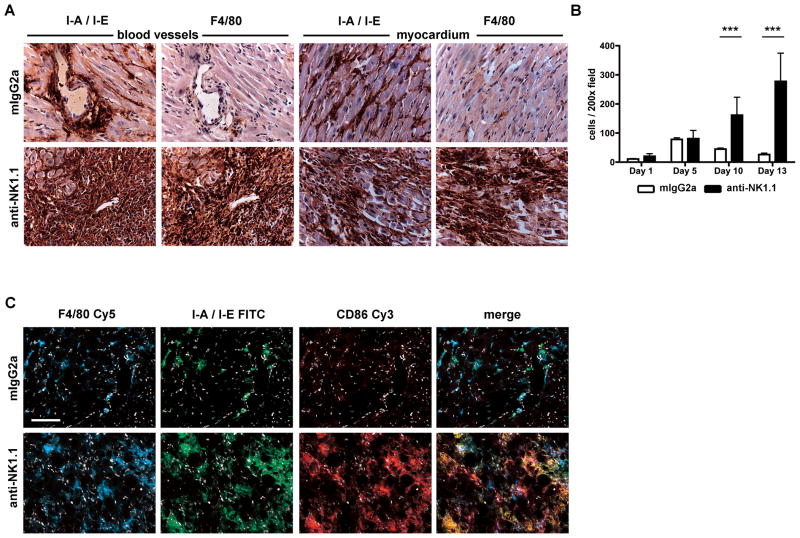
It was possible that NK cells regulated other infiltrating cell populations in the allograft tissue. To study this, we focused on characterizing the graft infiltrating cells. Immunohistochemical staining of grafts at day 13 revealed that MHC II+ F4/80+ macrophages constituted the majority of graft-infiltrating cells in the NK cell depleted recipients (Fig 5a). Immunohistochemical analysis of allograft myocardium showed no significant difference in macrophage infiltration between anti-NK1.1 mAb or isotype control treated recipients until ten days following transplantation. A 2-fold (p<0.005) and a 4-fold (p<0.005) relative increase in F4/80+ macrophage number was observed in anti-NK1.1 mAb treated recipients at ten and thirteen days respectively (Fig 5b). NK cell sufficient allografts contained MHC II+ cells around vessel walls and throughout the myocardium, but only a minority of these cells expressed F4/80, suggesting they were dendritic cells and not macrophages. Post transplant day ten infiltrating F4/80+ cells in NK cell depleted grafts co-stained for I-A/I-E, F4/80, and CD86, consistent with the profile of activated macrophages (Fig. 5c). No other significant changes in the percentage of CD11c+ dendritic cells, CD11b+Ly6C+ monocytes, or CD11b+Ly6G+ granulocytes could be observed in the allograft following anti-NK1.1 treatment 10 days following transplant.
Figure 5.
F4/80+ macrophages infiltrate NK cell depleted recipients at days 10 and 13 post-transplant. (A) Immunohistochemical analysis of paraffin-embedded allograft tissue 13 days post-transplant. Recipients received tolerogen + isotype control or anti-NK1.1 mAb. Serial sections stained for I-A/I-E and F4/80. Cardiac blood vessels and myocardium are shown. (B) Quantification of F4/80+ cell infiltration in recipient allografts receiving tolerogen plus isotype control (white bars) or anti-NK1.1 mAb (black bars) at days 1, 5, 10, and 13 post-transplant. Cells counted per 200X field of myocardium. Results are mean ± SEM (n = 3 grafts/group, 3 sections/graft, 5 fields/section). P values determined by Student’s t test. (C) Immunofluorescence microscopy of F4/80+ cells in recipients receiving tolerogen plus isotype control or anti-NK1.1 mAb 10 days following transplant. Representative of 3 independent experiments (n = 4 mice).
NKG2D blockade increases allograft macrophage infiltration and Rae-1γ expression
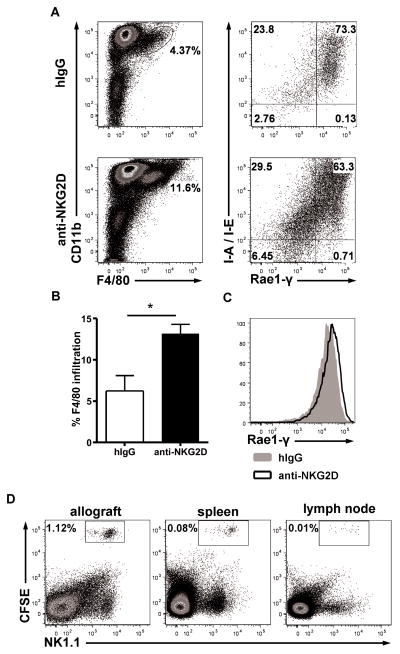
The absence of alloantibody and CD4 T cell responses following NK cell depletion suggested that NK cells directly regulate macrophage populations or their monocyte precursors. In addition to triggering effector responses, NK cell activating receptors, such as NKG2D, have been recently shown to regulate host immune cells including CD8 T cells (10, 29). To determine if NKG2D blockade interfered with tolerance induction, recipients received HMG2D, an anti-NKG2D blocking antibody, following transplantation. NKG2D blockade was not sufficient to cause acute rejection, but allografts analyzed by flow cytometry 10 days post-transplant contained a higher percentage of F4/80+ macrophages among infiltrating cells compared to recipients receiving isotype control (Fig. 6a–b). Additionally, F4/80+MHC-II+ cells expressed high levels of the NKG2D ligand Rae-1γ. HMG2D treatment further increased expression of Rae-1γ compared to recipients receiving isotype control antibody (Fig. 6c). Short-term adoptive transfer of CFSE-labeled NK cells in HMG2D treated transplant recipients was performed at day 10 to determine if NK cells actively migrate to the allograft at this timepoint post-transplant. 24 hours post-injection, NK cells were found in the allograft, the spleen, and to a lesser degree, the peripheral lymph nodes (Fig 6d). These observations suggest that under conditions of tolerance following transplantation, allograft-homing NK cells regulate macrophage infiltration in part by NKG2D-Rae-1γ receptor-ligand interactions.
Figure 6.
Increased F4/80+ macrophage infiltration and Rae-1γ expression in anti-NKG2D treated recipients 10 days following transplant. (A) Recipients received tolerogen plus isotype control or anti-NKG2D mAb. Graft-infiltrating cells were gated on CD45+DAPI- singlet cells. Rae-1γ expression was gated on CD45+DAPI-CD11b+F4/80+ cells. (B) F4/80+ graft-infiltrating cells as a percentage of total CD45+ cells. Results expressed as mean ± SEM (n = 6, p < 0.05). (C) Rae-1γ expression on F4/80+ graft-infiltrating cells comparing isotype control treated (gray filled) to anti-NKG2D treated (black) recipients. Staining is representative of four separate transplant pairs. (D) Day 10 tolerized transplant recipients receiving anti-NKG2D were transferred with CFSE-labeled NK cells Allograft and secondary lymphoid organs were harvested 24 hours post injection to identify NK cell migration. Cells were gated on the CD45+DAPI- lymphocyte gate.
Discussion
Our study demonstrated the requirement for NK cells for preventing acute allograft rejection under conditions of co-stimulatory blockade induced tolerance. Anti-NK1.1 mAb treated recipients showed prolonged depletion of NK cells and rejected allografts within 2 weeks following transplantation. These observations are consistent with an islet transplantation study that found NK cells necessary for tolerance induction in anti-CD40L mAb treated allograft recipients (17). Others have shown NK cells associate with regulating the expansion and activation of CD4+ and CD8+ T cell responses (24–26, 30, 31). Rejection in our model was associated with increased macrophage infiltration in the graft, but no significant alloantibody or CD4+ alloantigen specific response. Numbers of regulatory T cells were also unchanged between untreated and NK cell depleted recipients. Despite the absence of robust alloantigen-specific responses, allograft rejection in anti-NK1.1 treated recipients may still be dependent on T cells. Rejection in our model was associated with enhanced macrophage infiltration. Graft-infiltrating monocytes and macrophages secrete proteases and reactive oxygen and nitrogen species, as well as inflammatory cytokines to mediate tissue injury (32). Macrophage depletion using liposomal clodronate attenuated histological and functional parameters of acute rejection in a renal allograft model (33). One clinical study strongly correlated monocytic infiltration with renal dysfunction, while T cell infiltration did not (34). Renal transplant patients receiving the T cell depleting antibody alemtuzumab had undetectable levels of T cells for the first month post-transplant (35). These patients still experienced acute rejection episodes and allograft dysfunction that correlated with the early infiltration of monocytes and macrophage in the allograft. Thus, unregulated macrophage infiltration into allografts may be sufficient to mediate acute rejection despite attenuated or anergized alloantigen-specific T cell responses.
NK cells may contribute to transplantation tolerance by directly suppressing or eliminating monocyte or macrophage populations during tolerogenesis. The expression of NK cell activating receptor ligands in rejecting allografts suggests their role in facilitating acute rejection (36). However, a regulatory role for these ligands could apply during tolerance induction. NK cells regulate host immunity though activating receptors including NKG2D (24, 30, 37). Blockade of NKG2D did not affect tolerance induction in our experimental model and did not prevent the migration of NK cells to the allograft. However, increased infiltration of macrophages was observed compared to untreated tolerized recipients post-transplant. Cytotoxicity dependent mechanisms may be important in transplantation since Beilke et al. demonstrated that NK cells require intact perforin to establish islet transplantation tolerance (17). A study of human macrophages showed that lipopolysaccharide stimulation up-regulated the mRNA levels of the NKG2D stress ligands ULBP1-3 and MICA (38). Increased stimulation increased macrophage susceptibility to NK cell mediated killing. Similar cytotoxic mechanisms were observed with NK cells co-cultured with immature human dendritic cells (39–41). Our data indicate that allograft-infiltrating macrophages in tolerized recipients expressed high levels of the NKG2D ligand Rae-1γ. Blockade of NKG2D further increased Rae-1γ expression on these cells. While the contribution of other NKG2D expressing cells cannot be excluded in our model, we demonstrated that NK cells migrate to the allograft post-transplantation. The elevated expression of NKG2D ligand on macrophages may stimulate NK cell activation and promote cytotoxicity during the resolution of inflammation in the tolerized allograft.
Cytotoxic-independent mechanisms of macrophage regulation should also be considered in assessing roles of NK cells in transplantation tolerance. Liver transplant models associating IL-4 treatment to prolonged graft survival noted increased levels of NK cell derived IFNγ and indoleamine-pyrrole 2,3-dioxygenase (IDO) (42). NK cells may also directly compete with the migration of monocytes/macrophages towards chemokines including MIP-1a and IP-10 (43). In contrast, NK cells also have the potential to secrete chemokines such as CCL3/MIP-1α and CCL4/MIP-1β that can recruit other inflammatory cells (13). The expression and contribution of these factors by NK cells under conditions of tolerance requires further study.
Our finding that NK cell subsets expressing CD11b and CD27 differ in the blood and grafts of rejecting versus tolerized mice further highlights the importance of defining functional NK cell subsets. Studies focusing on CD11b and CD27 expression broadly describe NK cell subsets, their maturation, and the expression of cytokines and cytotoxic effector function potential (27, 44). However, NK cell subsets defined by these cell surface markers variably express activating and inhibitory receptors as well as chemokine receptors responsible for homing (5, 44). NK cell effector function mediated by cytotoxic granule release, Fas-FasL binding, or inhibitory co-receptor-ligand interactions such as PD-1-PD-L1 (5) are additional potential mechanisms regulating immunity.
NK cell research has primarily focused on non-self or stress-induced responses in infection and tumor models. However, there is increasing evidence that NK cells act as regulators of adaptive immunity. Uterine NK cells favor IL-10 secretion, lack Fc-receptors and are poorly cytotoxic. These tissue-resident NK cells protect the developing fetus from maternal immune responses (45). Gut mucosal NK cells regulate local immune responses by expressing IL-22 and limiting responses to intestinal pathogens (46). Similar regulatory roles may be expected in the allograft during rejection or in tolerance. A greater understanding of NK cell subsets and identification of NK cell regulatory mechanisms may uncover tolerance favoring pathways, and therapeutics that may be harnessed to improve transplant outcomes.
Materials and methods
Mice
CD1d deficient, BALB/c and C57BL/6 mice were purchased from The Jackson Laboratory. C57BL/6 T cell receptor transgenic TEa mice (47) were maintained in our facility. All mice were housed in a specific pathogen-free facility and all experiments used age- and sex-matched mice in accordance with protocols approved by the Mount Sinai Institutional Animal Care and Utilization Committee.
Monoclonal antibodies and treatment protocols
For tolerance induction, mice received 1×107 DST on day −7 and 250 μg CD40 ligand-specific mAb (MR1) (BioXCell, West Lebanon, NH) intravenously on days −7, −4, 0 and +4 relative to transplantation. NK cells were depleted using a single intravenous dose of 100 μg NK1.1-specific mAb (PK136) (BioXCell) on day −1. NK cell sufficient controls received mouse IgG2a isotype control mAb (C1.18.4) (BioXCell). NKG2D blockade was achieved using intraperitoneal dose of 200 μg anti-NKG2D blocking mAb (HMG2D) (BioXCell) every 3 days post-transplant.
Vascularized cardiac transplantation
BALB/c hearts were transplanted as fully vascularized heterotopic grafts into C57BL/6 mice as described (48). Graft function was monitored every other day by abdominal palpation.
Adoptive transfer of TCR-transgenic CD4+ TEa cells
CD4+ T cell subsets were isolated from the spleens and lymph nodes of T cell receptor transgenic TEa mice using the mouse CD4+ T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer’s protocol. Cells were then stained with 5 μM CFSE (Invitrogen, Carlsbad, CA), and 2×106 cells were adoptively transferred into C57BL/6 mice on the day of transplantation. CFSE-labeled TEa CD4+ T cells were evaluated day 5 post-transplantation by flow cytometry and immunofluorescence microscopy.
Adoptive transfer of NK cells
NK cells were isolated from the spleens of C57BL/6 mice using NK cell isolation kit II (Miltenyi Biotec). Isolated cells were then stained with 5 μM CFSE (Invitrogen), and 2 × 106 cells were adoptively transferred into C57BL/6 mice 10 days following transplantation.
Flow cytometry
Anti–mouse CD16/32 was used to block Fcγ III/II receptors and fluorochrome-conjugated antibodies specific for CD45, CD3ε, CD4, CD8β, CD25, CD11c, NK1.1, Gr-1, CD19, CD44, CD11b (eBioscience, San Diego, CA), NKp46 (R&D Systems, Minneapolis, MN), and F4/80 (Serotec, Raleigh, NC) were used for staining. Cells were stained according to manufacturers’ protocols. An LSR II (BD Biosciences, San Jose, CA) was used for flow cytometry, and data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Alloantibody assay
P815 (H-2d) mastocytoma cells were blocked with anti-CD16/32 mAb (eBioscience) and incubated with serum diluted 1:100. Samples were then stained with PE-conjugated secondary antibodies to mouse IgM and IgG (eBioscience). P815 cells incubated with naïve serum followed by isotype control PE-conjugated secondary antibody (bg) were used as a background control. Cells were analyzed by flow cytometry and mean fluorescence index (MFI) calculated by FlowJo.
Immunohistochemistry
Tissues were embedded in OCT compound (Sakura Finetek USA, Torrance, CA), and frozen at −80°C. 5μm sections were prepared and fixed in cold acetone, and blocked with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA). I-A/I-E (clone M5/114.15.2), F4/80 (clone CI:A3-1), CD86 (clone GL1), B220 (clone RA3-6B2), CD4 (clone GK1.5), CD8β (clone H35-17.2), and Foxp3 (clone FJK-16S) antibodies were purchased from eBioscience or Serotec. FITC, Cy3, and Cy5 conjugated anti-rat secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Quantification of graft infiltrating cells was performed by counting 5 fields per tissue section, 3 tissue sections per graft. Tissues were prepared for paraffin embedding following overnight fixation in 4% paraformaldehyde. 5μm paraffin-embedded sections were cleared using xylene, and antigens retrieved using a 0.05% trypsin incubation at 37°C for 30 minutes. Sections were blocked with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA) and stained with biotinylated primary antibodies (eBioscience) followed by avidin-HRP (eBioscience). Primary and secondary antibodies were stained using a 1:100 dilution. Staining was visualized using NovaRED peroxidase substrate and hemotoxylin QS counterstain (Vector Laboratories) according to the manufacturer’s protocols.
Real-time PCR
Graft tissue was perfused with PBS, then minced and digested with collagenase D (Roche, Indianapolis, IN) according to manufacturer’s protocol. Splenocytes were obtained by pulverization. Single cell suspensions were red blood cell lysed using LCK lysing buffer (Lonza, Walkersville, MD). NK cells, CD4 T cells, CD8 T cells were sorted on a MoFlo XDP cell sorter (Beckman Coulter, Miami, FL). Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA) then used for reverse transcription using OligoDT primers (Invitrogen) and Omniscript RT Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Quantitative real-time PCR was performed in duplicate using SYBR Green PCR Master Mix (Qiagen) and LightCycler 2.0 Real-Time PCR System (Roche). Relative expression was calculated as 2(CTcyclophilin-A − CTgene) where CT is cycling threshold with cyclophilin-A RNA as the endogenous control.
Luminex assay
Serum was harvested from mice day 10 post-transplant. Samples were tested for cytokine levels using a Th17 6-Plex and IL-4 set (Bio-Rad Laboratories, Hercules, CA). Samples were prepared according to manufacturer’s protocol and analyzed on a Bio-Plex 200 system (Bio-Rad). Cytokine concentrations were determined from experimental standard curves with a sensitivity < 10pg/mL for all cytokines measured.
Mixed lymphocyte reaction
BALB/c stimulator dendritic cells were enriched from total splenocytes using EasySep CD11c+ positive selection kit (StemCell Technologies, Vancouver, Canada). CD4+ and CD8+ responding T cells were enriched from C57Bl/6 splenocytes and lymph nodes by FACS sorting. CFSE (Invitrogen) labeled T cells were cultured with BALB/c CD11c+ dendritic cells at a 1:1 ratio for 5 days in complete medium.
Acknowledgments
We acknowledge the technical contributions of Dan Chen, Peter Boros, Jianhua Liu, and Yansui Li (Mount Sinai). Grant support: NIH R01 AI0720139.
Abbreviations
- DST
donor splenocyte transfusion
Footnotes
W.V. participated in performance of research, data analysis, and writing of the manuscript; B.B. contributed to TEa adoptive transfer experiments, and editing of the manuscript; G.L. participated in flow cytometry and editing of the manuscript; J.S.B. was responsible for study design, data interpretation, and writing of the manuscript.
Conflicts of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Martinez-Llordella M, Lozano JJ, Puig-Pey I, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118 (8):2845. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 120(6):1848. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenk S, Kish DD, He C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174 (6):3741. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 4.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353 (22):2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9 (5):503. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306 (5701):1517. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5 (12):1260. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 8.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5 (10):996. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009 doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang PA, Lang KS, Xu HC, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A. 109(4):1210. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perona-Wright G, Mohrs K, Szaba FM, et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe. 2009;6 (6):503. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narni-Mancinelli E, Jaeger BN, Bernat C, et al. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science. 335(6066):344. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Morita K, Watarai Y, et al. Early increased chemokine expression and production in murine allogeneic skin grafts is mediated by natural killer cells. Transplantation. 2000;69 (5):969. doi: 10.1097/00007890-200003150-00051. [DOI] [PubMed] [Google Scholar]
- 14.Uehara S, Chase CM, Kitchens WH, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175 (5):3424. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 15.Maier S, Tertilt C, Chambron N, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7 (5):557. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 16.Brillard E, Pallandre JR, Chalmers D, et al. Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25− T lymphocytes. Exp Hematol. 2007;35 (3):416. doi: 10.1016/j.exphem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11 (10):1059. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 18.Haspot F, Seveno C, Dugast AS, et al. Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. Am J Transplant. 2005;5 (10):2339. doi: 10.1111/j.1600-6143.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 19.Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112 (3):661. doi: 10.1182/blood-2007-10-120089. [DOI] [PubMed] [Google Scholar]
- 20.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203 (8):1851. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S, Scherer DC, Singh N, et al. Lipid antigen presentation in the immune system: lessons learned from CD1d knockout mice. Immunol Rev. 1999;169:31. doi: 10.1111/j.1600-065x.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Kojo S, Harada M, Ohkohchi N, Taniguchi M, Seino KI. Mechanism of NKT cell-mediated transplant tolerance. Am J Transplant. 2007;7 (6):1482. doi: 10.1111/j.1600-6143.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 23.Seino KI, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci U S A. 2001;98 (5):2577. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26 (5):593. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowdell KC, Cua DJ, Kirkman E, Stohlman SA. NK cells regulate CD4 responses prior to antigen encounter. J Immunol. 2003;171 (1):234. doi: 10.4049/jimmunol.171.1.234. [DOI] [PubMed] [Google Scholar]
- 26.Vankayalapati R, Klucar P, Wizel B, et al. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J Immunol. 2004;172 (1):130. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]
- 27.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113 (22):5488. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176 (3):1517. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 29.Ogasawara K, Hamerman JA, Ehrlich LR, et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20 (6):757. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Noval Rivas M, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 184(12):6790. doi: 10.4049/jimmunol.0902598. [DOI] [PubMed] [Google Scholar]
- 31.Hao J, Liu R, Piao W, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 207(9):1907. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magil AB. Monocytes/macrophages in renal allograft rejection. Transplant Rev (Orlando) 2009;23 (4):199. doi: 10.1016/j.trre.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76 (7):1015. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 34.Girlanda R, Kleiner DE, Duan Z, et al. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8 (3):600. doi: 10.1111/j.1600-6143.2007.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76 (1):120. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 36.Feng L, Ke N, Ye Z, et al. Expression of NKG2D and its ligand in mouse heart allografts may have a role in acute rejection. Transplant Proc. 2009;41 (10):4332. doi: 10.1016/j.transproceed.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 37.Rivas MN, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 184(12):6790. doi: 10.4049/jimmunol.0902598. [DOI] [PubMed] [Google Scholar]
- 38.Nedvetzki S, Sowinski S, Eagle RA, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109 (9):3776. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 39.Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163 (12):6365. [PubMed] [Google Scholar]
- 40.Carbone E, Terrazzano G, Ruggiero G, et al. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29 (12):4022. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195 (3):335. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Tay SS, Tran GT, et al. Donor IL-4-treatment induces alternatively activated liver macrophages and IDO-expressing NK cells and promotes rat liver allograft acceptance. Transpl Immunol. 22(3–4):172. doi: 10.1016/j.trim.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155 (8):3877. [PubMed] [Google Scholar]
- 44.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson M, Meadows SK, Wira CR, Sentman CL. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol. 2004;76 (3):667. doi: 10.1189/jlb.0204090. [DOI] [PubMed] [Google Scholar]
- 46.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457 (7230):722. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7 (2):197. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 48.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16 (4):343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]