Abstract
Peripheral nerves have the potential to regenerate axons and reinnervate end organs. Chronic denervation and disturbed nerve regeneration are thought to contribute to peripheral neuropathy, pain and pruritus in the skin. The capacity of denervated distal nerves to support axonal regeneration requires proliferation by Schwann cells, which guide regenerating axons to their denervated targets. However, adult peripheral nerve Schwann cells do not retain a growth-permissive phenotype, as is required to produce new glia. Therefore, it is believed that following injury, mature Schwann cells de-differentiate to a progenitor/stem cell phenotype to promote axonal re-growth. In this study, we show that Skin-derived precursors (SKPs), a recently identified neural-crest related stem cell population in the dermis of skin, are an alternative source of progenitors for cutaneous nerve regeneration. Using in vivo and in vitro 3-D cutaneous nerve regeneration models, we show that the SKPs are neurotropic toward injured nerves and that they have a full capacity to differentiate into Schwann cells and promote axon regeneration. The identification of SKPs as a physiologic source of progenitors for cutaneous nerve regeneration in the skin, where SKPs physiologically reside, has important implications for understanding early cellular events in peripheral nerve regeneration. It also provides fertile ground for the elucidation of intrinsic and extrinsic factors within the nerve microenvironment that likely play essential roles in cutaneous nerve homeostasis.
Keywords: Cutaneous nerve regeneration, SKPs, Skin-derived precursors, Peripheral nerve regeneration, Neurofibromatosis Type 1, NF1, Neurofibroma, Neurofibromatosis, Schwann cells, Cutaneous nerve homeostasis
Introduction
Skin is an important sensory organ that includes a complex network of cutaneous nerve plexuses. While afferent sensory innervation mediates the perceptions of touch, temperature, pressure and pain in skin, efferent autonomic nerves control glandular activities and smooth muscle contraction. Peripheral cutaneous nerves have remarkable regenerative capabilities in that damaged nerves are able to reconnect and re-establish their function, even after severe injury [1–3]. On the other hand, incomplete nerve regeneration and chronic denervation in the dermis of skin contribute to the pathogenesis of several human diseases, including peripheral neuropathy, pruritus, wound healing and hypohidrosis/anhidrosis [4–7].
The unique ability of the injured peripheral cutaneous nerves to support axonal regeneration depends on proliferating Schwann cells, which guide regenerating axons toward their denervated targets [2, 8, 9]. Schwann cells produce neurotrophic factors that support injured axons and prevent them from undergoing apoptosis [10, 11]. Proliferating Schwann cells also secrete chemoattractant factors that recruit other cell types, including fibroblasts which undergo cell sorting. In this process, Schwann cells and fibroblasts grow in ordered columns along the endoneurial tube, forming cellular corridors known as “bands of Bungner”, to guide regenerating axons across sites of injury [2, 3]. Injured and regenerating axons release from their growth cones neuregulins, which bind to erbB receptors on Schwann cells, mediating Schwann cell proliferation. Because adult peripheral nerve Schwann cells lack the capacity to sustain a growth-permissive phenotype, as is required to produce new glial cells, it is believed that mature Schwann cells de-differentiate into progenitor/stem-like cells to promote axonal re-growth following injury [2, 12]. However, the discovery of neural crest stem cells at sites of gliogenesis, including the sciatic nerve and dorsal root ganglia [13, 14] in the peripheral nervous system (PNS) and other tissues, including the neural crest-like stem cells in the skin [15–20], raises the possibility that resident adult stem cells may serve as an alternative source of progenitors for cutaneous nerve regeneration.
Miller and colleagues have pioneered the identification of neural crest-like stem cells in both human and mouse dermis. Termed skin-derived precursors (SKPs), they have the capacity to differentiate along neuronal and glial lineages [15–18]. The exact locations of the SKPs within the dermis have not been fully identified. However, it has been shown that SKPs reside near hair follicles [21–24]. Utilizing different culturing conditions, Herlyn and colleagues have also reported the existence of multipotent neural crest-like stem cells from the human dermis [25, 26]. The location and multipotency of these cells make them an attractive alternative source of progenitor cells for cutaneous nerve regeneration, where SKPs physiologically reside. In fact, SKPs have been shown to have the capacity to differentiate into Glial cells in respond to the nerve microenvironment within spinal cord and sciatic nerve injuries in mouse models [18, 27, 28]. We recently reported that SKPs are the cell of origin for dermal neurofibromas, which are unique and complex tumors associated with the genetic disease Neurofibromatosis Type 1. These tumors contain proliferating Schwann cells and other supporting elements of nerve fibers [29]. NF1-deficient SKPs have the capacity to generate plexiform or dermal neurofibromas, contingent on their local microenvironment, and they exhibit the same properties as the embryonic Schwann cell progenitors that give rise to plexiform neurofibromas [29]. However, the physiologic roles of SKPs within the dermis remain unknown. We report herein that the Skin-derived precursors are an alternative source of progenitors for cutaneous nerve regeneration. Using both in vivo and in vitro 3-D cutaneous nerve regeneration models, we show that SKPs are neurotropic to injured nerves and that they have the capacity to differentiate into Schwann cells to myelinate regenerating axons. Thus, this study identifies a new physiologic function of SKPs within the skin and reveals their critical roles in cutaneous nerve homeostasis.
Material and Methods
Mice
All mice were housed in the animal facility at the University of Texas Southwestern Medical Center at Dallas (UTSW). Animal care and use were in compliance with regulations of the Institutional Animal Care and Research Advisory Committee at UTSW. The LacZ reporter mice, ROSA26R [30], CMV-CreERT2, and nude mice were obtained from the Jackson Laboratories.
Cell culture and in vitro engineered skin rafts
SKPs were isolated as previously reported [15, 31]. Briefly, mice were anesthetized by intraperitoneal injection of 120 μl of a mixture of ketamine (10mg/ml) and xylazine (1mg/ml) solution. Skin was harvested from neck and back. Hair, fascia, adipose, blood vessel, and muscle tissues were carefully dissected out, and the skin tissues were cut into small pieces (2–3 mm), washed 3 times in Hanks’ Balanced Salt Solution (Invitrogen), and then digested with 0.1% trypsin at 37°C for 30 minutes. The skin tissues were then dissociated mechanically, passed through a 70-μm cell strainer and washed once with Dulbecco’s modified eagle medium (DMEM)/F12 + 10% Fetal Bovine Serum. The cell pellet was then washed three times with serum-free DMEM/F12 media, counted and plated at a density of 20 cells/μl on uncoated, ultra-low attachment 6-well plates (Corning) in proliferation media: DMEM/F12 containing penicillin/streptomycin (0.1%); fungizone (40 μg/ml); B27 (without vitamin A), epidermal growth factor (20 ng/ml), and basic fibroblast growth factor (40 ng/ml; Sigma). The sphere cells were fed every 3 to 4 days and passaged every 7 days.
Generation of in vitro engineered skin rafts/reconstructs has been well-established and characterized extensively [25, 32, 33]. They were generated as follow: inserts of six-well tissue culture plates (Corning Incorporated) were coated with 1 ml bovine collagen I (Organogenesis) and layered with 3 ml collagen I containing 1×105 human foreskin fibroblasts. After 7 days of incubation at 37°C, keratinocytes can be seeded on top of the dermal reconstructs. These skin equivalent rafts were kept submerged in medium for 2 days then raised to the air-liquid interface via feeding from medium bellow. Two to twelve weeks later, skin reconstructs were harvested and processed for histological and immunohistochemical analysis. SKPs, DRGs, nerves were introduced into the skin reconstructs by mixing with fibroblasts and then adding them to the dermal fibroblast/collagen layer.
Transplantation experiments
SKPs were isolated as above. Mice were allowed to recover from anesthesia after closure of excision wounds with 4–0 nylon suture. After 10–14 days in culture, SKPs were exposed to 1 μM of 4-OH-tamoxifen. Sphere cells were subsequently harvested for X-gal staining. Once recombination was confirmed, 1 × 106 viable LacZ-positive single cells from SKP spheres were resuspended in 40 μl of L15 medium (GIBCO). For sciatic nerve implantation, a skin incision was made above the right femur. Using iris scissors, a pocket was created within the quadriceps muscles to expose the sciatic nerve. The 40 μl of L15 medium containing 1 × 106 viable LacZ-positive SKPs was then deposited into this pocket so that SKPs can be in contact with the sciatic nerve. The quadriceps muscles were then closed with 4–0 Vicryl suture and the skin was closed with 5–0 prolene suture.
For cutaneous nerve regeneration assays, mice were anesthetized as above. A 1cm × 1.5 cm circular island of skin was excised from either the flank or back and autografted back to the same area with a 5–0 nylon suture. Viable LacZ-positive single cells from SKP spheres were resuspended in 40 μl of L15 medium (GIBCO) and implanted either intradermally/subcutaneously at the skin graft site of the same mouse from which the SKPs were originally isolated for autologous transplantation or to nude mice for allogeneic transplantation.
Histology and immunostaining
For hematoxylin and eosin (H&E) histology analyses, tissue specimens were harvested and fixed with 10% formalin in PBS for 1 day and subsequently embedded in paraffin. Sections (5 μm thick) were stained with H&E per manufacturer’s protocol (StatLab). For immunohistochemistry, paraffin sections were deparaffinized, rehydrated, and subjected to antigen retrieval prior to incubation with the primary antibodies. The primary antibodies were visualized by treating the sections with biotinylated secondary antibody and followed by amplification with peroxidase-conjugated avidin and 3,3′-diaminobenzidine substrate per manufacturer’s protocol (Vector Labs). For IHC staining of SKPs prior to transplantation, cells grown in 8-chamber slides were counter-stained with light hematoxylin solution after exposed to 3,3′-diaminobenzidine substrate. Sciatic nerve (SN) was used as positive control and 3T3 cells were used as negative control. The dilutions of primary antibodies used in this study were as follows: GAP43 (rabbit, 1:1,000 – 1;4000, Abcam) and MBP (goat, 1:1,000, Santa Cruz).
X-gal staining
For X-gal staining, tissues were harvested, fixed in 4% paraformaldehyde in PBS and equilibrated in 30% sucrose in PBS overnight at 4°C, washed 3 times with 1× PBS and stained with X-gal at 30°C overnight. The tissues were then post-fixed with 10% formalin overnight, paraffin embedded and sectioned. The X-gal reaction mixture was comprised of 1 mg/mL 4-chloro-5-bromo-3-indolyl-β-galactoside (X-gal), 4 mmol/L potassium ferrocyanide, 4 mmol/L potassium ferricyanide, and 2 mmol/L magnesium chloride in PBS.
Holmes stain for nerve fibers
Holmes staining, a silver nitrate method to detect nerve fibers and Neurofibrils in tissue section, was done as described previously [34]. Briefly, skin and nerve tissues were rehydrated, and placed in 20% silver nitrate in the dark at room temperature for 1 hour then rinsed in distilled water. The tissues were then placed in impregnating solution (Boric acid solution, Borax solution, silver nitrate and pyridine) and incubated overnight at 37°C, rinsed in distilled water and toned in 0.2% aqueous gold chloride for 3 minutes. The slides were then placed in 2% aqueous oxalic acid until the axons were thoroughly gray-black. The tissue slides were then rinsed again in distilled water, dehydrated, and mounted using a xylene base medium.
Results
SKPs are Neurotropic in vitro and in vivo
We recently reported that the SKPs are the cell of origin of dermal neurofibromas, a cutaneous tumor of Schwann cells commonly associated with the genetic disease Neurofibromatosis Type 1 (NF1). We show that NF1-deficient SKPs can give rise to classic plexiform or dermal neurofibromas contingent on the local nerve microenvironment and exhibit the same properties as the embryonic Schwann cell progenitors that give rise to plexiform neurofibromas [29].
To examine interactions between SKPs and the nerve microenvironment, we built a 3-D in vitro skin equivalent construct that models in vivo cutaneous gliogenesis, hence allowing us to extract detailed information on cell-cell communication networks and consequent cell behavior in complex, physiological 3D cultures. The human skin in vitro model is comprised of a stratified terminally differentiated epidermal compartment and a dermal compartment made up of fibroblasts embedded in collagen. This type of model is well-established, and it has been characterized extensively [25, 32, 33]. They were created as follow: inserts of six-well tissue culture plate were coated with 1 ml bovine collagen I and layered with 3 ml collagen I containing 1×105 human foreskin fibroblasts. After 7 days of incubation at 37°C, keratinocytes can be seeded on top of the dermal constructs. These skin equivalent rafts were kept submerged in medium for 2 days then raised to the air-liquid interface via feeding with medium from bellow. Two to twelve weeks later, these skin constructs can be harvested and processed for histological and immunohistochemical analysis. We concentrated our efforts on adding dermal components that would faithfully mimic in vivo counterparts, including of all of the key cell types important for peripheral gliogenesis and regeneration: SKPs, Schwann cells, neurons, fibroblasts, and dorsal root ganglia (DRGs). All of these cellular components could be introduced into the skin constructs by mixing with fibroblasts and adding the combination to the dermal fibroblast/collagen layer. Extracellular factors that are essential for this process included neuregulin, other Schwann cell factors, and nerve growth factors could be added to the media (Figure 1A).
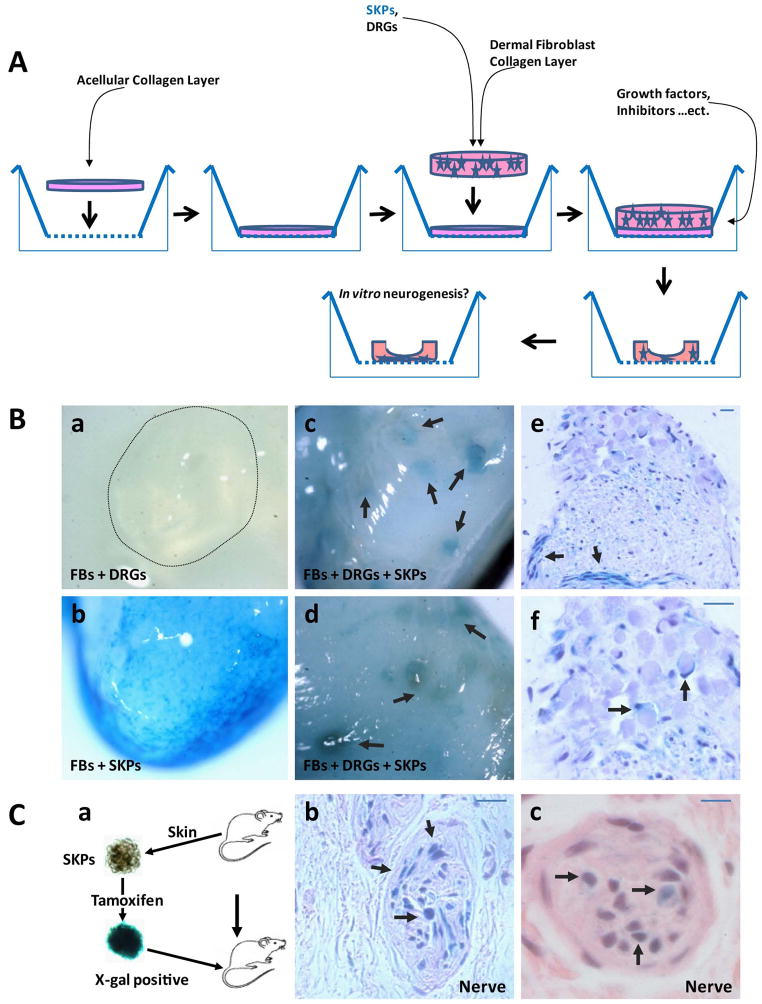
Figure 1. Implanted SKPs are Neurotropic In Vitro and In Vivo.
1A. In Vitro Engineered Skin Raft for gliogenesis/nerve regeneration.
Inserts of six-well tissue culture plates were coated with 1 ml bovine collagen I and layered with 3 ml collagen I containing 1×105 human foreskin fibroblasts. After 7 days of incubation at 37°C, keratinocytes were seeded on top of the dermal rafts. The skin equivalent rafts were kept submerged in medium for 2 days and then raised to the air-liquid interface via feeding with medium bellow. SKPs and DRGs/nerves were introduced into the skin constructs by mixing with fibroblasts and then adding them to the dermal fibroblast/collagen layer. Extracellular factors essential for this process, including neuregulin, other Schwann cell factors, and/or nerve growth factors were added to the media.
1B. X-gal staining of an in vitro engineered skin raft containing only fibroblasts, DRGs/nerves (a) or only fibroblasts and lacZ positive SKPs (b). In skin rafts that contain lacZ positive SKPs, fibroblasts and DRGs/nerves, the Lacz positive SKPs concentrate near the DRGs/nerves (c–d; arrows). Paraffin sections of these DRGs in (c) and (d) showing the LacZ positive SKPs infiltrating into the DRGs, forming cellular corridors, similar to the bands of Bungner (e, arrows) as well as differentiating into LacZ-postive Schwann-like cells that wrap around axons within the DRGs (f, arrows). Scale bar = 20 μm.
1C. SKPs were harvested from skin from the back & neck of CMV-CreERT2;Rosa26 mice and exposed to 4-OH-tamoxifen to induce recombination at the Rosa 26 locus and then reimplanted dermally in the same mice at the dorsal/sacral area (a). Grafted sites were harvested two months later for cutaneous nerve analysis. X-gal staining shows that blue lacZ positive SKPs migrate to and reside predominantly within the cutaneous nerves (b–c, arrows). Scale bar = 20 μm.
We performed X-gal staining to trace the locations of the SKPs within the skin constructs/rafts. We took advantage of the fact that only SKPs are lacZ positive and the rest of the cells in the raft are not. Consistently, we did not detect X-gal staining in the skin rafts that contained only fibroblasts, DRGs and nerves (Figure 1B, a). On the other hand, LacZ positive SKPs were distributed evenly throughout the rafts that had only SKPs and fibroblasts (Figure 1B, b). Strikingly, we observed that the Lacz positive SKPs concentrated mostly near the DRGs/nerves in skin rafts that contained SKPs, fibroblasts and DRGs/nerves (Figure 1B, c–d). These findings indicate that SKPs exhibit neurotropic behavior as they migrate toward nerves. We performed histological analyses of the constructs and observed that LacZ positive SKPs had infiltrated the DRGs, forming cellular corridors, as is seen in the bands of Bungner (Figure 1B, e, arrows), with some differentiating into LacZ-postive myelinating Schwann-like cells that would wrap around axons within the DRGs (Figure 1B, f, arrows).
We next sought to determine whether SKPs were neurotropic in vivo. We harvested skin from the backs and necks of CMV-CreERT2;Rosa26 mice for SKP isolation. We then exposed the harvested CMV-CreERT2;Rosa26 SKPs ex vivo to 4-OH-tamoxifen to induce recombination at the Rosa 26 locus and then reimplanted the LacZ+ SKPs dermally into the same mice from which the SKPs has been harvested, to avoid immunological rejection. These cells were placed in the dorsal/sacral area to prevent the recipient mice from damaging the sites of implantation (Figure 1C, a). Two months after grafting, we harvested the skin for histopathologic examination. Because the SKPs were lacZ positive and the hosts were not, we performed X-gal staining of the skin specimens. We observed that blue lacZ positive cells resided predominantly within cutaneous nerves (Figure 1C, b-c, arrows), indicating that they were derived from the transplanted SKPs. These observations indicate that the nerve microenvironment provides important triggers/niches to attract or enhance SKP migration and survival. We conclude from that SKPs are neurotropic in vitro and in vivo.
The nerve microenvironment promotes differentiation of SKPs into Schwann cells
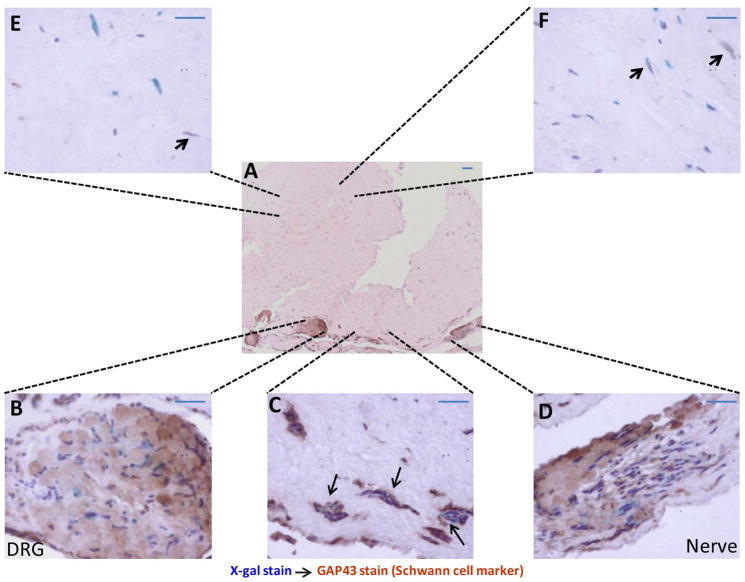
SKPs have been shown to express markers similar to those of neural crest stem cells and that they can differentiate along neuronal and glial lineages [35]. Therefore, we wanted to determine the fate of the SKPs as they migrated toward nerve microenvironments within our 3-D culturing system. We again generated dermal skin constructs that contained lacZ-postive SKPs, fibroblasts and DRGs/nerves. After 4–6 weeks in culture, we performed X-gal staining to trace the SKPs. The tissues were then processed for histological and immunohistochemical analysis. We consistently observed that the majority of blue cells (LacZ marker) were also positive for GAP 43 (a Schwann cell marker) within and in the vicinity of the DRGs/nerves (Figure 2A, B–D). We also observed tubal structures or cellular corridors of SKP-derived GAP 43 positive Schwann cells in these areas (Figure 2C, arrows). In contrast, there were only a few LacZ-GAP 43 double-positive cells in areas distant from the DRGs and nerves (Figure 2E, F, and arrow). This indicates that when placed in a favorable microenvironment (in proximity to a damaged peripheral nerve); the SKPs differentiate preferentially into Schwann cells and organize themselves into structures that resemble the bands of Bungner. This demonstrates that SKPs have a full potential to contribute to peripheral gliogenesis, and it suggests that they can be a source of progenitors for cutaneous nerve regeneration. Moreover, the essential role of the nerve microenvironment in this process is underscored.
Figure 2. Nerve microenvironment promotes the differentiation of SKPs into Schwann cells.
In vitro engineered skin rafts containing lacZ-postive SKPs, fibroblasts and DRGs/nerves were harvested after 4–6 weeks in culture, using X-gal staining to trace the location of the SKPs. Tissues were then processed for histological and immunohistochemical analysis. The majority of blue cells (marker for SKPs) are also positive for GAP 43 (a Schwann cell marker) within and in the vicinity of the DRGs/nerves (A–D). There are tubal structures or cellular corridors of SKP-derived GAP 43 positive Schwann cells in these areas (C, arrows). In contrast, there are only a few LacZ-GAP 43 double positive cells in areas distant from the DRGs and nerves (E, F and arrows). Scale bar = 20 μm.
SKPs contribute to peripheral gliogenesis
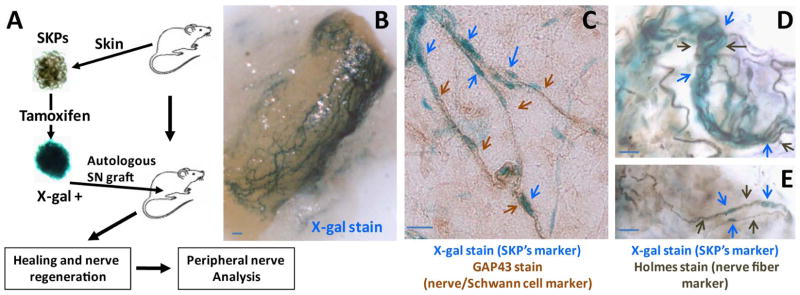
Next, we wished to determine whether SKPs have the capacity to contribute to peripheral gliogenesis. We again harvested skin from the backs of CMV-CreERT2;Rosa26 mice for SKP isolation, exposed the SKPs ex vivo to 4-OH-tamoxifen to induce recombination at the Rosa 26 locus, and reimplanted the LacZ+ SKPs back into genetically identical mice. Because we had observed that the nerve microenvironment promotes the differentiation of SKPs into Schwann cells, we reimplanted the SKPs into sciatic nerves for close proximity to a large peripheral nerve (Figure 3A). The transplanted LacZ+ SKPs gave rise to new nerve bundles and branches within 2 months after implantation in all of the nerves. To verify that the new nerves had arisen from the transplanted SKPs, we examined the nerves for lacZ positive cells. X-gal staining demonstrated a network of LacZ-positive nerves wrapping around each sciatic nerve, where the SKPs had been implanted (Figure 3B). Immunohistochemical analysis with GAP43, a Schwann cell marker and Holmes stain, a neurofibril/axon marker established that these LacZ-positive bundles and branches were nerve fibers (Figure 3C–E). Thus, when placed in a favorable microenvironment (in proximity to a peripheral nerve); the SKPs can give rise to bona fide peripheral nerves. These results confirm that SKPs have the full potential to contribute to gliogenesis in vivo and suggest that they may be an alternative source of progenitors for cutaneous nerve regeneration.
Figure 3. SKPs contributed to peripheral nerve regeneration.
SKPs were harvested from skin on the back & neck of CMV-CreERT2;Rosa26 mice and exposed to 4-OH-tamoxifen to induce recombination at the Rosa 26 locus and then reimplanted back to the same animals, adjacent to the sciatic nerve (A). X-gal staining demonstrates a network of LacZ-positive nerves wrapping around the sciatic nerve where SKPs were implanted (B). Immunohistochemical analysis with GAP43, a Schwann cell marker (C, brown arrows) and Holmes stain, a neurofibril/axon marker (D–E, black arrows) establish that the LacZ-positive bundles and branches (blue arrows) are nerve fibers. Scale bar = 20 μm.
SKPs as a source of progenitors for cutaneous nerve regeneration
Although SKPs have been shown to have the capacity to participate in peripheral gliogenesis (Figure 3) and to differentiate into Schwann cells within spinal cord and sciatic nerve [18, 27, 28], the physiologic roles of SKPs within the dermis remain unknown. In fact, we recently reported the pathogenic role of SKPs as the cell of origin of dermal neurofibroma, a complex cutaneous tumor of the Schwann cells associated with the genetic disorder Neurofibromatosis Type 1 [29]. However, it is certain that these SKPs do not evolve and migrate during embryogenesis from the neural crest to the skin for the sole purpose of making neurofibromas. Thus, we sought to determine whether SKPs could be a source of stem/progenitor cells for cutaneous nerve regeneration/gliogenesis in the dermis, where they reside physiologically. To address this question, we first wanted to verify that SKPs can participate in regeneration of an injured cutaneous nerve plexus. We generated axonal transections within the dermis by complete excision of a 1.5 cm circular island of skin on the back of C57BL6 mice. We then returned each graft autologously back into the same position, covering each with sterile petrolatum gauze, and secured with sutures. Subsequently, we implanted LacZ-positive SKPs, which were previously harvested from the same animal, into the graft and allowed the wounds to heal (Figure 4A,a). Immunohistochemical analysis with GAP43 showed that SKPs do not contain Schwann cells prior to transplantation (Figure 4A, b–d), indicating that they were not propagating Schwann cells in SKPs growth conditions.
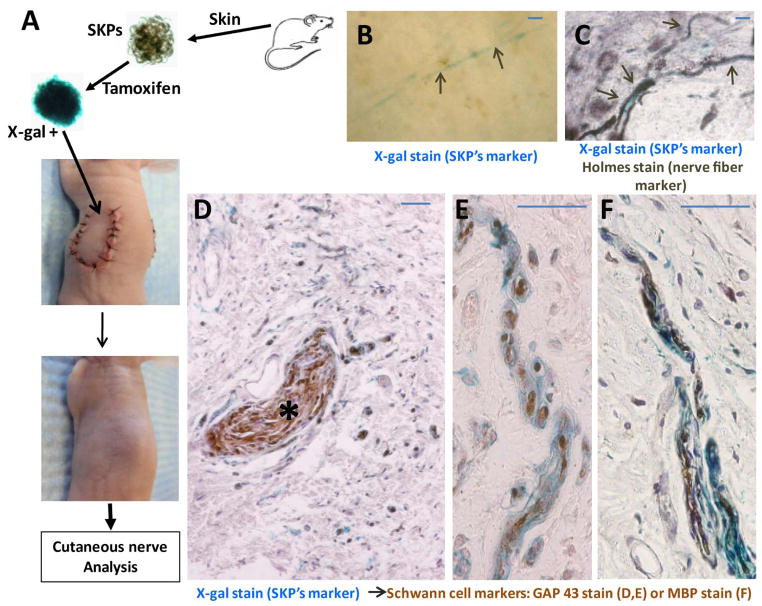
Figure 4. SKPs as a source of autologous progenitors for cutaneous nerve regeneration.
Cutaneous nerve injuries were generated with axonal transections within the dermis by complete excision of a 1.5 cm circular island of skin on the back. The excised skin was then grafted autologously back into the same position, covered with sterile petrolatum gauze and secured with sutures. LacZ-positive SKPs harvested from the same animal were implanted into the graft and the wounds allowed to heal for cutaneous nerve regeneration (A–a). Immunohistochemical analysis with GAP43 showed that SKPs do not contain Schwann cells prior to transplantation as they are GAP43 negative (A–b,c,d). X-gal staining of the excised skin graft 4–6 weeks after implantation demonstrates a network of LacZ-positive new nerves sprouting out of the proximal stumps (dotted lines) into the graft where the SKPs were implanted (B, arrows). Holmes stain, a neurofibril/axon marker (C) and immunohistochemical analysis with GAP43, a Schwann cell marker (D–E) establish that these LacZ-positive branches (arrows) are nerve fibers. Scale bar = 20 μm.
After such transection injuries of this sort, the cutaneous axon stumps would normally retract a short distance and then one or more sprouts would emerge from the proximal stumps through the bands of Bungner to reestablish connection with distal stumps or end organs [36]. We observed in our grafts that the new nerves sprouting out of the proximal stumps into grafted skin were blue when subjected to X-gal staining (Figure 4B, arrows). Holmes stain (Figure 4C) and immunohistochemical analysis with the Schwann cell marker GAP43 (Figure 4D, E) confirmed that that these LacZ-positive bundles were nerve fibers. We observed identical results in similar experiments where the allogeneic C57BL6, LacZ-positive SKPs were implanted into the skin grafts of BALB/c nude mice (Figure 5). In addition, immunohistochemical staining with Myelin Basic Protein (MBP) (Figure 5F) established that the LacZ-positive branches (Figure 5) are myelinating nerve fibers. These data indicate that the LacZ-positive SKPs can be a source of stem/progenitor cells that differentiate into Schwann cells and participate in the regeneration of injured cutaneous nerve plexus, documenting a critical role for SKPs in cutaneous nerve homeostasis.
Figure 5. SKPs can serve as a source of allogeneic progenitors for cutaneous nerve regeneration.
Cutaneous nerve injuries were made in athymic mice (BALB/c background) with axonal transections within the dermis through excision of a 1.5 cm circular island of skin on the back. The excised skin was then grafted autologously back into the same position. The LacZ-positive SKPs harvested from different mice (C57BL/6 background) were implanted into the graft and the wounds allowed to heal for cutaneous nerve regeneration (A). X-gal staining of the excised skin graft 4–6 weeks after implantation demonstrates LacZ-positive cutanous nerves in the grafts, where the SKPs were implanted (B, arrows). Holmes stain, a neurofibril/axon marker (C) and immunohistochemical analysis with GAP43, a Schwann cell marker (D–E) and MBP (F) establish that the LacZ-positive branches (arrows) are myelinating nerve fibers. Scale bar = 20 μm.
Discussion
Regeneration of peripheral and cutaneous nerves includes essential contributions by Schwann cells, in collaboration with regrowing axons [2, 8, 9]. Therefore, the ability of an injured peripheral nerve to support axonal regeneration depends on proliferating Schwann cells [2, 3]. During the development of peripheral nerves, neural crest cells generate myelinating and non-myelinating Schwann cells in a process that parallels embryonic development. Migrating neural crest cells move through immature connective tissues before the time of nerve formation at mouse embryonic days E9–E11 and differentiate into Schwann cell precursors between E12–E13. These Schwann cell precursors then become immature Schwann cells, which are generated from E14 until early neonatal stages. The differentiation of all immature Schwann cells into mature Schwann cells is largely postnatal [37, 38]. Given that adult Schwann cells lack the capacity to sustain a proliferative phenotype as is required to produce new glial cells, it is believed that mature Schwann cells de-differentiate into precursor/stem-like cells following nerve injury to promote axonal re-growth [2, 12].
Major advances in the development of in vitro and in vivo assays have enabled identification and characterization of many types of adult stem cells and their ability to undergo self-renewal division, as well as to produce daughter cells that differentiate into tissue-specific lineages. Therefore, these populations of stem cells play essential roles in tissue homeostasis. SKPs are self-renewing, multipotent neural stem/precursor cells, identified in both human and mouse dermis [15–18], using neurosphere culture conditions. They are distinct from other known stem/precursor cells within the skin [39]. However, the exact biological origin and function of SKPs in the dermis are not fully understood. Using Wnt1-Cre;ROSA26flox/stop/flox compound transgenic mice, which express β-galactosidase permanently in derivatives of neural crest stem cells, Fernandes et al. reported that SKPs derived from these mice are β-galactosidase positive [35]. In addition, SKPs were shown to express a variety of Neural Crest-associated transcription factors including Twist, Sox9, Pax3, Slug and Snail [35]. Moreover, under differentiating conditions, SKPs can generate neural lineage (neurons, Schwann cells) and mesodermal (smooth muscle) cells, as well as adipocytes, the same cell types that are known to derive from neural crest stem cells. Thus, these data strongly support the concept that SKPs are multipotent neural crest-related and possibly neural crest-derived precursors present in the skin. Such relatedness to the origin of peripheral and cutaneous nerves raises the question of whether these resident adult stem cells maybe an alternative source of progenitors for cutaneous nerve development and regeneration.
We undertook this study to test the hypothesis that SKPs participate in cutaneous nerve homeostasis. Skin is one of the most innervated organs in the body with complex networks of sensory nerves. In addition, injuries to cutaneous nerve plexuses are common, whether from traumatic wounds, thermal or chemical damage, acute compression, surgical transection or diseases associated polyneuropathy. Functional recovery is regained upon axonal regeneration. Here, we show that SKPs can survive and differentiate to a glial phenotype within milieu of the injuried cutaneous nerves, providing compelling evidence for the physiologic role of SKPs in the dermis as a source of progenitors for cutaneous nerve regeneration. Using both in vivo and in vitro 3-D cutaneous nerve regeneration models, we observed that the SKPs are neurotropic to injured nerves and that the microenvironment promotes differentiation of SKPs into Schwann cells. LacZ-labeled Schwann cells organized into tubal structures similar to the bands of Bungner (Figure 1B,e and Figure 2C, arrows) and participated in the generation of new nerves, sprouting out of the transected proximal nerve stumps in the dermis (Figure 4,5). We also observed the recognized feature of axon regeneration that following injury each nerve projects many sprouts resulting in new regenerating axon fibers that outnumber the original fibers in proximal nerve stumps (Figure 4); [40, 41]. Sprouting from transected or interrupted fibers, as described in our assay, is often termed “regenerative sprouting”. On the other hand, sprouting from uninjured fibers is called collateral sprouting [36]. Over time, some of these sprouts of interrupted fibers increase in caliber and extend longitudinally to innervate end organs, providing recovery of protective sensibility in denervated regions of skin. Those that fail to reach targets will eventually degenerate. This process of cutaneous nerves regeneration depends on proliferating Schwann cells [2, 3]. Our studies revealed that SKPs may be an alternative source of progenitors for this population of proliferative Schwann cells, indicating that SKPs are, in fact, an active participant in cutaneous nerve homeostasis.
Adult stem cells play essential roles in tissue homeostasis by undergoing self-renewal division, in which a stem cell replicates itself as well as producing a daughter cell that differentiates into tissue-specific lineage. This process is under strict regulation by both intrinsic factors and the microenvironment and deregulation of this tightly controlled self-renewal procedure, for example, via accumulation of mutations in key signaling pathways, may represent early events leading to stem cell expansion and tumorigenesis. In fact, recent progress in the field of stem cell research points to the importance of stem cells in the initiation and maintenance of many cancers, including leukemia, sarcomas, blastomas and carcinomas [42–46]. However, for many types of cancer, the cell(s) of origin - i.e. the cell(s) that first acquires the genetic mutation and undergoes initial expansion to form a tumor - remain unknown. Evidence is accumulating that embryonic Schwann cells or their progenitors are the cells of origin of neurofibromas, a complex tumor associated with Neurofibromatosis Type 1 (NF1) [47–53]. NF1 is among the most common human genetic diseases, and it is caused by mutation in the NF1 tumor suppressor gene, which encodes a GTPase Activating Protein (GAP) that negatively regulates p21-RAS signaling [54, 55]. Cutaneous or dermal neurofibromas are the most common tumor in NF1. They appear at puberty and are formed exclusively in the skin. They occur as a result of proliferation of Schwann cells an all supporting elements of the nerve fibers. Recently, we identified SKPs as the cell of origin of NF1-associated dermal neurofibromas and generated a novel mouse model for this complex cutaneous tumor [29]. We showed that NF1-deficient SKPs can give rise to classic dermal neurofibromas contingent on their local microenvironment and exhibit the same properties as the embryonic Schwann cell progenitors that give rise to plexiform neurofibromas [29]. These studies provide evidence that SKPs, or their derivatives, can be the cell of origin of dermal neurofibromas and that multiple additional signals from non-neoplastic cells in the tumor microenvironment, including the nerve microenvironment, play essential roles in dermal neurofibroma formation.
Summary
In conclusion, SKPs are multipotent neural crest-related and possibly -derived precursors in the skin that play an important role in cutaneous nerve homeostasis. Under physiologic condition, SKPs maybe an alternative source of progenitors for cutaneous nerve development and regeneration. However, under NF1 pathologic condition, SKPs maybe the cell of origin of the tumor dermal neurofibroma (Figure 6). Further elucidating the biology of SKPs will give us a better understanding of cutaneous nerve homeostasis and its associated diseases, including peripheral neuropathy, pruritus, pain as well as the pathogenesis of neurofibromatosis.
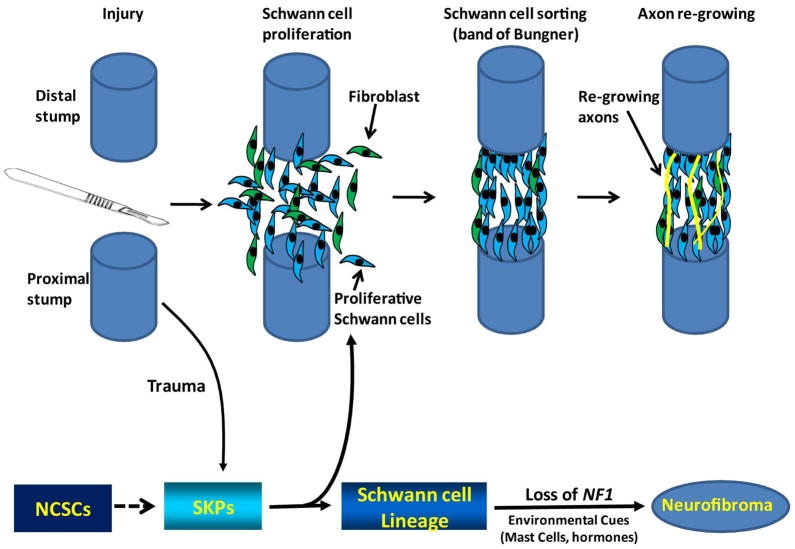
Figure 6. Physiologic and pathophysiologic roles of SKPs in the skin.
SKPs are multipotent neural crest stem cell (NCSC)-related precursors in the skin that may play important roles in cutaneous nerve homeostasis. Under physiologic conditions, cutaneous nerve transection may recruit neighboring SKPs into the injured sites and promote the differentiation of SKPs into Schwann cells. The proliferating Schwann cells also secrete chemoattractant factors that recruit other cell types, including fibroblasts that undergo cell sorting, where Schwann cells and fibroblasts grow in ordered columns along the endoneurial tube creating cellular corridors, known as “bands of Bungner”, to guide regenerating axons across the injured gaps [2, 3]. On the other hand, under NF1 pathologic condition, neurons/trauma may produce “physiologic factors” that preferentially induce SKPs to differentiate toward the Schwann cell lineage. During Schwann cell differentiation, Loss of Heterozygosity of NF1 expression in the SKPs or early Schwann cell differentiation, in addition to other microenvironmental cues (such as hormones, neurons, inflammation, etc.), leads to neurofibroma formation [29]. This is in alignment with the human clinical scenario, because it is known that trauma to the skin of NF1 patients can induce dermal neurofibroma formation [56].
Acknowledgments
We thank all members of the Le lab for helpful suggestions and discussions and P.R. Bergstresser for critical review of the manuscript. L.Q. Le holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. This work is partially supported by funding from the Dermatology Foundation, Disease-Oriented Clinical Scholar Program, National Cancer Institute of the National Institutes of Health Grant# R01 CA166593 and U.S. Department of Defense Grant# W81XWH-12-1-0161 to L.Q. Le. L.Q. Le is grateful to L.F. Parada and K.B. Yancey (UT Southwestern Medical Center) for mentorship and career guidance.
Footnotes
Author contributions: L.L.: conception and experimental designs, data collection and/or assembly, data analysis and interpretation, manuscript writing and final approval of manuscript; Z.C. and S.P.: experimental designs, data collection and/or assembly, data analysis and interpretation, manuscript writing; C.L.: data collection; Z.C. and S.P. contributed equally to this article.
Disclosure of potential conflicts of interest.
The authors have no potential conflicts of interest.
References
- 1.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Parrinello S, Napoli I, Ribeiro S, et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zochodne DW. Neurobiology of Peripheral Nerve Regeneration. New York: Cambridge University Press; 2008. [Google Scholar]
- 4.Ringkamp M, Schepers RJ, Shimada SG, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosemberg S, Marie SK, Kliemann S. Congenital insensitivity to pain with anhidrosis (hereditary sensory and autonomic neuropathy type IV) Pediatr Neurol. 1994;11:50–56. doi: 10.1016/0887-8994(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 6.Scollard DM. The biology of nerve injury in leprosy. Lepr Rev. 2008;79:242–253. [PubMed] [Google Scholar]
- 7.Yasuda H, Terada M, Maeda K, et al. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69:229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen YY, McDonald D, Cheng C, et al. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64:613–622. doi: 10.1097/01.jnen.0000171650.94341.46. [DOI] [PubMed] [Google Scholar]
- 9.Webber C, Zochodne D. The nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51–59. doi: 10.1016/j.expneurol.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 11.Tofaris GK, Patterson PH, Jessen KR, et al. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherer SS, Salzer JL. Axon-Schwann Cell Interactions during Peripheral Nerve Degeneration and Regeneration. Oxford: Oxford University Press; 2001. [Google Scholar]
- 13.Morrison SJ, White PM, Zock C, et al. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 14.Hagedorn L, Suter U, Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development. 1999;126:3781–3794. doi: 10.1242/dev.126.17.3781. [DOI] [PubMed] [Google Scholar]
- 15.Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 16.Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes KJ, Kobayashi NR, Gallagher CJ, et al. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. doi: 10.1016/j.expneurol.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie IA, Biernaskie J, Toma JG, et al. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 20.Singh RP, Cheng YH, Nelson P, et al. Retentive multipotency of adult dorsal root ganglia stem cells. Cell Transplant. 2009;18:55–68. doi: 10.3727/096368909788237177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amoh Y, Li L, Campillo R, et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005;102:17734–17738. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amoh Y, Li L, Katsuoka K, et al. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005;102:5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchugonova A, Duong J, Zhang N, et al. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–2050. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- 24.Biernaskie J, Paris M, Morozova O, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Fukunaga-Kalabis M, Yu H, et al. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2010;123:853–860. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zabierowski SE, Fukunaga-Kalabis M, Li L, et al. Dermis-derived stem cells: a source of epidermal melanocytes and melanoma? Pigment Cell Melanoma Res. 2011;24:422–429. doi: 10.1111/j.1755-148X.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 27.Biernaskie J, Sparling JS, Liu J, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh SK, Gordon T, Addas BM, et al. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp Neurol. 2010;223:221–228. doi: 10.1016/j.expneurol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Le LQ, Shipman T, Burns DK, et al. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 31.Biernaskie JA, McKenzie IA, Toma JG, et al. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803–2812. doi: 10.1038/nprot.2006.422. [DOI] [PubMed] [Google Scholar]
- 32.MacNeil S, Shepherd J, Smith L. Production of tissue-engineered skin and oral mucosa for clinical and experimental use. Methods Mol Biol. 2011;695:129–153. doi: 10.1007/978-1-60761-984-0_9. [DOI] [PubMed] [Google Scholar]
- 33.Margulis A, Zhang W, Garlick JA. In vitro fabrication of engineered human skin. Methods Mol Biol. 2005;289:61–70. doi: 10.1385/1-59259-830-7:061. [DOI] [PubMed] [Google Scholar]
- 34.Prophet E, Mills B, Arrington J, et al., editors. Laboratory Methods in Histotechnology. Washington, D.C: American Registry of Pathology; 1992. [Google Scholar]
- 35.Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 36.Griffin JW, Pan B, Polley MA, et al. Measuring nerve regeneration in the mouse. Exp Neurol. 2010;223:60–71. doi: 10.1016/j.expneurol.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 37.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 38.Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56:1590–1605. doi: 10.1002/glia.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008;363:185–198. doi: 10.1098/rstb.2006.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aitken JT, Sharman M, Young JZ. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947;81:1–22. 22. [PubMed] [Google Scholar]
- 41.Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 42.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 43.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 44.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 45.Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756:25–52. doi: 10.1016/j.bbcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Gao JX. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2008;12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Ghosh P, Charnay P, et al. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng H, Chang L, Patel N, et al. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Williams JP, Rizvi TA, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph NM, Mosher JT, Buchstaller J, et al. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 2008;13:129–140. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cichowski K, Shih TS, Schmitt E, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 52.Vogel KS, Klesse LJ, Velasco-Miguel S, et al. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le LQ, Liu C, Shipman T, et al. Susceptible stages in Schwann cells for NF1-associated plexiform neurofibroma development. Cancer Res. 2011;71:4686–4695. doi: 10.1158/0008-5472.CAN-10-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballester R, Marchuk D, Boguski M, et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63:851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- 55.Xu GF, O’Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 56.Riccardi VM. Cutaneous manifestation of neurofibromatosis: cellular interaction, pigmentation, and mast cells. Birth Defects Orig Artic Ser. 1981;17:129–145. [PubMed] [Google Scholar]