Abstract
We have used quantitative autoradiography to localize in rat brain beta 1- and beta 2-adrenergic receptors. These receptors were labeled in vitro with 125I-labeled pindolol, an antagonist of beta-adrenergic receptors that binds nonselectively to both beta 1 and beta 2 subtypes. The selective inhibition of 125I-labeled pindolol binding with specific antagonists of beta 1 and beta 2 receptors allowed the visualization of beta-adrenergic receptor subtypes. High levels of beta 1 receptors were observed in the cingulate cortex, layers I and II of the cerebral cortex, the hippocampus, the Islands of Calleja, and the gelatinosus, mediodorsal, and ventral nuclei of the thalamus. High levels of beta 2 receptors were found in the molecular layer of the cerebellum, over pia mater, and in the central, paraventricular, and caudal lateral posterior thalamic nuclei. Approximately equal levels of beta 1 and beta 2 receptors occurred in the substantia nigra, the olfactory tubercle, layer IV of the cerebral cortex, the medial preoptic nucleus, and all nuclei of the medulla. The pronounced differences in the ratio of beta 1 to beta 2 receptors among brain regions suggests that the subtypes of beta-adrenergic receptors may play different roles in neuronal function.
Full text
PDF
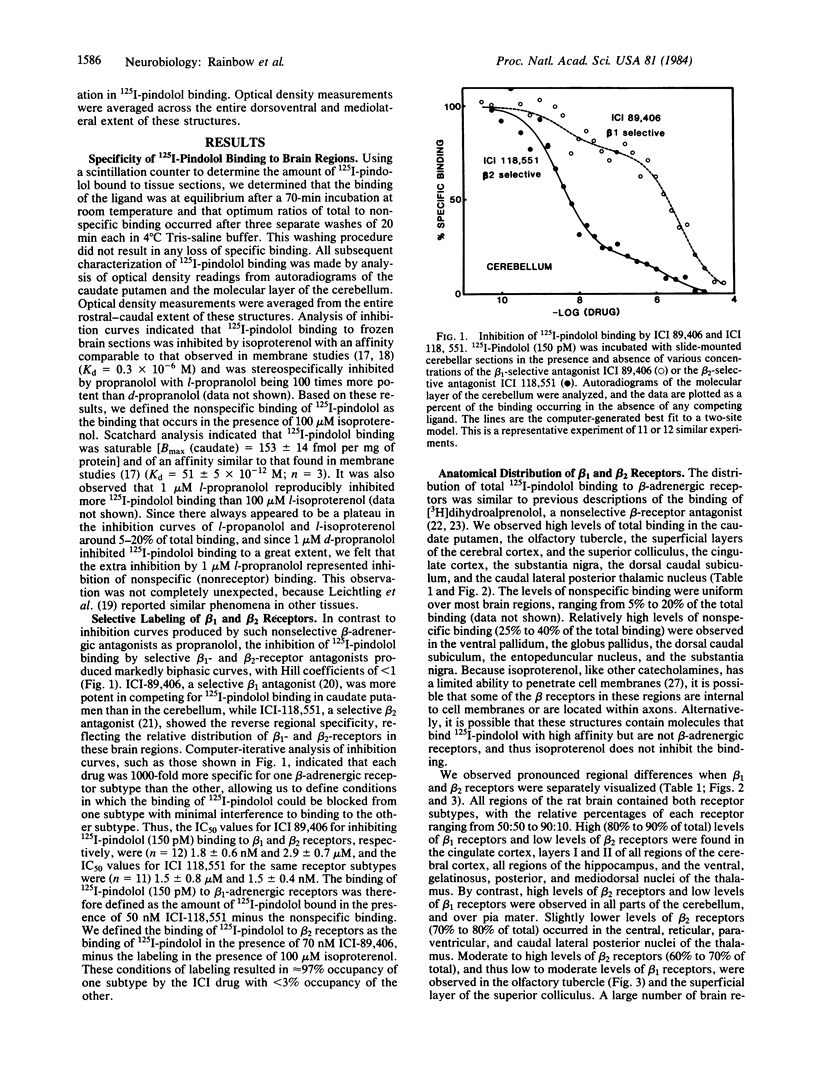
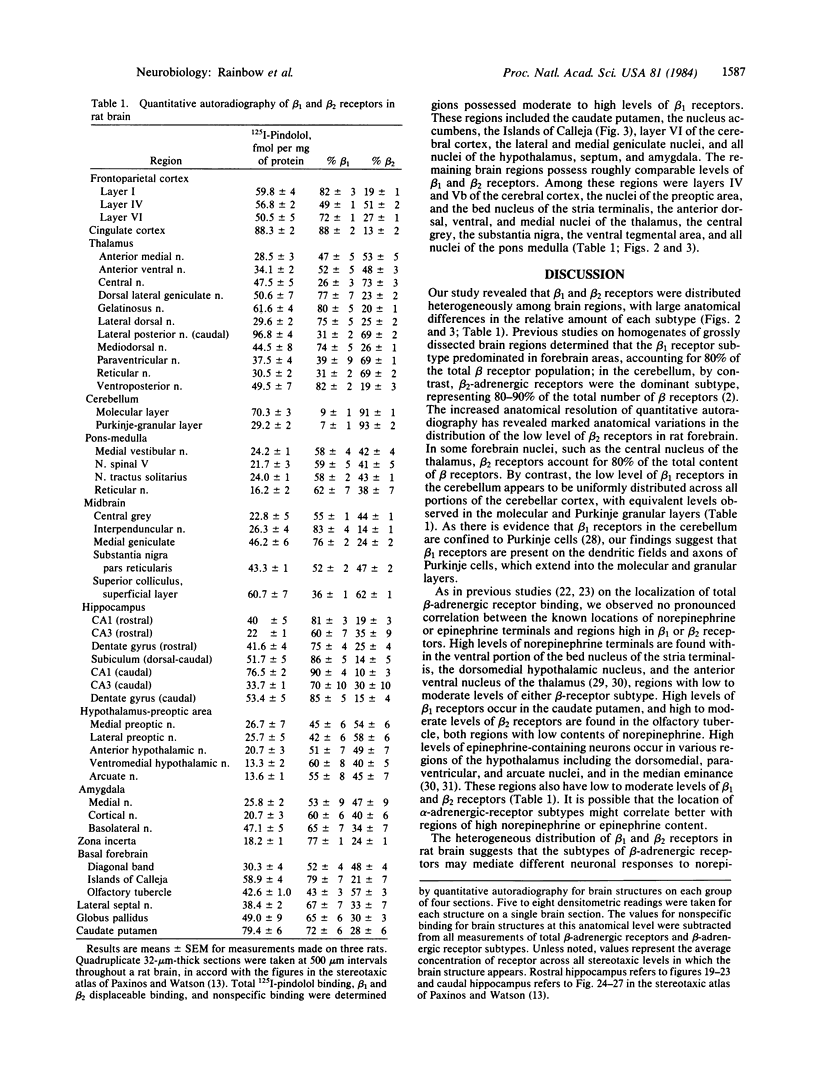
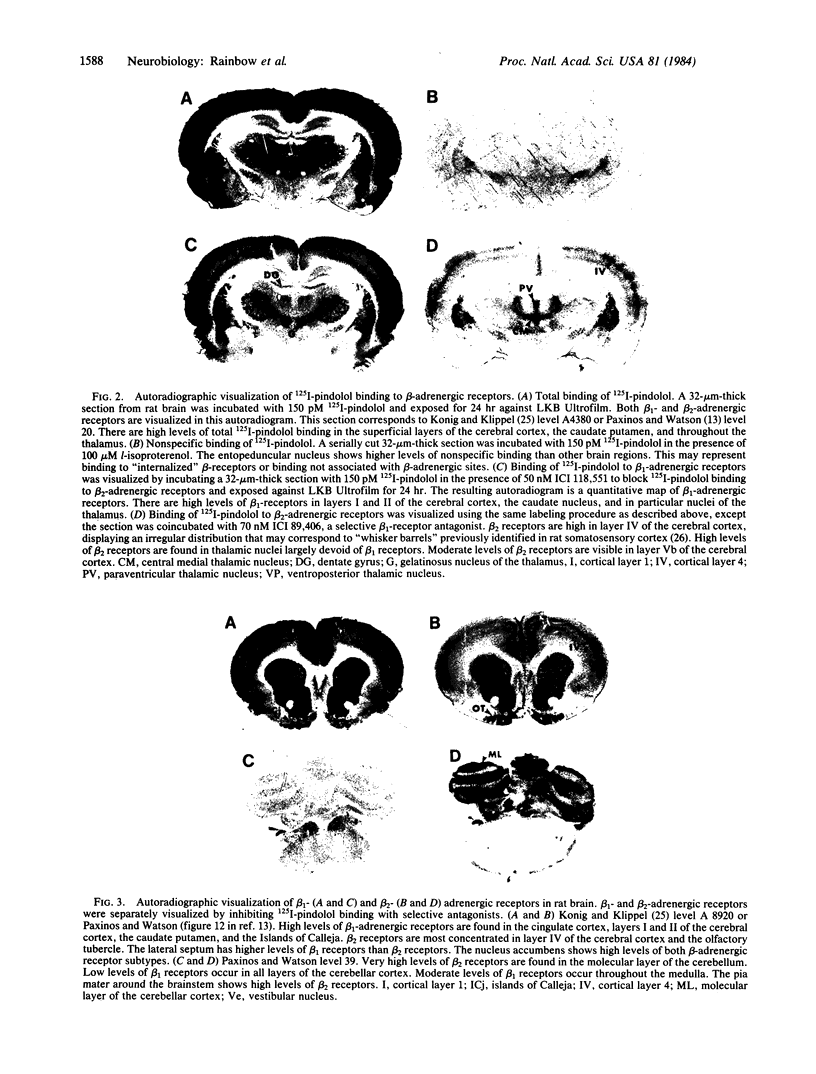

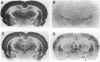
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barovsky K., Brooker G. (-)-[125I]-iodopindolol, a new highly selective radioiodinated beta-adrenergic receptor antagonist: measurement of beta-receptors on intact rat astrocytoma cells. J Cyclic Nucleotide Res. 1980;6(4):297–307. [PubMed] [Google Scholar]
- Durham D., Woolsey T. A. Barrels and columnar cortical organization: evidence from 2-deoxyglucose (2-DG) experiments. Brain Res. 1977 Nov 25;137(1):169–174. doi: 10.1016/0006-8993(77)91022-8. [DOI] [PubMed] [Google Scholar]
- Engel G., Hoyer D., Berthold R., Wagner H. (+/-)[125Iodo] cyanopindolol, a new ligand for beta-adrenoceptors: identification and quantitation of subclasses of beta-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1981;317(4):277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- Hancock A. A., DeLean A. L., Lefkowitz R. J. Quantitative resolution of beta-adrenergic receptor subtypes by selective ligand binding: application of a computerized model fitting technique. Mol Pharmacol. 1979 Jul;16(1):1–9. [PubMed] [Google Scholar]
- Hegstrand L. R., Minneman K. P., Molinoff P. B. Multiple effects of guanosine triphosphate on beta adrenergic receptors and adenylate cyclase activity in rat heart, lung and brain. J Pharmacol Exp Ther. 1979 Aug;210(2):215–221. [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Leichtling B. H., Su Y. F., Wimalasena J., Harden T. K., Wolfe B. B., Wicks W. D. Studies of cAMP metabolism in cultured hepatoma cells: presence of functional adenylate cyclase despite low cAMP content and lack of hormonal responsiveness. J Cell Physiol. 1978 Aug;96(2):215–223. doi: 10.1002/jcp.1040960210. [DOI] [PubMed] [Google Scholar]
- Mattsson H., Andersson T., Carlsson E., Hedberg A., Lundgren B., Olsson T. beta 1-and beta 2-adrenoceptor stimulatory effects of prenalterol. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(4):302–308. doi: 10.1007/BF00498518. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Dibner M. D., Wolfe B. B., Molinoff P. B. beta1- and beta2-Adrenergic receptors in rat cerebral cortex are independently regulated. Science. 1979 May 25;204(4395):866–868. doi: 10.1126/science.35829. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Hedberg A., Molinoff P. B. Comparison of beta adrenergic receptor subtypes in mammalian tissues. J Pharmacol Exp Ther. 1979 Dec;211(3):502–508. [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. Simultaneous determination of beta-1 and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol Pharmacol. 1979 Jul;16(1):34–46. [PubMed] [Google Scholar]
- Minneman K. P., Pittman R. N., Yeh H. H., Woodward D. J., Wolfe B. B., Molinoff P. B. Selective survival of beta 1-adenergic receptors in rat cerebellum following neonatal x-irradiation. Brain Res. 1981 Mar 23;209(1):25–34. doi: 10.1016/0006-8993(81)91169-0. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Wolfe B. B., Molinoff P. B. Selective changes in the density of beta 1-adrenergic receptors in rat striatum following chronic drug treatment and adrenalectomy. Brain Res. 1982 Dec 9;252(2):309–314. doi: 10.1016/0006-8993(82)90398-5. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Kuhar M. J. Beta-adrenergic-receptor localization by light microscopic autoradiography. Science. 1980 Jun 20;208(4450):1378–1380. doi: 10.1126/science.6246585. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Niehoff D. L., Kuhar M. J. Receptor autoradiography with tritium-sensitive film: potential for computerized densitometry. Neurosci Lett. 1981 Sep 1;25(2):101–105. doi: 10.1016/0304-3940(81)90315-3. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Pan H. S., Young A. B., Frey K. A., Dauth G. W. Quantitative autoradiography of [3H]muscimol binding in rat brain. Science. 1981 Nov 27;214(4524):1036–1038. doi: 10.1126/science.6272394. [DOI] [PubMed] [Google Scholar]
- Quirion R., Hammer R. P., Jr, Herkenham M., Pert C. B. Phencyclidine (angel dust)/sigma "opiate" receptor: visualization by tritium-sensitive film. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5881–5885. doi: 10.1073/pnas.78.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow T. C., Bleisch W. V., Biegon A., McEwen B. S. Quantitative densitometry of neurotransmitter receptors. J Neurosci Methods. 1982 Jan;5(1-2):127–138. doi: 10.1016/0165-0270(82)90059-0. [DOI] [PubMed] [Google Scholar]
- Reivich M., Jehle J., Sokoloff L., Kety S. S. Measurement of regional cerebral blood flow with antipyrine-14C in awake cats. J Appl Physiol. 1969 Aug;27(2):296–300. doi: 10.1152/jappl.1969.27.2.296. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Palkovits M., Brownstein M. J., Axelrod J. Localisation of phenylethanolamine N-methyl transferase in the rat brain nuclei. Nature. 1974 Apr 19;248(5450):695–696. doi: 10.1038/248695a0. [DOI] [PubMed] [Google Scholar]
- Stiles G. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Mammalian beta-adrenergic receptors. Structural differences in beta 1 and beta 2 subtypes revealed by peptide maps. J Biol Chem. 1983 Sep 10;258(17):10689–10694. [PubMed] [Google Scholar]
- Stiles G. L., Strasser R. H., Lavin T. N., Jones L. R., Caron M. G., Lefkowitz R. J. The cardiac beta-adrenergic receptor. Structural similarities of beta 1 and beta 2 receptor subtypes demonstrated by photoaffinity labeling. J Biol Chem. 1983 Jul 10;258(13):8443–8449. [PubMed] [Google Scholar]
- Swanson L. W., Hartman B. K. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975 Oct 15;163(4):467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Niehoff D. L., Kuhar M. J., Palacios J. M. Quantitative receptor autoradiography using [3H]ultrofilm: application to multiple benzodiazepine receptors. J Neurosci Methods. 1982 Jul;6(1-2):59–73. doi: 10.1016/0165-0270(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K. Guanine nucleotides modulate the affinity of antagonists at beta-adrenergic receptors. J Cyclic Nucleotide Res. 1981;7(5):303–312. [PubMed] [Google Scholar]
- Wolfe B. B., Minneman K. P., Molinoff P. B. Selective increases in the density of cerebellar beta-1-adrenergic receptors. Brain Res. 1982 Feb 25;234(2):474–479. doi: 10.1016/0006-8993(82)90890-3. [DOI] [PubMed] [Google Scholar]
- Yeh H. H., Woodward D. J. Beta-1 adrenergic receptors mediate noradrenergic facilitation of Purkinje cell responses to gamma-aminobutyric acid in cerebellum of rat. Neuropharmacology. 1983 May;22(5):629–639. doi: 10.1016/0028-3908(83)90155-7. [DOI] [PubMed] [Google Scholar]