Abstract
Two principal neurotransmitters are involved in the regulation of mammalian neuronal activity, namely, γ-aminobutyric acid (GABA), an inhibitory neurotransmitter, and L-glutamic acid, an excitatory neurotransmitter. Low GABA levels in the brain have been implicated in epilepsy and several other neurological diseases. Because of GABA’s poor ability to cross the blood-brain barrier (BBB), a successful strategy to raise brain GABA concentrations is the use of a compound that does cross the BBB and inhibits or inactivates GABA aminotransferase (GABA-AT), the enzyme responsible for GABA catabolism. Vigabatrin, a mechanism-based inactivator of GABA-AT, is currently a successful therapeutic for epilepsy, but has harmful side effects, leaving a need for improved GABA-AT inactivators. Here, we report the synthesis and evaluation of a series of heteroaromatic GABA analogues as substrates of GABA-AT, which will be used as the basis for the design of novel enzyme inactivators.
Keywords: γ-aminobutyric acid, GABA aminotransferase, enzyme inhibition, synthetic substrates, heteroaromatic compounds
1. Introduction
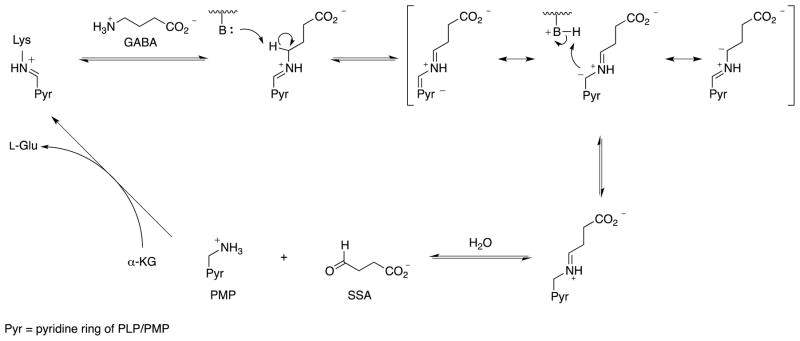
The two major neurotransmitters involved in the regulation of brain neuronal activity are γ-aminobutyric acid (GABA), an inhibitory neurotransmitter, and L-glutamic acid, an excitatory neurotransmitter.1 GABA concentration is regulated by two pyridoxal 5′-phosphate (PLP)-dependent enzymes: L-glutamic acid decarboxylase (GAD), which catalyzes the conversion of L-glutamate to GABA, and GABA aminotransferase (GABA-AT), which degrades GABA to succinic semialdehyde (SSA). GABA-AT catabolizes GABA via transamination while converting PLP to pyridoxamine 5′-phosphate (PMP), which is restored to PLP by conversion of α-ketoglutarate to L-glutamate (GABA-AT, E.C. 2.6.1.19; Scheme 1).2 When the concentration of GABA diminishes below a certain level in the brain, convulsions result; raising the brain GABA levels terminates the seizure.3–5 A reduction in the concentrations of GABA and of the enzyme GAD has been implicated not only in the symptoms associated with epilepsy6,7 but also with several other neurological diseases such as Huntington’s chorea8 Parkinson’s disease,9 Alzheimer’s disease,10 and tardive dyskinesia.11 GABA brain levels cannot be increased by direct administration of GABA, however, because it does not cross the blood-brain barrier (BBB). A strategy that has been effective in raising brain GABA concentrations is the use of a compound that crosses the blood-brain barrier and inhibits or inactivates GABA-AT.
Scheme 1.
Catalytic mechanism for GABA-AT.
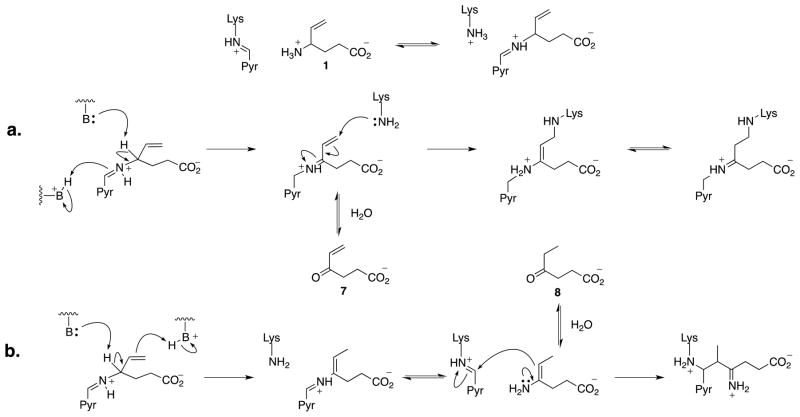
The FDA approved a selective mechanism-based inactivator12,13 of GABA-AT, vigabatrin (1; 4-aminohex-5-enoic acid; γ-vinylGABA), which is currently marketed for the treatment of epilepsy.14–18 In addition, vigabatrin has been shown to be effective in the treatment of addiction.19,20 Unfortunately, treatment with vigabatrin has the potential for side effects, with 25–50% of patients exhibiting irreversible abnormalities in peripherial vision following chronic administration.21 Vigabatrin also suffers from low potency (KI = 10 mM)14 and poor BBB permeability, resulting in doses of 1–3 g/day, leaving a clear need for improved GABA-AT inactivators.12,13,22 Vigabatrin was determined to inactivate GABA-AT through two mechanistic pathways (Scheme 2): a Michael addition mechanism (pathway a) and an enamine mechanism (pathway b).23 Both inactivation pathways have the possibility of generating potentially bioactive side products (7 and 8) via hydrolysis prior to inactivation. These side products could be related to vigabatrin’s side effects; however, the biochemical mechanisms underlying them are still currently unknown.24
Scheme 2.
Mechanisms of inactivation of GABA-AT by vigabatrin (1). Pyr stands for the pyridoxal 5′-phosphate ring system.
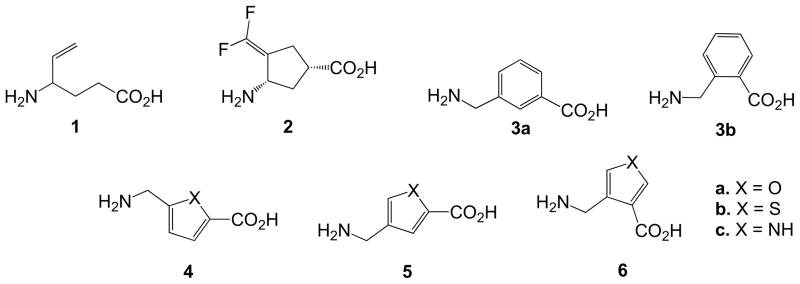
Previously, in an effort to increase potency and eliminate the generation of side products, conformationally restricted analogues of vigabatrin were synthesized and evaluated as potential inactivators of GABA-AT.25–29 Of those tested, 2 was found to be a very potent GABA-AT inactivator with a potency that is 187 times greater than that of vigabatrin.26 Because of the low lipophilicity of this amino acid compound, a series of benzene-containing amino acid analogues of GABA (3, Figure 1) were evaluated as substrates that could be used as the basis for the development of inactivators with improved lipophilicity30 and, possibly, bioavailability, and it was found that some of these compounds were substrates of GABA-AT.31 On the basis of this result, the remarkable potency of 2, and the generally better activity observed for exocyclic-functionalized cyclopentyl analogues over cyclohexyl analogues,27,32 a series of 5-membered heteroaromatic analogues was designed from structures 3 as potential substrates for GABA-AT (4–6, Figure 1). Because mechanism-based inactivators must undergo a catalytic transformation by the enzyme to a species that inactivates the enzyme,13 identification of substrates is imperative for the development of new inactivator scaffolds. Here we report the synthesis and evaluation of 4–6 as substrates and inhibitors of GABA-AT, the first step toward the design of mechanism-based inactivators.
Figure 1.
Previously described GABA-AT inactivators (1 and 2), aromatic substrates (3), and the new series of heteroaromatic analogues (4–6).
2. Results and Discussion
2.1. Chemistry
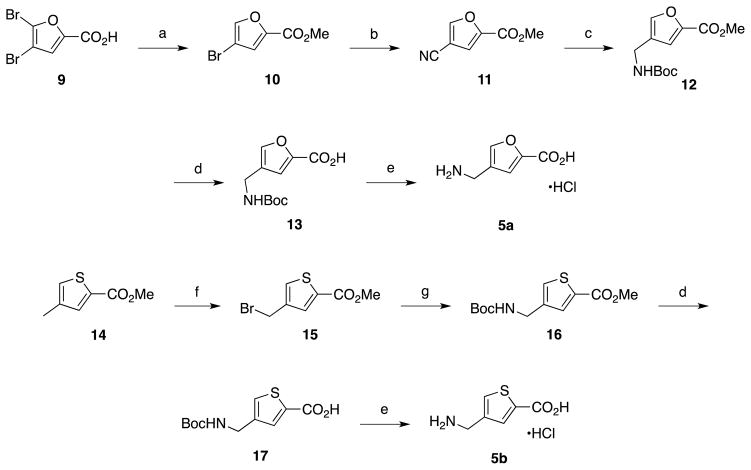
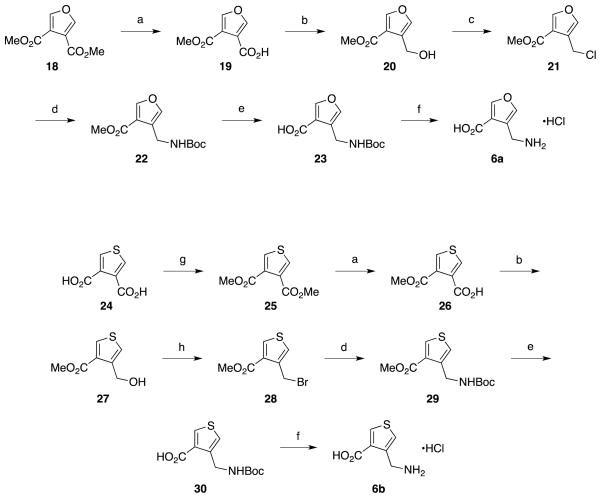
Compounds 4a and 4b were commercially available but were converted to their corresponding hydrochloride salts prior to analysis. Compounds 5a and 5b were not commercially available and were synthesized as shown in Scheme 3. The synthesis of 5a began with 4,5-dibromofuran-2-carboxylic acid (7). Selective dehalogenation of 9 using zinc metal, followed by esterification, provided 10 in good yields. Palladium-catalyzed cyanation of 10 generated nitrile 11, which was then reduced and Boc protected to yield 12. Saponification of the ester followed by Boc deprotection of 12 gave the desired amino acid product (5a) as the hydrochloride salt. Compound 5b was synthesized starting with methyl 4-methylthiophene-2-carboxylic acid (14). Radical bromination of 14 provided bromomethyl intermediate 15 in good yields.33 Displacement of the bromide with azide followed by catalytic hydrogenation and Boc protection provided 16. Saponification of the ester followed by Boc deprotection yielded the desired amino acid (5b) as the hydrochloride salt.
Scheme 3.
Synthesis of the 2,4-disubstituted furan and thiophene amino acids. Reagents and conditions: (a) i) Zn (s), NH4OH, H2O, ii) H2SO4, MeOH, reflux; b) Pd(PPh3)4, Zn(CN)2, DMF; c) Boc2O, NaBH4, NiCl2·6H2O (cat.), MeOH; d) LiOH·H2O, MeOH, H2O; e) 6 N HCl (aq.); f) NBS, benzoyl peroxide, CCl4; g) i) NaN3, DMF, ii) Boc2O, H2 (g), Pd-C, EtOAc.
The 3,4-disubstituted furan and thiophene analogues (6a and 6b) were synthesized as shown in Scheme 4. Synthesis of 6a began with dimethyl ester 18. Monosaponification provided the ester-acid 19. Selective reduction of the carboxylic acid gave alcohol 2034 that was then converted to the chloromethyl intermediate 21. Substitution of the chloride with azide followed by catalytic hydrogenation and Boc protection generated 22. Saponification of the ester followed by Boc deprotection yielded the desired amino acid (6a) as the hydrochloride salt. Compound 6b was synthesized starting with the bis-esterification of 3,4-thiophenedicarboxylic acid (24) followed by a synthetic route similar to the one used to generate 6a.
Scheme 4.
Synthesis of 3,4-disubstituted furan and thiophene amino acids. Reagents and conditions: a) 1 eq. NaOH, MeOH; b) BH3·THF, THF; c) PPh3, CCl4, CH2Cl2; d) i)NaN3, DMF, ii) Boc2O, H2 (g), Pd-C (cat.), EtOAc; e) LiOH· H2O, MeOH, H2O; f) 6 N HCl (aq); g) H2SO4, MeOH; h) PPh3, CBr4, CH2Cl2.
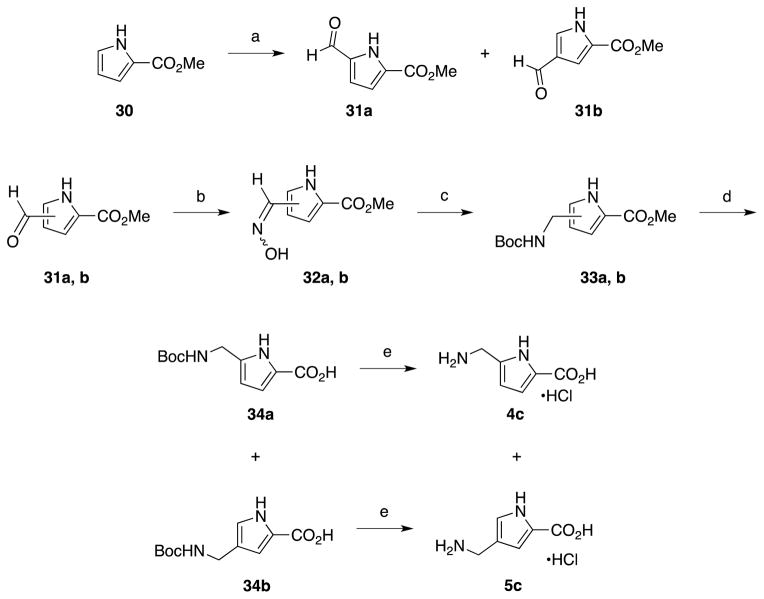
The pyrrole amino acids (4c and 5c) were synthesized in parallel from a common precursor (30) as shown in Scheme 5. Vilsmeier formylation of 30 generated aldehydes 31a and 31b as a mixture that was readily separated with flash chromatography. Treatment of either with hydroxylamine provided the corresponding aldoximes (32a and b).35 Catalytic hydrogenation of the aldoximes followed by Boc protection gave 33a or b. Saponification of the ester36 followed by Boc deprotection provided desired products 4c and 5c as hydrochloride salts.
Scheme 5.
Synthesis of 2,4- and 2,5-disubstituted pyrrole amino acids. Reagents and conditions: a) POCl3, DMF, CH2Cl2; b) NH2OH·HCl, K2CO3, H2O; c) Boc2O, H2 (g), Pd-C (cat.), EtOAc; d) LiOH·H2O, MeOH, H2O; e) 6 N HCl (aq).
Despite having minimal literature precedent, the synthesis of 3,4-substituted pyrrole 6c was attempted but initial efforts were unsuccessful. This was attributed to the instability of this substitution pattern, leading to decomposition under ambient conditions, and was not pursued further.
2.2. Enzymatic Assay Results
All of the synthesized compounds (4–6) were tested as competitive inhibitors of GABA-AT using a coupled enzyme assay.37 None of the compounds, at 5 mM concentration, inhibited any of the coupling enzymes in the assays, namely, L-glutamate oxidase, horseradish peroxidase, and succinic semialdehyde dehydrogenase. All of the compounds have Ki values (Table 1) similar to the Km of GABA, which suggests binding to the active site with similar affinity. Next, the compounds were evaluated as substrates, with kinetic constants Km and kcat determined by Hanes and Woolf plots (kinetic constants determined by direct Michaelis-Menten fitting were not significantly different).38,39 Compounds 4a, 5b, 5c, and 6a exhibit Km values similar to GABA while the Km values of compounds 4b and 4c were much higher. Transamination was observed for 6b indicating substrate activity; however, a Km could not be accurately determined. Compound 5a presented the lowest Km at 0.31 ± 0.07 mM, approximately 4-fold lower than that of GABA. All of the compounds presented very slow turnover rates, having kcat values that are orders of magnitude lower than that of GABA. Compound 4a has the highest kcat/Km value indicating it is the most efficient GABA-AT substrate of the series. The clogP values, a measure of lipophilicity, indicates that almost all of the compounds are more lipophilic than 1 and 2, but not as lipophilic as 3a.
Table 1.
Kinetic constants for substrate activity and inhibition of GABA-AT for 4–6 and GABA.
| Compound | Ki (mM) | Km (mM) | kcat (min−1) | kcat/Km (min−1/mM−1) | clogP |
|---|---|---|---|---|---|
| 4a | 1.40 ± 0.14 | 2.1 ± 0.1 | 3.4×10−2 ± 4×10−3 | 0.016 | 0.19 |
| 4b | 2.49 ± 0.28 | 6.3 ± 0.2 | 1.0×10−2 ± 3×10−3 | 0.0016 | 0.83 |
| 4c | 2.14 ± 0.31 | 9.5 ± 0.3 | 1.5×10−3 ± 2×10−4 | 0.00016 | 0.09 |
| 5a | 1.64 ± 0.44 | 0.31 ± 0.07 | 2.3×10−3 ± 1×10−4 | 0.0074 | −0.12 |
| 5b | 1.35 ± 0.08 | 1.7 ± 0.3 | 5.2×10−3 ± 5×10−4 | 0.00031 | 0.52 |
| 5c | 1.59 ± 0.14 | 1.4 ± 0.3 | 3.4×10−3 ± 3×10−4 | 0.0024 | −0.22 |
| 6a | 2.27 ± 0.51 | 2.8 ± 0.5 | 8.3×10−4 ± 5×10−5 | 0.00030 | −0.43 |
| 6b | 2.86 ± 0.51 | > 10 | n.d.a | n.d.a | 0.21 |
| GABA | - | 1.3 ± 0.3 | 49 ± 5 | 38.6 |
clogP, the calculated logP, was determined using molinspiration.com software. The clogP for 1 was −0.39, for 2 was −0.37, and for 3a was 1.01.
n.d. = not determined
The normal catalytic mechanism of GABA-AT involves Schiff base formation between GABA and the lysine-bound PLP coenzyme followed by active-site base removal of the γ-proton to form a resonance-stabilized carbanion (Scheme 1). Reprotonation on the coenzyme followed by hydrolysis provides SSA and PMP, which is converted back to PLP with conversion of α-ketoglutarate to L-glutamate. Despite having Ki values that suggest similar active-site binding affinity, all compounds exhibited markedly low substrate activity when compared with GABA. It was hypothesized that their rigid structure may require a binding conformation that orients the requisite proton unfavorably relative to the active-site base responsible for deprotonation, the rate-determining step for turnover of GABA,40 and therefore decreasing turnover rates.
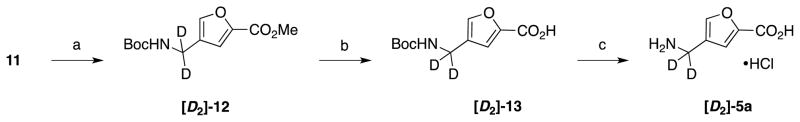
To identify the rate-determining step for turnover of these analogues, a kinetic isotope effect experiment was conducted using deuterium labeled 5a, the analogue exhibiting the lowest Km value of the series. The labeled analogue ([D2]-5a) was synthesized from 11 (Scheme 3) as shown in Scheme 6, and evaluated as a substrate alongside unlabeled 5a. The Hk/Dk value for substrate turnover was determined to be 1.22 ± 0.07, which is considerably lower than would be expected for a primary isotope effect if deprotonation were rate limiting.41 Alternatively, a Hk/Dk of 1.22 ± 0.07 is suggestive of a secondary kinetic isotope effect and is typically observed with a change in hydridization α- or β- to the heavy atom label, with a Hk/Dk > 1 indicative of a transition from sp3 to sp2 (or sp2 to sp) hybridization.
Scheme 6.
Synthesis of deuterated 5a. Reagents and conditions: a) Boc2O, NaBD4, NiCl2·6H2O (cat.), MeOH; b) LiOH·H2O, MeOH, H2O; c) 6 N HCl (aq.).
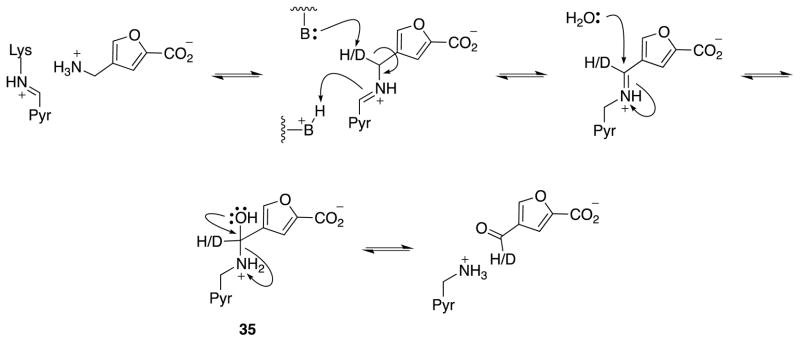
In the catalytic mechanism of GABA-AT with substrate GABA, and presumably with this series of compounds, product release is achieved by hydrolysis of the PMP-product Schiff base formed after transamination. Hydrolysis of Schiff bases is known to proceed through the formation of a hemiaminal intermediate followed by elimination of the amine to form a carbonyl (Scheme 7). Hemiaminals, like hemiacetals, are known to be stabilized by electron-withdrawing substituents.42–46 Hemiaminals have also been shown to be stabilized by both intermolecular and intramolecular hydrogen bonding.47–50 The hemiaminal formed during product hydrolysis (35, Scheme 7) is stabilized by the electron-withdrawing nature of the heterocyles and potentially favorable interactions within the active site. On the basis of the secondary isotope effect observed for [D2]-5a, and the known stability of aryl substituted hemiaminals, the breakdown of this intermediate may be rate limiting, leading to a normal secondary isotope effect (change in hybridization from sp3 to sp2), which may supercede the expected primary isotope effect from C-H(D) bond cleavage.51 While these results are intriguing and somewhat unexpected, further work would be required to truly identify the substrate mechanism for these analogs; it is not unreasonable that the isotope effect is associated with a nonchemical step. Ultimately, for a compound to be an effective mechanism-based inactivator of an enzyme, large rate constants (kinact) are not necessary. Therefore, we are currently working on the syntheses of potential mechanism-based inactivators using the structure of the substrates identified in this study as the basis for design.
Scheme 7.
Presumed substrate mechanism of 5a or [D2]-5a.
3. Conclusion
A series of heteroaromatic compounds were designed as potential substrates and inhibitors of GABA-AT. All analogues were found to act as competitive inhibitors having Ki values near the Km of GABA, suggesting similar active site binding affinity. In addition, all analogues were observed to act as substrates; however, turnover rates were found to be much lower than that of GABA. An isotope effect experiment with one of the analogues suggests that the hemiaminal formed during hydrolysis of the Schiff base between the product and coenzyme PMP is rate limiting as a result of increased stabilization by the heterocycle. These results are intriguing because, as shown previously in Scheme 2, vigabatrin has the potential to produce metabolites (7 and 8) generated from unwanted Schiff base hydrolysis prior to inactivation. The results reported here will be useful in the design of improved vigabatrin analogues, as well as other novel mechanism-based inactivators of GABA-AT, which do not generate potentially harmfully metabolites.
4. Experimental Procedures
4.1. General Methods
Analogues 4a and 4b were purchased from Matrix Scientific and ChemBridge, respectively. All syntheses were conducted under anhydrous conditions in an atmosphere of argon, using flame-dried apparatus and employing standard techniques in handling air-sensitive materials, unless otherwise noted. All solvents were distilled and stored under an argon or nitrogen atmosphere before use. All reagents were used as received. Analytical thin layer chromatography was visualized by ultraviolet light. Flash column chromatography was carried out under a positive pressure of nitrogen. 1H NMR spectra were recorded on 500 MHz spectrometers. 1H NMR and 13C NMR spectra were obtained with a Bruker AVANCE III 500 spectrometer. Data are presented as follows: chemical shift (in ppm on the δ scale relative to δ = 0.00 ppm for the protons in TMS), integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad), coupling constant (J/Hz). Coupling constants were taken directly from the spectra and are uncorrected. 13C NMR spectra were recorded at 125 MHz, and all chemical shift values are reported in ppm on the δ scale, with an internal reference of δ 77.16 or 49.0 for CDCl3 or CD3OD, respectively. High resolution mass spectra (HRMS) were measured with an Agilent 6210 LC-TOF (ESI, APCI, APPI) mass spectrometer. The purity of the synthesized final compounds was determined by HPLC analysis to be ≥ 95%. The column used was a Phenomenex Luna 5 μm 200Å, 4.6 × 250 mm. The column was thoroughly equilibrated at 100% solvent A, minimum 5 volumes. The compounds were eluted with a gradient from solvent A (90:10, CH3CN:50 mM NH4OAc pH 5.8) to solvent B (50:50, CH3CN:10 mM NH4OAc pH 5.8), 0–2.5 min, 0% B isocratic; 2.5–10 min, 0–100% B; 10–20 min, 100% B isocratic. The compounds had the following retention times: 4c, 11.57 min; 5a, 11.24 min; 5b, 11.25 min; 5c, 11.57 min; 6a, 11.20 min; 6b, 11.27 min; [D2-5a], 11.47 min.
4.2. Preparation and characterization of new compounds
4.2.1. 5-(Aminomethyl)furan-2-carboxylic acid hydrochloride (4a)
5-(Aminomethyl)furan-2-carboxylic acid (25.0 mg, 0.18 mmol) was dissolved in 2 N HCl (5 mL) followed by solvent removal under reduced pressure. This was repeated twice to yield 5-(aminomethyl)furan-2-carboxylic acid hydrochloride as an off white powder (31.5 mg, 100%). 1H NMR (500 MHz, MeOD) δ 7.22 (d, J = 3.3 Hz, 1H), 6.71 (d, J = 3.3 Hz, 1H), 4.25 (s, 2H). 13C NMR (126 MHz, MeOD) δ 161.27, 152.05, 147.49, 119.76, 113.69, 36.80. HRMS (ESI): calcd for C6H7NO3 [M-H]− 140.0353; found 140.0343
4.2.2. 5-(Aminomethyl)thiophene-2-carboxylic acid hydrochloride (4b)
5-(Aminomethyl)thiophene-2-carboxylic acid (20.0 mg, 0.13 mmol) was dissolved in 2 N HCl (5 mL) followed by solvent removal under reduced pressure. This was repeated twice to yield 5-(aminomethyl)thiophene-2-carboxylic acid hydrochloride as a light yellow powder (24.6 mg, 100%). 1H NMR (500 MHz, MeOD) δ 7.71 (d, J = 3.7 Hz, 1H), 7.26 (d, J = 3.7 Hz, 1H), 4.37 (s, 2H). 13C NMR (126 MHz, MeOD) δ 164.70, 142.48, 137.95, 134.51, 131.04, 38.68. HRMS (ESI): calcd for C6H7NO2S [M-H]− 156.0125; found 156.0122.
4.2.3. Methyl 4-bromofuran-2-carboxylate (10)
4,5-Dibromofuran-2-carboxylic acid (9, 1.00 g, 3.7 mmol) was suspended in water (11 mL) and NH4OH (3.5 mL) with vigorous stirring at ambient temperature. Powdered zinc metal (1.30 g, 20.3 mmol) was added, and the mixture was allowed to stir at ambient temperature for 3 h. The reaction mixture was filtered through a pad of Celite and acidified (pH 2) with 2 N HCl. The filtrate was extracted with EtOAc (4 × 50 mL), dried (Na2SO4), and concentrated to dryness under reduced pressure to provide 665 mg of a white powder. To this crude intermediate dissolved in MeOH (12 mL) was added concentrated sulfuric acid (80 μL) while stirring. The resulting solution was heated to reflux and stirred overnight. The reaction mixture was allowed to cool to room temperature followed by concentration under vacuum. The resulting crude residue was then partitioned between saturated aqueous NaHCO3 and diethyl ether, and the aqueous layer was further extracted with diethyl ether (2 × 40 mL). The combined ether solutions were washed with brine (20 mL), dried (Na2SO4) and concentrated to dryness under reduced pressure to provide 652 mg (3.18 mmol, 86%) of the desired product as a clear oil. 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J = 1.0 Hz, 1H), 7.18 (d, J = 1.0 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 158.05, 144.89, 144.44, 120.20, 101.18, 52.19. HRMS (ESI): calcd for C6H5BrO3 [M+Na]+ 226.9314; found 226.932.
4.2.4. Methyl 4-cyanofuran-2-carboxylate (11)
Methyl 4-bromofuran-2-carboxylate (10, 141 mg, 0.74 mmol), Pd(PPh3)4 (85 mg, 0.07 mmol), and Zn(CN)2 (52 mg, 0.44 mmol) were suspended in anhydrous DMF (5 mL) under an argon atmosphere. The resulting mixture was heated to 80 °C and stirred overnight. After cooling to room temperature the reaction mixture was partitioned between water and diethyl ether. The aqueous layer was extracted with ether (3 × 10 mL). The combined organics were washed with brine (10 mL), dried (Na2SO4), and concentrated under reduced pressure to give a yellow oil, which was chromatographed (ethyl acetate/hexanes, 1:6) to afford a white solid (85 mg, 76%). 1H NMR (500 MHz, CDCl3) δ 8.06 (d, J = 0.9 Hz, 1H), 7.33 (d, J = 0.9 Hz, 1H), 3.94 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 157.59, 151.84, 145.92, 117.76, 111.60, 100.03, 52.69. HRMS (LC-TOF): calcd for C7H5NO3 151.0269; found 151.0285.
4.2.5. Methyl 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylate (12)
Methyl 4-cyanofuran-2-carboxylate (11, 100 mg, 0.66 mmol), Boc2O (289 mg, 1.32 mmol), and NiCl2·6H2O (16 mg, 0.07 mmol) were dissolved in methanol and cooled to 0 °C with stirring. Sodium borohydride (176 mg, 4.63 mmol) was added in portions over 30 min. After addition of sodium borohydride, the reaction was allowed to warm to room temperature, and to stir for 1 h, at which point diethylenetriamine (72 μL, 0.66 mmol) was added. The mixture was allowed to stir for an additional 30 min before solvent evaporation. The residue was dissolved in EtOAc and washed with saturated aqueous NaHCO3 (2×30 mL), dried (Na2SO4), concentrated under reduced pressure, and chromatographed (ethyl acetate/hexanes, 1:5) to afford the desired product as a white solid (86 mg, 51%). 1H NMR (500 MHz, CDCl3) δ 7.47 (s, 1H), 7.12 (s, 1H), 4.90 (br s, 1H), 4.14 (m, 2H), 3.86 (s, 3H), 1.42 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 159.03, 155.75, 144.89, 143.51, 125.52, 118.07, 79.81, 52.00, 35.28, 28.35, 28.21. HRMS (ESI): calcd for C12H17NO5 [M+Na]+ 278.0999; found 278.1001.
4.2.6. 4-((tert-Butoxycarbonyl)aminomethyl)furan-2-carboxylic acid (13)
To a solution of methyl 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylate (12, 86 mg, 0.34 mmol) in MeOH (2 mL) and water (2 mL) was added LiOH·H2O (16 mg, 0.37 mmol) and allowed to stir overnight. The reaction mixture was diluted in water (10 mL) and extracted with EtOAc (2×10 mL), which was discarded. The aqueous phase was adjusted to pH 1 with hydrochloric acid (2 N), and the suspension was washed with ethyl acetate (3 × 10 mL). The combined organics were washed with brine (10 mL), dried (Na-2SO4), concentrated under reduced pressure, and chromatographed (5% methanol with 0.5% acetic acid in dichloromethane) to afford a white solid (72 mg, 89%). 1H NMR (500 MHz, MeOD) δ 7.60 (s, 1H), 7.15 (s, 1H), 4.08 (s, 2H), 1.44 (s, 9H). HRSM (ESI): calcd for C11H14NO5 [M-H]− 240.0877; found 240.0868.
4.2.7. 4-(Aminomethyl)furan-2-carboxylic acid hydrochloride (5a)
To 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylic acid (13, 50 mg, 0.21 mmol) was added 6 N HCl (8 mL). The solution was stirred at room temp for 4 h. The solvent was then evaporated under reduced pressure followed by recrystallized from ethanol/ethyl ether to give the product as white crystals (31 mg, 82%). 1H NMR (500 MHz, MeOD) δ 7.89 (s, 1H), 7.34 (s, 1H), 4.06 (s, 2H). 13C NMR (126 MHz, MeOD) δ 161.22, 147.55, 147.36, 121.28, 118.95, 34.85. HRMS (ESI): calcd for C6H7NO3 [M-H]− 140.0353; found 140.0351.
4.2.8. Methyl 4-(bromomethyl)thiophene-2-carboxylate (15)33
Methyl 4-methylthiophene-2-carboxylate (14, 1 g, 6.4 mmol), N-bromosuccinimide (1.25 g, 7.0 mmol), and benzoyl peroxide (155 mg, 0.64 mmol) were dissolved in carbon tetrachloride (30 mL). The reaction mixture was refluxed with stirring for 2 h, followed by cooling to 0 °C and filtered. The reaction mixture was then concentrated and chromatographed (ethyl acetate/hexanes, 1:6) to provide the desired product as a clear oil (1.34 g, 89%). 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 1.6 Hz, 1H), 7.47 (d, J = 1.5 Hz, 1H), 4.44 (s, 2H), 3.86 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.18, 138.87, 134.62, 134.06, 130.85, 52.37, 26.43. HRMS (ESI): calcd for C7H7BrO2S [M-Br]*+ 155.0161; found 155.0160.
4.2.9. Methyl 4-(((tert-butoxycarbonyl)amino)methyl)thiophene-2-carboxylate (16)
Methyl 4-(bromomethyl)thiophene-2-carboxylate (14, 1 g, 4.25 mmol) and sodium azide (1.38 g, 21.3 mmol) were dissolved in dimethylformamide (5 mL) and stirred at 60 °C under argon atmosphere for 1 h. After cooling, the reaction was partitioned between diethyl ether (30 mL) and water (30 mL). The aqueous phase was extracted with ether (3 × 20 mL), washed with brine (20 mL), dried (Na2SO4), and concentrated to dryness under reduced pressure. The crude product was dissolved in ethyl acetate (50 mL) in the presence of 10% Pd/C (100 mg) and Boc2O (563 mg, 2.58 mmol). The mixture was placed under a H2 atmosphere and vigorously stirred overnight at room temperature. The reaction mixture was then filtered through a Celite pad, concentrated in vacuo and chromatographed (ethyl acetate/hexanes, 1:5) to yield the desired product as a clear oil (738 mg, 64%). 1H NMR (500 MHz, CDCl3) δ 7.69 (s, 1H), 7.34 (s, 1H), 4.95 (br s, 1H), 4.27 (m, 2H), 3.86 (s, 2H), 1.44 (s, 7H). 13C NMR (126 MHz, CDCl3) δ 162.52, 155.74, 140.84, 134.05, 133.19, 128.76, 79.79, 52.24, 39.79, 28.38. HRMS (ESI): calcd for C12H17NO4S [M+Na]+ 294.0770; found 294.0779.
4.2.10. 4-((tert-Butoxycarbonyl)aminomethyl)thiophene-2-carboxylic acid (17)
Compound 17 was synthesized using a similar procedure to that of 13 (90%). 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 1.6 Hz, 1H), 7.44 (s, 1H), 4.92 (s, 1H), 4.32 (d, J = 6.1 Hz, 2H), 1.47 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 166.67, 155.78, 141.10, 134.41, 133.53, 130.19, 79.96, 39.75, 28.36. HRMS (ESI): calcd for C11H15NO4S [M-H]− 256.0649; found 256.0640.
4.2.11. 4-(Aminomethyl)thiophene-2-carboxylic acid hydrochloride (5b)
Compound 5b was synthesized using a similar procedure to that of 5a (84%). 1H NMR (500 MHz, MeOD) δ 7.86 (s, 1H), 7.84 (s, 1H), 4.16 (s, 2H). 13C NMR (126 MHz, MeOD) δ 164.65, 137.39, 135.76, 134.66, 133.81, 38.94. HRMS (ESI): calcd for C6H6NO2S [M-H]− 156.0125; found 156.0117.
4.2.12. 4-(Methoxycarbonyl)furan-3-carboxylic acid (19)
To a solution of dimethyl furan-3,4-dicarboxylate (18, 2.00 g, 10.9 mmol) in methanol (10 mL) was added sodium hydroxide (434 mg, 10.9 mmol) and stirred overnight at room temperature. Solvent was removed, and the crude product was partitioned between diethyl ether (30 mL) and water (30 mL). The aqueous phase was extracted with ether (3 × 20 mL), and the combined organic layers were discarded. The aqueous phase was adjusted to pH 1 with hydrochloric acid (2 N) and extracted with EtOAc (3 × 20 mL). The combined organics were washed with brine (10 mL), dried (Na2SO4), and concentrated under reduced pressure to yield the desired product as a white solid (1.72 g, 93%). 1H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 1.8 Hz, 1H), 8.13 (d, J = 1.8 Hz, 1H), 4.01 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.13, 161.30, 152.67, 150.73, 118.92, 115.08, 77.30, 53.64. HRMS (ESI): calcd for C6H7O5 [M+Na]+ 193.0107; found 193.0109.
4.2.13. Methyl 4-(hydroxymethyl)furan-3-carboxylate (20)34
A solution of 4-(methoxycarbonyl)furan-3-carboxylic acid (18, 2.0 g, 11.8 mmol) in anhydrous THF (50 mL) under an argon atmosphere was cooled to 0 °C. Borane tetrahydrofuran (12.3 mL, 1.0 M solution in THF, 12.3 mmol) was added dropwise via syringe with stirring. The reaction was allowed to warm to ambient temperature and stir for 4 h, after which it was cooled to 0 °C and quenched with the dropwise addition of water until gas evolution had ceased. After bulk solvent removal, the resulting crude residue was then partitioned between saturated aqueous NaHCO3 and diethyl ether, and the aqueous layer was further extracted with diethyl ether (2 × 40 mL). The combined ether solutions were washed with brine (20 mL) and dried (Na2SO4), concentrated to dryness under reduced pressure and chromatographed (ethyl acetate/hexanes, 2:5) to afford a clear oil (1.58 g, 86%). 1H NMR (500 MHz, CDCl3) δ 7.98 (s, 1H), 7.42 (s, 1H), 4.64 (s, 2H), 3.84 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 164.60, 149.18, 141.22, 125.16, 117.44, 55.21, 51.69. HRMS (ESI): calcd for C7H8O4 [M-H]− 155.0350; found 155.0339.
4.2.14. Methyl 4-(chloromethyl)furan-3-carboxylate (21)
To a solution of methyl 4-(hydroxymethyl)furan-3-carboxylate (19, 500 mg, 3.2 mmol) and triphenylphospine (1.26 g, 4.8 mmol) in anhydrous CH2Cl2 was added carbon tetrachloride (739 mg, 4.8 mmol). The reaction was stirred under an argon atmosphere at room temp for 18 h. The solvent was removed in vacuo, and the crude product was chromatographed (ethyl acetate/hexanes, 2:5) to afford a clear oil (356 mg, 64%). 1H NMR (500 MHz, CDCl3) δ 8.14 (d, J = 3.4 Hz, 1H), 7.22 (d, J = 3.3 Hz, 1H), 4.74 (d, J = 6.8 Hz, 2H), 3.90 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 163.12, 149.20, 143.04, 122.62, 117.20, 51.57, 35.97. HRMS (LC-TOF): calcd for C7H7O3Cl 174.0084; found 174.0078.
4.2.15. Methyl 4-(((tert-butoxycarbonyl)amino)methyl)furan-3-carboxylate (22)
Methyl 4-(chloromethyl)furan-3-carboxylate (21, 300 mg, 1.72 mmol) and sodium azide (560 mg, 8.60 mmol) were dissolved in dimethylformamide (5 mL) and stirred at 60 °C under argon atmosphere for 1 h. After cooling, the reaction was partitioned between diethyl ether (30 mL) and water (30 mL). The aqueous phase was extracted with ether (3 × 20 mL), washed with brine (20 mL), dried (Na2SO4), and concentrated to dryness under reduced pressure. The crude product was dissolved in ethyl acetate (20 mL) in the presence of 10% Pd/C (30 mg) and Boc2O (563 mg, 2.58 mmol). The mixture was placed under a H2 atmosphere and was vigorously stirred overnight at room temp. The reaction mixture was then filtered through a Celite pad, concentrated in vacuo, and chromatographed (ethyl acetate/hexanes, 1:5) to yield the desired product as a clear oil (303 mg, 69%). 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 1.7 Hz, 1H), 7.43 (s, 1H), 5.55 (t, J = 6.5 Hz, 1H), 4.29 (d, J = 6.4 Hz, 2H), 3.84 (s, 3H), 1.43 (br s, 9H). 13C NMR (126 MHz, CDCl3) δ 164.02, 155.72, 149.03, 141.88, 122.75, 117.49, 79.21, 51.52, 34.24, 28.33. HRMS (ESI): calcd for C12H17NO5 [M+Na]+ 278.0999; found 278.0999.
4.2.16. 4-((tert-Butoxycarbonyl)aminomethyl)furan-3-carboxylic acid (23)
Compound 23 was synthesized using a similar procedure to that of 13 (88%). 1H NMR (500 MHz, CDCl3) δ 8.07 (d, J = 1.8 Hz, 1H), 7.44 (d, J = 2.1 Hz, 1H), 4.98 (br s, 1H), 4.27 (s, 2H), 1.43 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 166.43, 158.13, 150.83, 142.98, 124.79, 119.18, 80.30, 35.84, 28.72. HRSM (ESI): calcd for C11H14NO5 [M-H]− 240.0877; found 240.0866.
4.2.17. 4-(Aminomethyl)furan-3-carboxylic acid hydrochloride (6a)
Compound 6a was synthesized using a similar procedure to that of 5a (78%).1H NMR (500 MHz, MeOD) δ 8.23 (s, 1H), 7.81 (s, 1H), 4.19 (s, 2H). 13C NMR (126 MHz, MeOD) δ 166.66, 151.40, 145.78, 119.50, 118.94, 34.45. HRMS (ESI): calcd for C6H7NO3 [M-H]− 140.0353; found 140.0347.
4.2.18. Dimethyl thiophene-3,4-dicarboxylate (25)
To a stirred solution of thiophene-3,4-dicarboxylic acid (24, 1.5 g, 8.71 mmol) in methanol (15 mL) was added conc. sulfuric acid (1 mL) dropwise. The reaction was heated to reflux and stirred under nitrogen overnight. The reaction mixture was allowed to cool to room temperature followed by concentration under vacuum. The resulting crude residue was then partitioned between saturated aqueous NaHCO3 and diethyl ether, and the aqueous layer was further extracted with diethyl ether (2 × 40 mL). The combined ether solutions were washed with brine (20 mL), dried (Na2SO4), and concentrated to dryness under reduced pressure to provide the desired product as a clear oil (1.46 g, 84%). 1H NMR (500 MHz, CDCl3) δ 7.88 (s, 2H), 3.88 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 163.41, 133.11, 131.90, 52.33. HRMS (LC-TOF): calcd for C8H8O4S 200.0143; found 200.0130.
4.2.19. 4-(Methoxycarbonyl)thiophene-3-carboxylic acid (26)
To a solution of dimethyl thiophene-3,4-dicarboxylate (25, 825 mg, 4.12 mmol) in methanol (10 mL) was added sodium hydroxide (165 mg, 4.12 mmol) and stirred overnight at room temperature. Solvent was removed, and the crude product was partitioned between diethyl ether (15 mL) and water (15 mL). The aqueous phase was extracted with ether (3 × 10 mL), and the combined organic layers were discarded. The aqueous phase was adjusted to pH 1 with hydrochloric acid (2 N) and extracted with EtOAc (3 × 10 mL). The combined organics were washed with brine (10 mL), dried (Na-2SO4), and concentrated under reduced pressure to yield the desired product as a white solid (667 mg, 87%). 1H NMR (500 MHz, CDCl3) δ 8.49 (d, J = 3.7 Hz, 1H), 8.37 (d, J = 3.7 Hz, 1H), 4.04 (s, 4H). 13C NMR (126 MHz, CDCl3) δ 166.95, 161.38, 139.26, 138.45, 133.68, 128.05, 53.96. HRMS (ESI): calcd for C7H6O4S [M-H]− 184.9914; found 184.9900.
4.2.20. Methyl 4-(hydroxymethyl)thiophene-3-carboxylate (27)
A solution of 4-(methoxycarbonyl)thiophene-3-carboxylic acid (25, 595 mg, 3.2 mmol) in anhydrous THF (20 mL) under argon atmosphere was cooled to 0 °C. Borane tetrahydrofuran (3.4 mL, 1.0 M solution in THF, 3.4 mmol) was added dropwise via syringe with stirring. The reaction was allowed to warm to ambient temperature and to stir for 4 h after which it was cooled to 0 °C and quenched with dropwise addition of water until gas evolution had ceased. After bulk solvent removal, the resulting crude residue was then partitioned between saturated aqueous NaHCO3 and diethyl ether, and the aqueous layer was further extracted with diethyl ether (2 × 15 mL). The combined ether solutions were washed with brine (15 mL), dried (Na2SO4), concentrated to dryness under reduced pressure, and chromatographed (ethyl acetate/hexanes, 2:5) to afford a clear oil (342 mg, 62%). 1H NMR (500 MHz, CDCl3) δ 8.13 (d, J = 3.4 Hz, 1H), 7.22 (d, J = 3.4 Hz, 1H), 4.74 (s, 2H), 3.88 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 164.30, 142.94, 135.67, 130.70, 124.12, 60.03, 52.11. HRMS (ESI): calcd for C7H8O3S [M-H]− 171.0121; found 171.0108.
4.2.21. Methyl 4-(bromomethyl)thiophene-3-carboxylate (28)
Carbon tetrabromide (769 mg, 2.32 mmol) was added to a stirred solution of methyl 4-(hydroxymethyl)thiophene-3-carboxylate (27, 265 mg, 1.54 mmol) in dry CH2Cl2 (10 mL) under argon atmosphere. After stirring for 10 min, triphenylphosphine (608 mg, 2.32 mmol) was added and the resulting reaction mixture was stirred for 1 h at room temperature. Solvent was removed and the crude product was separated between diethyl ether (15 mL) and water (15 mL). Aqueous phase was extracted with ether (3 × 10 mL). The combined ether solutions were washed with brine (15 mL), dried (Na2SO4), concentrated to dryness under reduced pressure, and chromatographed (ethyl acetate/hexanes, 1:5) to afford a clear oil (232 mg, 64%). 1H NMR (500 MHz, CDCl3) δ 8.14 (d, J = 3.4 Hz, 1H), 7.39 (d, J = 3.3 Hz, 1H), 4.84 (s, 2H), 3.89 (s, 4H). 13C NMR (126 MHz, CDCl3) δ 162.75, 138.74, 135.27, 130.28, 127.08, 51.85, 26.81. HRMS (ESI): calcd for C7H7BrO2S [M-Br]+ 155.0161; found 155.0162.
4.2.22. Methyl 4-(((tert-butoxycarbonyl)amino)methyl)thiophene-3-carboxylate (29)
Compound 29 was synthesized using a similar procedure to that of 22 (72%). 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 3.4 Hz, 1H), 7.25 (d, J = 3.4 Hz, 1H), 5.56 (t, J = 6.7 Hz, 1H), 4.45 (d, J = 6.6 Hz, 2H), 3.87 (s, 3H), 1.43 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 163.62, 155.78, 140.35, 135.16, 130.65, 124.81, 79.24, 51.83, 39.30, 28.44. HRMS (ESI): calcd for C12H17NO4S [M+Na]+ 294.0770; found 294.0775.
4.2.23. 4-((tert-Butoxycarbonyl)aminomethyl)thiophene-3-carboxylic acid (30)
Compound 30 was synthesized using a similar procedure to that of 13 (92%). 1H NMR (500 MHz, MeOD) δ 8.21 (d, J = 3.4 Hz, 1H), 7.22 (d, J = 3.4 Hz, 1H), 4.94 (s, 1H), 4.44 (s, 2H), 1.44 (s, 9H). 13C NMR (126 MHz, MeOD) δ 166.05, 158.31, 142.29, 136.60, 132.45, 124.26, 80.32, 40.93, 28.78. HRMS (ESI): calcd for C11H15NO4S [M-H]− 256.049; found 256.044.
4.2.24. 4-(Aminomethyl)thiophene-3-carboxylic acid hydrochloride (6b)
Compound 6b was synthesized using a similar procedure to that of 5a (82%). 1H NMR (500 MHz, MeOD) δ 8.37 (d, J = 3.2 Hz, 1H), 7.66 (d, J = 3.1 Hz, 1H), 4.32 (s, 2H). 13C NMR (126 MHz, MeOD) δ 166.30, 137.67, 135.13, 132.94, 129.99, 38.99. HRMS (ESI): calcd for C6H7NO2S [M-H]− 156.0125; found 156.0113.
4.2.25. Methyl 5-formyl-1H-pyrrole-2-carboxylate (31a) and methyl 4-formyl-1H-pyrrole-2-carboxylate (31b)
Prepared as described in the literature from methyl pyrrole-2-carboxylate as a 5:2 ratio of 31a and 31b, respectively.52
31a
1H NMR (500 MHz, CDCl3) δ 9.85 (s, 1H), 9.78 (br s, 1H), 7.58 (dd, J = 3.3, 1.5 Hz, 1H), 7.32 (dd, J = 2.5, 1.5 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 185.75, 161.37, 128.53, 127.79, 124.91, 114.31, 52.24.
31b
1H NMR (500 MHz, CDCl3) δ 10.24 (br s, 1H), 9.67 (s, 1H), 6.95-6.91 (m, 2H), 3.91 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 180.64, 161.04, 134.65, 128.31, 119.89, 115.82, 52.41.
4.2.26. Methyl 5-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate (32a)35
Methyl 5-formyl-1H-pyrrole-2-carboxylate (31a, 2.50 g, 16.3 mmol) was dissolved in water at 65 °C, and a solution of hydroxylamine hydrochloride (1.70 g, 24.5 mmol) and potassium carbonate (1.40 g, 10.1 mmol) in water (30 mL) was added dropwise. Upon cooling a white precipitate formed, which was filtered and recrystallized from water/ethyl acetate (3:1) to give the desired product in a 3:5 mixture of the two diastereomers as a white powder (1.56 g, 57%). 1H NMR (500 MHz, CDCl3) δ 10.58/9.98 (br s, 1H), 8.24/8.20 (br s, 1H), 6.90 (m, 1H), 6.49/6.41 (dd J = 3.9, 2.5 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.34/161.24, 142.17/138.02, 129.29/127.53, 124.86/124.64, 116.51/115.12, 115.57/114.56, 52.14/51.94. HRMS (ESI): calcd for C7-H8N2O3 [M+H]+ 169.0608; found 169.0610.
4.2.27. Methyl 5-(((tert-butoxycarbonyl)amino)methyl)-1H-pyrrole-2-carboxylate (33a)
Methyl 5-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate (32a, 3.0 g, 17.8 mmol) was dissolved in ethyl acetate (100 mL) in the presence of 10% Pd/C (300 mg) and Boc2O (4.28 g, 19.6 mmol). The mixture was placed under a H2 atmosphere and vigorously stirred overnight at room temperature. The reaction mixture was filtered through a Celite pad, concentrated in vacuo, and chromatographed (ethyl acetate/hexanes, 1:5) to yield the desired product as a clear oil (3.88 g, 84%). 1H NMR (500 MHz, CDCl3) δ 9.92 (s, 1H), 6.81 (dd, J = 3.7, 2.5 Hz, 1H), 6.07 (dd, J = 3.2 Hz, 1H), 5.17 (s, 1H), 4.26 (d, J = 6.1 Hz, 2H), 3.84 (s, 3H), 1.45 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 161.51, 156.68, 135.29, 122.43, 115.34, 108.69, 80.19, 51.45, 37.49, 28.34. HRMS (ESI): calcd for C12H18N2O4 [M+H] 255.1339; found 255.1342.
4.2.28. 5-(((tert-Butoxycarbonyl)amino)methyl)-1H-pyrrole-2-carboxylic acid (34a)36
Compound 34a was synthesized using a similar procedure to that of 13 (82%). 1H NMR (500 MHz, MeOD) δ 6.77 (d, J = 3.7 Hz, 1H), 6.05 (d, J = 3.7 Hz, 1H), 4.19 (s, 2H), 1.44 (s, 10H). 13C NMR (126 MHz, MeOD) δ 164.37, 158.57, 137.15, 123.41, 117.03, 109.13, 80.45, 38.12, 28.75. HRMS (ESI): calcd for C11H15N2O4 [M-H]− 239.1037; found 239.1025.
4.2.29. 5-(Amino)methyl)-1H-pyrrole-2-carboxylic acid hydrochloride (4c)
Compound 4c was synthesized using a similar procedure to that of 5a (74%). 1H NMR (500 MHz, MeOD) δ 6.84 (d, J = 3.8 Hz, 1H), 6.33 (d, J = 3.8 Hz, 1H), 4.14 (s, 2H). 13C NMR (126 MHz, MeOD) δ 163.85, 129.69, 125.46, 116.96, 111.69, 36.77. HRMS (ESI): calcd for C6H7N2O2 [M-H]− 139.0513; found 139.0512.
4.2.30. Methyl 4-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate (32b)35
Methyl 4-formyl-1H-pyrrole-2-carboxylate (31b, 500 mg, 3.26 mmol) was converted to methyl 4-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate (280 mg, 51%) using conditions identical to those used to prepare methyl 5-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate from methyl 5-formyl-1H-pyrrole-2-carboxylate. 1H NMR (500 MHz, MeOD) δ 8.00/7.26 (s, 1H), 7.67/7.22 (d, J = 1.5 Hz, 1H), 7.20/7.06 (d, J = 1.7 Hz, 1H), 3.84/3.83 (s, 3H). 13C NMR (126 MHz, MeOD) δ 162.99/162.88, 145.50/142.15, 129.01/125.20, 124.79/123.59, 119.92/118.28, 117.80/113.65, 52.00. HRMS (ESI): calcd for C7H8N2O3 [M-H]− 167.0462; found 167.0463.
4.2.31. 4-(((tert-Butoxycarbonyl)amino)methyl)-1H-pyrrole-2-carboxylate (33b)
Methyl 4-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate (32b, 167 mg, 0.99 mmol) was converted to methyl 4-(((tert-butoxycarbonyl)amino)methyl)-1H-pyrrole-2-carboxylate (207 mg, 82%) using conditions identical to those used to prepare 5-(((tert-butoxycarbonyl)amino)methyl)-1H-pyrrole-2-carboxylate from methyl 5-((hydroxyimino)methyl)-1H-pyrrole-2-carboxylate. 1H NMR (500 MHz, MeOD) δ 6.87 (d, J = 1.6 Hz, 1H), 6.78 (d, J = 1.8 Hz, 1H), 4.06 (s, 2H), 3.80 (s, 3H), 1.44 (s, 9H). 13C NMR (126 MHz, MeOD) δ 163.18, 158.47, 124.81, 123.35, 123.00, 115.81, 80.04, 51.72, 37.91, 28.81. HRMS (ESI): calcd for C12H18N2O4 [M+Na]+ 277.1159; found 277.1158.
4.2.32. 4-(((tert-Butoxycarbonyl)amino)methyl)-1H-pyrrole-2-carboxylic acid (34b)36
Compound 34b was synthesized using a similar procedure to that of 13 (86%) 1H NMR (500 MHz, MeOD) δ 6.86 (d, J = 1.7 Hz, 1H), 6.78 (d, J = 1.8 Hz, 1H), 4.07 (s, 2H), 1.44 (s, 9H). 13C NMR (126 MHz, MeOD) δ 164.33, 158.49, 124.70, 123.97, 122.77, 115.84, 80.03, 37.97, 28.80. HRMS (ESI): calcd for C11H16N2O4 [M-H]− 239.1037; found 239.1026.
4.2.33. 4-(Aminomethyl)-1H-pyrrole-2-carboxylic acid hydrochloride (5c)
Compound 5c was synthesized using a similar procedure to that of 5a (82%) 1H NMR (500 MHz, MeOD) δ 7.10 (d, J = 1.8 Hz, 1H), 6.95 (d, J = 1.8 Hz, 1H), 4.00 (s, 2H). 13C NMR (126 MHz, MeOD) δ 163.86, 125.28, 124.57, 117.94, 116.33, 37.13. HRMS (ESI): calcd for C6H8N2O2 [M-H]− 139.0513; found 139.0503.
4.2.34 Methyl 4-((tert-butoxycarbonyl)amino(2H2)methyl)furan-2-carboxylate ([D2]-12)
Methyl 4-cyanofuran-2-carboxylate (11, 190 mg, 1.26 mmol) was converted to methyl 4-((tert-butoxycarbonyl)amino(2H2)methyl)furan-2-carboxylate (130 mg, 40%) by treatment with sodium borodeuteride (368 mg, 8.80 mmol), Boc2O (550 mg, 2.52 mmol), and NiCl2·6H2O (31 mg, 0.13 mmol), following the same procedure used to generate methyl 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylate (12). 1H NMR (500 MHz, CDCl3) δ 7.45 (s, 1H), 7.10 (s, 1H), 4.99 (s, 1H), 3.83 (s, 3H), 1.39 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 159.06, 155.84, 144.88, 143.57, 125.56, 125.49, 118.14, 79.75, 52.01, 35.29, 35.04, 34.87, 28.38. HRMS (ESI): calcd for C12H15D2NO5 [M+Na]+ 280.1124; found 280.1116.
4.2.35. 4-((tert-Butoxycarbonyl)amino(2H2)methyl)furan-2-carboxylic acid ([D2]-13)
Methyl 4-((tert-butoxycarbonyl)amino(2H2)methyl)furan-2-carboxylate ([D2]-12, 130 mg, 0.51 mmol) was converted to 4-((tert-butoxycarbonyl)amino(2H2)methyl)furan-2-carboxylic acid (85 mg, 69%) using conditions identical to those used to convert methyl 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylate (12) to 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylic acid (13). 1H NMR (500 MHz, MeOD) δ 7.60 (s, 1H), 7.16 (s, 1H), 1.44 (s, 9H). 13C NMR (126 MHz, MeOD) δ 161.71, 158.38, 146.53, 145.05, 127.46, 127.40, 127.34, 119.19, 80.34, 35.78, 35.69, 35.53, 35.36, 28.72. HRMS (ESI): calcd for C11H13D2NO5 [M+Na]+ 266.0968; found 266.0954.
4.2.36. 4-Amino(2H2)methyl)furan-2-carboxylic acid hydrochloride ([D2]-5a)
4-((tert-Butoxycarbonyl)amino(2H2)methyl)furan-2-carboxylic acid ([D2]-13, 85 mg, 0.35 mmol) was converted to 4-amino(2H2)methyl)furan-2-carboxylic acid hydrochloride (63 mg, 100%) using conditions identical to those used to convert 4-((tert-butoxycarbonyl)aminomethyl)furan-2-carboxylic acid (13) to 4-aminomethyl)furan-2-carboxylic acid hydrochloride (5b) with ≥95% isotopic purity by 1H NMR spectroscopy. 1H NMR (500 MHz, D2O) δ 7.81 (s, 1H), 7.30 (s, 1H). 13C NMR (126 MHz, D2O) δ 158.59, 143.51, 142.27, 116.42, 116.36, 116.30, 115.55, 30.53, 30.30, 30.12. HRMS (ESI): calcd for C6H5D2NO3 [M-H]− 142.0479; found 142.0471.
4.3. Enzyme and Assays
4.3.1. Purification of GABA-AT from Pig Brain
GABA-AT was isolated and purified from pig brain by a modified procedure.53 The purified GABA-AT used in these experiments was found to have a concentration of 6.41 mg/mL with a specific activity of 1.84 units/mg.
4.3.2. Evaluation of Compounds as Inhibitors of GABA-AT
Inhibition constants were determined by monitoring GABA-AT activity in the presence of 0–5 mM concentrations of synthesized analogues using a coupled assay with the enzyme succinic semialdehyde dehydrogenase (SSADH). The assay solution consisted of 10 mM GABA, 5 mM α-ketoglutarate, 1 mM NADP+, 5 mM β-mercaptoethanol, and excess SSADH in 50 mM potassium pyrophosphate buffer, pH 8.5. Enzyme activity was determined by observing the change in absorbance at 340 nm at 25 °C.37 Competitive inhibition constants were determined by Dixon plots of obtained data. Prior to their evaluation, initial experiments were performed to confirm the synthesized analogues (at 5 mM concentration) do not inhibit the coupling enzymes utilized in the substrate and inhibition assays.
4.3.3. Evaluation of Compounds as Substrates for GABA-AT
Compounds were tested using an experiment in which the conversion of α-ketoglutarate (α-KG) to L-glutamic acid was monitored as an indication of the rate of PLP reduction to PMP, which in turn corresponds to the rate of amine oxidation to the corresponding aldehyde. Enzyme reactions were prepared at varying concentrations of compounds in 100 μL pyrophosphate buffer (50 mM, pH 8.5) containing 5 mM α-ketoglutarate, 2 mM 2-mercaptoethanol, and 0.13 mg/mL purified GABA-AT and allowed to incubate at room temperature for 16 h. The L-glutamic acid content was determined by combining 50 μL of each incubation mixture with 50 μL of Tris-HCl buffer (100 mM, pH 7.5) containing 100 μM Ampliflu™ Red (Sigma-Aldrich), 0.25 units/mL horseradish peroxidase and 0.08 units/mL L-glutamate oxidase in a 96-well black walled plate. After incubation at 37 °C for 30 min fluorescence was recorded with the aid of a microplate reader (BioTek Synergy H1) with 530 nm excitation and 590 nm emission wavelengths, where fluorescence is proportional to the L-glutamate concentration. Compounds 4a, 4b, 5a, 5c, and GABA were evaluated in triplicate at 90 μM, 225 μM, 450 μM, 900 μM, 2.25 mM and 4.5 mM concentrations. Compounds 4c, 5b, 6a and 6b were evaluated in triplicate at 900 μM, 2.25 mM, 4.5 mM, 9.0 mM and 13.5 mM concentrations. Substrate kinetic constants were determined by Hanes-Woolf plots using linear regression analysis (GraphPad Prism). Cornish-Bowden replots indicated that the compounds were competitive inhibitors.
Supplementary Material
Acknowledgments
The authors are grateful to the National Institutes of Health for financial support (GM066132 and DA030604). The authors would also like to thank Park Packing Co. (Chicago, IL) for generously providing fresh pig brains for this study.
Abbreviations
- GABA
γ-aminobutyric acid
- GABA-AT
GABA aminotransferase
- GAD
L-glutamic acid decarboxylase
- PLP
pyridoxal-5′-phosphate
- SSA
succinic semialdehyde
- PMP
pyridoxamine 5′-phosphate
- BBB
blood-brain barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krnjevic K. Physiol Rev. 1974;54:418–540. [Google Scholar]
- 2.Baxter CF, Roberts E. J Biol Chem. 1958;233:1135–1139. [PubMed] [Google Scholar]
- 3.Karlsson A, Fonnum F, Malthe-Sørenssen D, Storm-Mathisen J. Biochem Pharmacol. 1974;23:3053–3061. doi: 10.1016/0006-2952(74)90281-0. [DOI] [PubMed] [Google Scholar]
- 4.Gale K. Epilepsia. 1989;30:S1–S11. doi: 10.1111/j.1528-1157.1989.tb05825.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Gelder NM, Elliott KAC. J Neurochem. 1958;3:139–143. doi: 10.1111/j.1471-4159.1958.tb12620.x. [DOI] [PubMed] [Google Scholar]
- 6.Bakay RAE, Harris AB. Brain Res. 1981;206:387–404. doi: 10.1016/0006-8993(81)90539-4. [DOI] [PubMed] [Google Scholar]
- 7.Ribak CE, Harris AB, Vaugh JE, Roberts E. Science. 1979;205:211–214. doi: 10.1126/science.109922. [DOI] [PubMed] [Google Scholar]
- 8.Wu JY, Bird ED, Chen MS, Huang WM. Neurochem Res. 1979;4:575–586. doi: 10.1007/BF00964435. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd KG, Shemen L, Hornykiewicz O. Brain Research. 1977;127:269–278. doi: 10.1016/0006-8993(77)90540-6. [DOI] [PubMed] [Google Scholar]
- 10.Sherif FM, Ahmed SS. Clin Biochem. 1995;28:145–154. doi: 10.1016/0009-9120(94)00074-6. [DOI] [PubMed] [Google Scholar]
- 11.Gunne LM, Haeggstroem JE, Sjoequist B. Nature. 1984;309:347–349. doi: 10.1038/309347a0. [DOI] [PubMed] [Google Scholar]
- 12.Silverman RB. Mechanism-Based Enzyme Inactivation: Chemistry and Enzymology. I and II. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- 13.Silverman RB. Methods Enzymol. 1995;249:240–283. doi: 10.1016/0076-6879(95)49038-8. [DOI] [PubMed] [Google Scholar]
- 14.Lippert B, Metcalf BW, Jung MJ, Casara P. Eur J Biochem. 1977;74:441–445. doi: 10.1111/j.1432-1033.1977.tb11410.x. [DOI] [PubMed] [Google Scholar]
- 15.Loscher W. Neuropharmacology. 1982;21:803–810. doi: 10.1016/0028-3908(82)90068-5. [DOI] [PubMed] [Google Scholar]
- 16.Tassinari CA, Micheluccia R, Ambrosetto G, Salvi F. Arch Neurol. 1987;44:907–910. doi: 10.1001/archneur.1987.00520210009010. [DOI] [PubMed] [Google Scholar]
- 17.Brown TR, Mattson RJ, Penry JK, Smith DB, Treiman DM, Wilder BJ, Ben-Menachem E, Miketta RM, Sherry KM, Szabo GK. Br J Clin Pharmacol. 1989;27:95S–100S. doi: 10.1111/j.1365-2125.1989.tb03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivenius MR, Ylinen A, Murros K, Matilainen R, Riekkinen P. Epilepsia. 1987;28:688–692. doi: 10.1111/j.1528-1157.1987.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 19.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 20.Peng X-Q, Li X, Gilbert JG, Pak AC, Ashby CR, Jr, Brodie JD, Dewey SL, Gardner EL, Xi Z-X. Drug Alcohol Depend. 2008;97:216–225. doi: 10.1016/j.drugalcdep.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maguire MJ, Hemming K, Wild JM, Hutton JL, Marson AG. Epilepsia. 2010;51:2423–2431. doi: 10.1111/j.1528-1167.2010.02772.x. [DOI] [PubMed] [Google Scholar]
- 22.Highlights of Prescribing Information (for Sabril) http://www.lundbeck.com/upload/us/files/pdf/Products/Sabril_PI-IS_US_EN.pdf.
- 23.Nanavati SM, Silverman RB. J Am Chem Soc. 1991;113:9341–9349. [Google Scholar]
- 24.Heim MK, Gidal BE. Acta Neurol Scand. 2012. [DOI] [PubMed] [Google Scholar]
- 25.Qiu J, Silverman RB. J Med Chem. 2000;43:706–720. doi: 10.1021/jm9904755. [DOI] [PubMed] [Google Scholar]
- 26.Pan Y, Qiu J, Silverman RB. J Med Chem. 2003;46:5292–5293. doi: 10.1021/jm034162s. [DOI] [PubMed] [Google Scholar]
- 27.Pan Y, Calvert K, Silverman RB. Bioorg Med Chem. 2004;12:5719–5725. doi: 10.1016/j.bmc.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Silverman RB. J Med Chem. 2006;49:7404–7412. doi: 10.1021/jm0608715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan H, Silverman RB. Bioorg Med Chem Lett. 2007;17:1651–1654. doi: 10.1016/j.bmcl.2006.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie TJ, Macdonald SJF, Young RJ, Pickett SD. Drug Discov Today. 2011;16:164–171. doi: 10.1016/j.drudis.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Clift MD, Silverman RB. Bioorg Med Chem Lett. 2008;18:3122–3125. doi: 10.1016/j.bmcl.2007.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Silverman RB. Bioorg Med Chem. 2006;14:2242–2252. doi: 10.1016/j.bmc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Peschke B, Madsen K, Hansen BS, Johansen NL. Bioorg Med Chem Lett. 1997;7:1969–1972. [Google Scholar]
- 34.McCoy LL, Mal D. J Org Chem. 1984;49:939–942. [Google Scholar]
- 35.Anderson HJ, Lee SF. Can J Chem. 1965;43:409–414. [Google Scholar]
- 36.Zhang Y, Yin Z, He J, Cheng JP. Tetrahedron Lett. 2007;48:6039–6043. [Google Scholar]
- 37.Silverman RB, Levy MA. Biochemistry. 1981;20:1197–1203. doi: 10.1021/bi00508a022. [DOI] [PubMed] [Google Scholar]
- 38.Woolf B, Haldane JBS, Stern KG. Algemeine Chemie der Enzyme. Steinkopf; Dresden: 1932. pp. 119–120. [Google Scholar]
- 39.Hanes CS. Biochem J. 1932;26:1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu PH, Duren DA, Davis BA, Boulton AA. J Neurochem. 1987;48:440–446. doi: 10.1111/j.1471-4159.1987.tb04112.x. [DOI] [PubMed] [Google Scholar]
- 41.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; 2006. p. 428.p. 431. [Google Scholar]
- 42.Forlani L, Marianucci E, Todesco PE. J Chem Res. 1984:126. [Google Scholar]
- 43.Chudeck JA, Foster R, Young D. J Chem Soc Perkin Trans II. 1985:1285. [Google Scholar]
- 44.Billard T, Langlois BR, Blond G. Eur J Org Chem. 2001:1467–1471. doi: 10.1021/jo015587u. [DOI] [PubMed] [Google Scholar]
- 45.Matsui M, Yamada K, Funabiki K. Tetrahedron. 2005;61:4671–4677. [Google Scholar]
- 46.Barys M, Ciunik Z, Drabnet K, Kwiecien A. New J Chem. 2010;34:2605–2611. [Google Scholar]
- 47.Velázquez M, Salgado-Zamora H, Pérez C, Campos-A ME, Mendoza P, Jiménez H, Jiménez R. J Mol Struc. 2010;979:56–61. [Google Scholar]
- 48.Heine A, DeSantis G, Luz JG, Mitchell M, Wong CH, Wilson IA. Science. 2001;294:369–374. doi: 10.1126/science.1063601. [DOI] [PubMed] [Google Scholar]
- 49.Iwasawa T, Hooley RJ, Rebek J., Jr Science. 2007;317:493–496. doi: 10.1126/science.1143272. [DOI] [PubMed] [Google Scholar]
- 50.Hooley RJ, Iwasawa T, Rebek J., Jr J Am Chem Soc. 2007;129:15330–15339. doi: 10.1021/ja0759343. [DOI] [PubMed] [Google Scholar]
- 51.Bordwell FG, Boyle WJ., Jr J Am Chem Soc. 1975;97:3447–3452. [Google Scholar]
- 52.Schmuck C. Tetrahedron. 2001;57:3063–3068. [Google Scholar]
- 53.Koo YK, Nandi D, Silverman RB. Arch Biochem Biophys. 2000;374:248. doi: 10.1006/abbi.1999.1623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.