Abstract
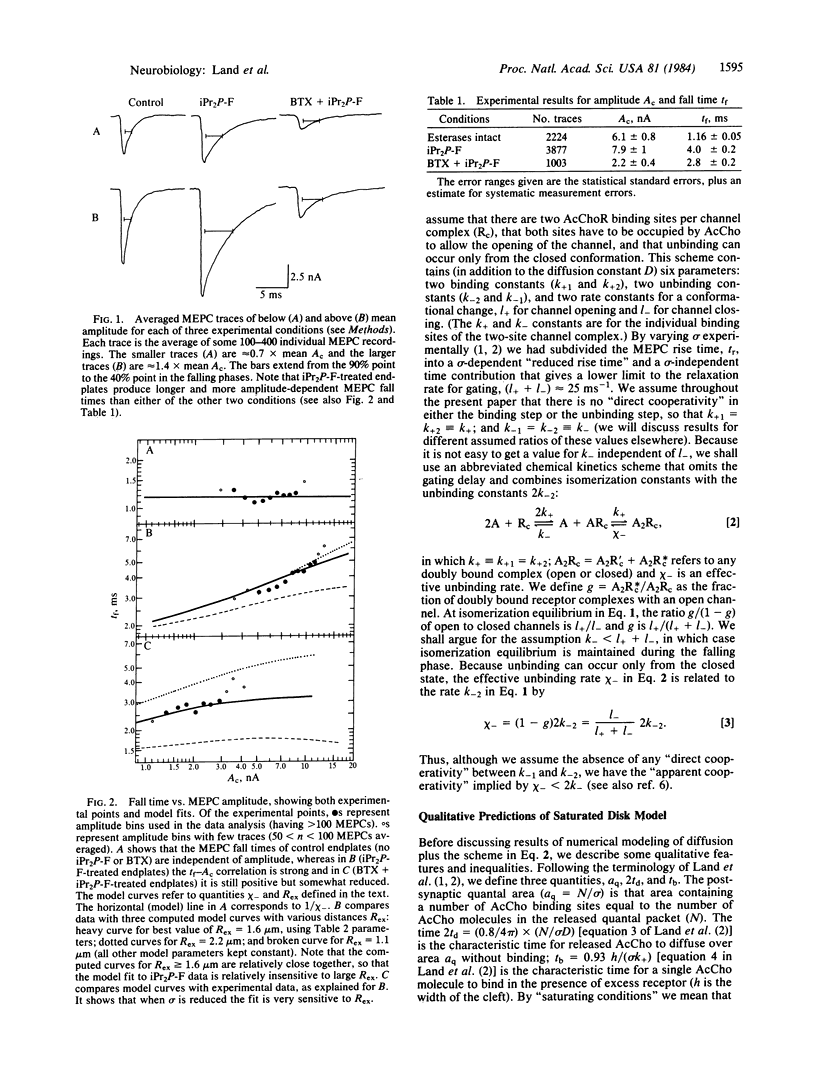
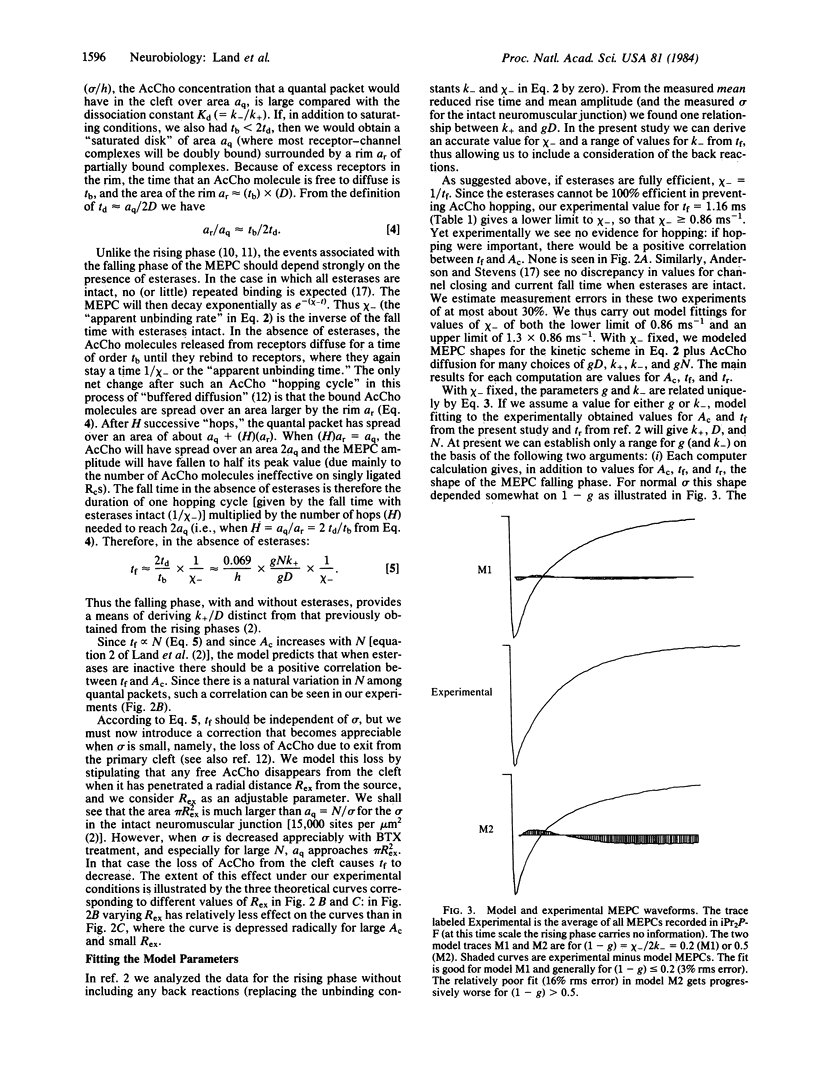
In previous papers we studied the rising phase of a miniature endplate current (MEPC) to derive diffusion and forward rate constants controlling acetylcholine (AcCho) in the intact neuromuscular junction. The present study derives similar values (but with smaller error ranges) for these constants by including experimental results from the falling phase of the MEPC. We find diffusion to be 4 X 10(-6) cm2 s-1, slightly slower than free diffusion, forward binding to be 3.3 X 10(7) M-1 s-1, and the distance from an average release site to the nearest exit from the cleft to be 1.6 micron. We also estimate the back reaction rates. From our values we can accurately describe the shape of MEPCs under different conditions of receptor and esterase concentration. Since we suggest that unbinding is slower than isomerization, we further predict that there should be several short "closing flickers" during the total open time for an AcCho-ligated receptor channel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. End-plate channel opening and the kinetics of quinacrine (mepacrine) block. J Physiol. 1980 Sep;306:283–306. doi: 10.1113/jphysiol.1980.sp013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash D. J., Aoshima H., Hess G. P. Acetylcholine-induced cation translocation across cell membranes and inactivation of the acetylcholine receptor: chemical kinetic measurements in the millisecond time region. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3318–3322. doi: 10.1073/pnas.78.6.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Uchitel O. D. Diffusion of acetylcholine in the synaptic cleft of normal and myasthenia gravis human endplates. Nature. 1980 Jul 31;286(5772):500–502. doi: 10.1038/286500a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E. Characterization of drug iontophoresis with a fast microassay technique. Biophys J. 1976 Jul;16(7):705–717. doi: 10.1016/S0006-3495(76)85723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion translocation: chemical kinetic investigations of the mechanism. Annu Rev Biophys Bioeng. 1983;12:443–473. doi: 10.1146/annurev.bb.12.060183.002303. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. M., Dionne V. E. Temperature dependence of ion permeation at the endplate channel. J Gen Physiol. 1983 May;81(5):687–703. doi: 10.1085/jgp.81.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. Diffusion of acetylcholine in agar gels and in the isolated rat diaphragm. J Physiol. 1960 Oct;153:562–572. doi: 10.1113/jphysiol.1960.sp006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor site density affects the rising phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3736–3740. doi: 10.1073/pnas.77.6.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7200–7204. doi: 10.1073/pnas.78.11.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Koblin D. D., Sheridan R. E. Role of voltage-sensitive receptors in nicotinic transmission. Biophys J. 1978 Mar;21(3):181–194. doi: 10.1016/S0006-3495(78)85518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Equilibrium binding of [3H]tubocurarine and [3H]acetylcholine by Torpedo postsynaptic membranes: stoichiometry and ligand interactions. Biochemistry. 1979 Nov 27;18(24):5464–5475. doi: 10.1021/bi00591a032. [DOI] [PubMed] [Google Scholar]
- Neumann E., Chang H. W. Dynamic properties of isolated acetylcholine receptor protein: kinetics of the binding of acetylcholine and Ca ions. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3994–3998. doi: 10.1073/pnas.73.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J Biol Chem. 1980 Nov 10;255(21):10144–10156. [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


