Abstract
Clinical investigators in academic medical centers often perceive federal regulations as a significant obstacle to conducting clinical research. The regulatory authority of the FDA extends to clinical studies of medical devices. Consequently, researchers wishing to conduct device research using FDA-approved as well as non-approved devices must comply with federal regulations for Investigational Device Exemptions (IDE) as described in Title 21 of the Code of Federal Regulations Part 812. FDA regulatory oversight is structured to match the risk to the subject to the risk of the device. Medical device studies can be categorized as 1) meeting exemption criteria, 2) being a non-significant risk device, or 3) being a significant risk device. All IDE studies must meet regulations for the protection of human subjects, but no additional federal filing on the part of the investigator is necessary for those that meet exempt criteria. Non-significant risk device studies require meeting abbreviated IDE regulatory requirements for the conduct of the study, but no prior FDA approval is required. Significant risk device studies require that the investigator also function as a sponsor and to file an IDE with the FDA for approval before starting. A Sponsor-Investigator filing an IDE follows the format and content described in 21 CFR 812.20. The study may begin 30 days after the date of submission receipt unless the FDA notifies the sponsor otherwise. While the IDE is active, the Sponsor-Investigator must meet the requirements for the conduct of the study and the required monitoring and reporting to the FDA.
Keywords: Food and Drug Administration, Investigational Device Exemption, federal regulations, significant risk device
INTRODUCTION
Clinical investigators in academic medical centers often perceive federal regulations as a significant obstacle to conducting clinical research. Those investigators who would like to conduct a clinical study of a medical device may be hesitant to proceed due to what may seem to be an intimidating bulwark of federal regulations governing medical devices. This review is intended to advise an investigator in understanding and meeting these regulatory requirements. More specifically, this review is focused on a clinical studies conducted by an individual investigator in an academic health institution whose objective is to study an FDA-approved, unmodified medical device that will be used or investigated in a manner that is different from its FDA approved label, and therefore doesn’t fulfill exemption criteria. It is anticipated that the investigator can acquire an understanding of the regulations in order to permit the conduct of a broad range of projects that otherwise might not be attempted. Indeed, relevant regulations accompanied with clear guidance do permit the conduct of a wide variety of trials and should not be seen as a significant impediment to important clinical research.
THE REGULATORY ENVIRONMENT AND THE FDA ROLE
The addition of Food and Drug Administration (FDA) oversight for the conduct of device research complements the basic regulatory requirements for the protection of human subjects with the addition of specific regulatory requirements for devices. (1, 2) The FDA is the federal agency within the Department of Health and Human Services that is charged with oversight of the safety of medical devices granted under the Federal Food, Drug, and Cosmetic Act (FD&C Act). (3) The Center for Devices and Radiological Health (CDRH) is the branch of the agency charged with oversight of medical devices. They are responsible for the regulation of manufacturing, repackaging, relabeling, and importation of medical devices as well as radiation-emitting electronic products sold in the United States.
The modern era of device regulation began in 1976 with the Medical Device Amendment to the 1906 FD&C Act. Prior to the amendments, the Act prohibited the adulteration and misbranding of the medical devices but there were no requirements for the review of the medical devices prior to their marketing. Subsequent to the Amendment relevant regulations regarding medical devices, including the requirement for the pre-marketing review as well as device classification, have been introduced, and are included in Title 21 of the Code of Federal Regulations Part 812. (2) Table 1 lists the sections of the regulations relevant to device studies for Sponsor-Investigators as discussed in this review.
TABLE 1.
Federal Regulations That Apply to the IDE Application Process for Sponsor-Investigators
| Code of Federal Regulations Title 21, PART 812 SUBCHAPTER H--MEDICAL DEVICES | |
|---|---|
| Subparts and sections discussed in text | |
| Subpart A — | General Provisions |
| Sec. 812.2 | Applicability |
| Sec. 812.2 (b) | Abbreviated Requirements |
| Sec. 812.2 (c) | Exempted investigations |
| Sec. 812.3 | Definitions |
| Sec. 812.5 | Labeling of investigational devices |
| Sec. 812.7 | Prohibition of promotion and other practices |
| Sec. 812.19 | Address for IDE correspondence |
| Subpart B — | Application and Administrative Action |
| Sec. 812.20 | Application |
| Sec. 812.25 | Investigational plan |
| Sec. 812.27 | Report of prior investigations |
| Sec. 812.30 | FDA action on applications |
| Sec. 812.35 | Supplemental applications. |
| Subpart C — | Responsibilities of Sponsors |
| Sec. 812.40 | General responsibilities of sponsors |
| Sec. 812.42 | FDA and IRB approval |
| Sec. 812.46 | Monitoring investigations |
| Subpart D — | IRB Review and Approval |
| Sec. 812.62 | IRB approval |
| Sec. 812.66 | Significant risk device determinations |
| Subpart E — | Responsibilities of Investigators |
| Sec. 812.100 | General responsibilities of investigators |
| Sec. 812.110 | Specific responsibilities of investigators |
| Subpart G — | Records and Reports |
| Sec. 812.140 | Records |
| Sec. 812.150 | Reports |
| Other Relevant Code of Federal Regulations | |
| 45 CFR Part 46 | Protection of Human Subjects |
| 21 CFR Part 50 | Protection of Human Subjects |
| 21 CFR Part 56 | Institutional Review Boards |
| 21 CFR Part 54 | Financial Disclosure by Clinical Investigators |
| 21 CFR Part 801 | Labeling (Devices) |
| 21 CFR Part 58 | Good Laboratory Practice for Nonclinical Laboratory Studies |
| 21 CFR Part 803 | Medical Device Reporting |
| 21 CFR Part 809 | In Vitro Diagnostic Products For Human Use |
| 21 CFR Part 814 | Premarket Approval of Medical Devices |
| 21 CFR Part 820 | Quality System Regulation |
| 21 CFR Part 822 | Postmarket Surveillance |
Investigational Device Exemptions (IDE), New Drug Application (NDA), Institutional Review Board (IRB), Food and Drug Administration (FDA)
The considerable heterogeneity of medical devices, from something as simple as dental floss to the complexity of an artificial organ, should give great appreciation to the need for guidance in understanding and applying the regulations governing these devices. Indeed, the FDA has issued extremely helpful guidance documents and maintains a comprehensive and highly usable website that is intended to assure access to understandable information. Clinical investigators as well as commercial developers should make use of these resources.
DEVICE AND STUDY CLASSIFICATIONS - IMPACT ON IDE REQUIREMENTS
Medical device is defined in the FD&C Act. Specifically, “A medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part, or accessory, which is … intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, … intended to affect the structure or any function of the body of man, and which does not achieve its primary intended purposes through chemical action within or on the body of man … and which is not dependent upon being metabolized for the achievement of its primary intended purposes”.(4) It is worth noting the latter part of the definition of a device may help in differentiating a device from a drug.
Based on the 1976 Medical Device Amendment, all marketed devices are classified into the three regulatory groups based on the inherent risk that their use presents to the patient. Accordingly, the level of regulations for the oversight of the device development and marketing is based on the classification risk. An essential component of device classification is the intended use of the device such that when a device is approved the labeling includes approved intended uses. An investigator conducting device research should understand the regulatory approach to these classifications in order to appreciate why any modifications to the device or applications outside of the labeled use of a device may dramatically change the associated risk and hence the classification. Both must be taken into account in assessing risk in the proposed research. (5, 6)
All classes of devices are subject to “general controls” which are the baseline general quality standards of the FD&C Act. (7) As complexity increases then manufacturing control and process verification necessary to meet FDA regulations increases proportionally. (8) Class I devices are the lowest risk and are subject only to general controls and are usually exempt from premarket notification and FDA approval prior to marketing. The manufacturers nonetheless must meet registration, device listing, manufacturing, and labeling requirements under “general controls”. (7) This contrasts with Class II and Class III devices requiring “special controls” to provide a reasonable assurance of safety and effectiveness. This may include special labeling requirements, performance standards, and postmarket surveillance. Most Class II devices require premarket notification (PMN also referred to as 510(k)) clearance by the FDA prior to marketing. Frequently these devices are based on an existing Class II device and, if judged substantially equivalent, can be marketed without further review. Occasionally, these devices will require submission of clinical data. Lastly, Class III devices are considered the highest risk and require a comprehensive review by the FDA via a Premarket Approval (PMA) prior to approval for marketing. The evidence from animal studies and clinical trials submitted under the PMA must show a reasonable assurance of the safety and effectiveness of the device. The FDA has established an extensive reference list of devices and guidance for determining the risk category for an unclassified device. (9)
REGULATORY SCOPE OF DEVICE RESEARCH
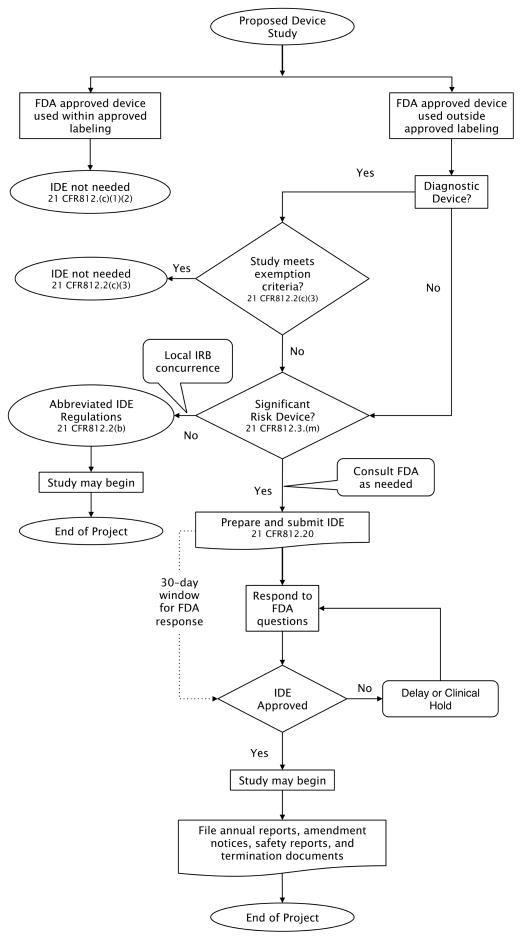
Regulatory authority for medical devices by the FDA extends to investigations of medical devices. The FDA has structured the regulatory oversight to match the risk to the subject with the risk of the device. Researchers at an academic health center wishing to conduct a study with the objective of assessing the safety and/or effectiveness of the device must comply with the Investigational Device Exemptions (IDE) in 21 CFR 812. (2) The regulations apply not only to studies of an unapproved device but also to the use of an approved device in an unapproved population or manner. For device research the FDA designates three types of device studies: 1) studies exempt from the IDE regulations, 2) nonsignificant risk (NSR) studies which require conduct as an abbreviated IDE application and 3) significant risk (SR) studies which require approval of IDE application (10). Figure 1 illustrates the IDE application scenarios for Sponsor-Investigators. It is important to understand that any modifications made to an approved device render the subsequent clinical study subject to the IDE regulations; this special case is not addressed further in this review.
FIGURE 1.
Flowchart for a clinical device study that may require an IDE application for a Sponsor-Investigator.
The FDA defines the Sponsor as a person or other entity that initiates the investigation (11). A Sponsor can be an individual as well as commercial enterprise or an academic institution. A Sponsor-Investigator is an individual who both initiates and actually conducts, alone or with others, a clinical investigation and under whose immediate direction the investigational device is administered, dispensed, or used. Therefore, the Sponsor-Investigator assumes the responsibilities of both the Sponsor and the Investigator. For individual clinical investigators who want to initiate and conduct research with a medical device complying with FDA regulations may seem insurmountable. But, since trials initiated by a clinical investigator at an academic health center would typically be small single site investigations, it is possible for an individual investigator to meet the regulatory responsibilities of both. Additionally, many institutions have resources available to assist in IND/IDE studies.(12)
Determining when an IDE is needed
Exempt Device Studies
The FDA sets criteria to define trials that are “exempt” from device regulation in Sec.812.2(c) of the IDE regulation. (2) Clinical investigations whose objective is to study the safety and/or effectiveness of an FDA-approved device in a manner within the approved labeling, meet the definition of exempt device research and require no additional FDA review or approval. Studies testing consumer preference of commercial devices, devices solely for veterinary use, research in laboratory animals, or in custom made devices also usually meet the exemption criteria in Sec.812.2(c)(1–2,4–7). Non-diagnostic device exemption criteria are listed in Sec.812.2(c) and the investigator should carefully review to see if the proposed study meets the detailed criteria. As with all research involving human subjects, these studies must conform to the existing Federal Policy for the Protection of Human Research Subjects. (1)
Exempt Diagnostic Device Studies
Exemption criteria also address diagnostic devices. Diagnostic device studies are exempt if the device meets labeling requirements, the testing is non-invasive, sampling procedures do not present a significant risk, the testing does not by design or intent introduce energy into a subject, and the testing is not used as a diagnostic procedure without confirmation by another medically established diagnostic product or procedure. Notably, there are specific definitions of “non-invasive” sampling as well as other device-specific definitions in Sec. 812.3. Here, the “significant risk” may lie in the sampling method. Therefore, interventions such as the biopsy of major organ, use of general anesthesia or significant prolongation of anesthesia time, prolonged hospitalization, increased risk of infection, etc., would present a significant risk to the patient and would not be considered an “exempt” study.
Significant Risk and Nonsignificant Risk Medical Device Studies
If the study does not meet exemption criteria, then an evaluation of the risk of the device as used in the study must be made. The first step is to determine if the device study meets the criteria of the significant risk (SR) study. Based on the definition in the regulations, a SR device is “intended as an implant and presents a potential for serious risk to the health, safety, or welfare of a subject; purported or represented to be for use supporting or sustaining human life and presents a potential for serious risk to the health, safety, or welfare of a subject; for a use of substantial importance in diagnosing, curing, mitigating, or treating disease, or otherwise preventing impairment of human health and presents a potential for serious risk to the health, safety, or welfare of a subject; or otherwise presents a potential for serious risk to the health, safety, or welfare of a subject.” (21 CFR 812.3) If the device does not meet the criteria above, it is considered to be a non-significant risk (NSR) device. The SR determination is based not only on a device itself but also in how it is used in the proposed investigation. For example, if the use of the device increases time for surgery, or requires that the subject undergoes an otherwise unnecessary procedure, or that the device is being used in combination with another surgical procedure that increases risk to the health, safety or welfare of the subject, the study should be considered SR study and FDA review of an IDE would be required. The Sponsor makes the initial assessment as to whether the device is NSR or SR. Thus, the Sponsor-Investigator makes the NSR/SR declaration to present to the governing IRB. The IRB then reviews the Sponsor-Investigator’s risk designation and either agrees or disagrees. For a NSR device, the local IRB will also make the determination as to whether the proposed use of the device in a study may constitute a change in the consideration of the device as NSR versus a SR. In this instance the local IRB is the FDA proxy for the SR/NSR determination. The FDA is available to help in making the determination, and in those cases the FDA is the final judge. The FDA has issued guidance to aid in making this determination.(10, 13)
Nonsignificant Risk Device Study
If the local IRB concurs that the device and use in the proposed study do not constitute a significant risk study, then by default it is a non-significant risk device study (NSR). Importantly, NSR research studies are not without regulatory requirements. They fall into the category of an abbreviated IDE and must meet regulatory requirements set forth in CFR 812.2(b). These include complying with the labeling of an IDE and prohibitions regarding promotion of Investigational Devices (CFR Part 812.7); as well as meeting the more general investigator obligations to obtain local IRB approval of the study and the agreement that the device is not a significant risk device; to have documentation of written informed consent approved by the reviewing IRB; to meet monitoring, recording keeping, and reporting requirements. Note that the IRB still must review and approve the study but no specific notification or approval from the FDA is required.
Significant Risk Device Study
Any device determined to be a “significant risk device” by the FDA will remain so designated until the FDA rules otherwise. Likewise, the proposed use of a device outside of its labeling may also confer a “significant risk” determination. Studies using investigational SR devices are required to file an IDE with the FDA for approval before starting.
The FDA provides helpful resources and guidance. There is specific guidance to assist Sponsor-Investigators as well as IRB members in assessing SR/NSR status of the studies. (10) Also, the FDA maintains a comprehensive and accessible website that is extremely useful in guiding device research under the heading of “Device Advice: Comprehensive Regulatory Advice“.(14) If the guidance and other webinars posted on the FDA website are insufficient for investigators and/or IRBs to make a SR/NSR determination, they may solicit an opinion from the FDA by way of an e-mail, a fax or a letter. (15) The inquiry should include a detailed device description (for each device, if more than one is in the study); the protocol for the study; a description of how the device will be used, if not included in the protocol; a description of the population, if not included in the protocol; and the sponsor’s name and contact person(s), including titles, address, phone number, fax number, and email address. The FDA will provide a written response documenting the decision making process and the response.
FILING AN INVESTIGATIONAL DEVICE EXEMPTION APPLICATION
General Principles
A Sponsor-Investigator filing an IDE follows the format and content described in 21 CFR 812.20. The FDA has provided a very useful guide for an IDE Application that includes a list of required elements, formats, a list of common problems, and a very helpful checklist.(16) If a Sponsor-Investigator has questions regarding the regulatory pathway that would be applicable for their investigation, early communication with the FDA is encouraged. Communication can be by telephone, emails, conference calls, videoconferences, or face-to-face discussions.(15) This can include both informal as well as formal meetings. To make these meetings most productive, preparing specific questions ahead of the meeting can greatly enhance efficiency and the ability of the FDA officials to be able to respond.(17) For Sponsor-Investigators at academic health centers, communication with both the FDA (Table 2) and, if available, the institution’s regulatory support office can greatly ease the application process.(12)
TABLE 2.
Contact information and mailing address for the CDRH at FDA
| Contact information for CDRH | 1-800-638-2041 301-796-7100 Fax: 301-847-8149 dsmica@fda.hhs.gov Food and Drug Administration 10903 New Hampshire Avenue WO66-5429 Silver Spring, MD 20993 |
|
| |
| IDE submissions to CDRH | Food and Drug Administration Center for Devices and Radiological Health Document Mail Center - WO66-G609 10903 New Hampshire Avenue Silver Spring, Maryland 20993-0002 |
|
| |
| FDA website for CDRH contact information | About the Center for Devices and Radiological Health. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/default.htm |
FDA issued guidance provides a thorough description of the required information.(16) There is also an optional form that can help structure the application (Form 3514). (18) Throughout the application, the investigator should focus on providing information to the agency reviewers that shows that the research participant’s safety is paramount. The guidance states “The sponsor must demonstrate in the application that there is reason to believe that the risks to human subjects from the proposed investigation are outweighed by the anticipated benefits to subjects and the importance of the knowledge to be gained, that the investigation is scientifically sound, and that there is reason to believe that the device as proposed for use will be effective.”(13) Since the study will be using an unmodified FDA approved device, there will be no need to present the FDA the information regarding the manufacture of the device, other than referral to its latest approved label. The objective is to present information that supports the premise that the proposed use in the study presents no unjustified additional risk and offers the possibility of benefit when compared to the approved labeled use of the device while maintaining subject safety. Lastly, the complexities of conducting a multi-site study as a Sponsor-Investigator are beyond the scope of this review and are not addressed.
IDE Content and FDA Guidance
The cover page should identify the submission as an “Original Investigational Device Exemption Application” and the sponsor should sign the page. Likewise, to facilitate review, include a Table of Contents for the application. The IDE should be prefaced with a cover letter. The letter should provide a brief snapshot of the project. Namely, include the name and manufacturer of the device, the intended use of the device, the name of the sponsor-investigator including contact information plus a secondary contact, reference any additional information such as a pre-submission meeting (previously called a Pre-IDE meeting) or prior conferences with the FDA, whether any waiver from the IDE requirements is requested and justification, and whether there are existing referenced files for the device that the manufacturer grants access (include a letter from the manufacturer to so indicate). Otherwise, indicate that the study will use a commercially available device and request to have the submission of manufacturing data waived.
Required Elements for an IDE (Sec. 812.20)
Include in the following order:
Name and address of sponsor. In this case the information provided should be of the Sponsor-Investigator. Even if the manufacturer supplies the device, and/or is financial sponsor of the study, the term “sponsor” in this context represents the person that assumes regulatory responsibilities for the study conduct. It is important that the contact information be complete and accurate. The correspondence address, email address, and telephone number should be the most effective contact information for the Sponsor-Investigator.
Report of prior investigations. For Sponsor-Investigators using a commercial device, the manufacturer has already submitted the information supporting the safety and efficacy of the device for the labeled indications needed by the FDA. Therefore, it is advisable to refer to the latest FDA approved label and provide the URL link. The FDA will, however, also want information supporting the proposed use in the study that is not covered in the approval data. Include relevant reports of all prior clinical, animal, and laboratory testing of the device. The information presented should comprehensive and adequate to justify the proposed investigation. Include a bibliography of all publications, whether adverse or supportive, that are relevant to an evaluation of the safety or effectiveness of the device, copies of all published and unpublished adverse information, and, if requested by an IRB or FDA, copies of other significant publications. A qualified medical librarian should be able to assure that you have a focused literature search. Also provide any unpublished information, if available. If nonclinical laboratory studies are included, indicate whether or not they were conducted under Good Laboratory Practice regulations.(19)
-
The Investigational Plan. A critical part of the IDE is the scientific inquiry that drives the investigation. An investigation that is unlikely to yield valid information would not justify putting any subject at risk to conduct. Hence, the investigational plan must be well thought out and carefully presented. If this is a pilot or feasibility study, clearly indicate the limited nature of the investigation. It must include the following items:
Purpose. Include the name and intended use of the device, the objectives and duration of the investigation.
Protocol. Include a description of the methods, objectives, and analysis. Device trials do not always lend themselves to control groups or blinding as used in drug trials. A clear indication of analytical approach will help the reviewer evaluate the scientific soundness. A qualified biostatistician can greatly help in developing a protocol. Include clearly stated objectives, primary and secondary endpoints, methods to assure an appropriate subject population, methods of data acquisition, and methods of analysis including the statistical approach.
Risk Analysis. Develop a description and analysis of the risks and approach to minimizing these risks. Include a justification of the risks. Provide a description of the participant population including number, age, sex, and condition. If any vulnerable populations will be included, e.g. pediatric subjects, provide a justification.
Description of the device. Reference the FDA approved label.
Monitoring Procedures. The Sponsor-Investigator is responsible for monitoring the investigation. This is true for single site studies as well as multi-site. However, if conducting a single-site study, based on current FDA guidance, you are not required to submit the written monitoring procedure with your application.
Any additional records and reports.
Since the study will employ a device already in commercial production, there is no need to include information on the manufacture, processing, or packaging of the device, other than to refer to its approved label. If there are deviances to labeled storage or installation of the device, then include details in the application.
Provide an example of the Investigator’s agreement. The Sponsor-Investigator’s obligations as a sponsor include accountability for all investigators conducting research on the IDE. The sponsor-investigator should select qualified investigators with appropriate training and experience and the FDA should be informed of all the investigators conducting the study. These investigators will be expected to sign a commitment to: conduct the investigation in accordance with the agreement, the investigational plan, applicable FDA regulations, and conditions of approval imposed by the reviewing IRB or FDA; agreement to supervise all testing of the device involving human subjects; and willingness to ensure that the requirements for obtaining informed consent are met. Include a list of the investigators including names and addresses, a copy of the investigator’s curriculum vita including relevant experience, including the dates, location(s), extent, and type of relevant experience of all investigators who have signed the agreement. Accurate financial disclosure information consistent with disclosures to the local IRB should be included. The FDA has issued guidance for IRBs, clinical investigators, and sponsors. (20)
The Sponsor-Investigator should include a statement that all investigators who will participate in the investigation have signed the agreement and a statement that the list of investigators is inclusive and that any investigator will sign the agreement before being added.
Provide information about the IRB that has or will review the investigation, including the name, address, and chairperson. If previous actions have been taken by the IRB, a certification of those should be included.
State that this will be a single site study. Note that to the FDA a “site” is under the jurisdiction of a single IRB, so, a single site may have more than one physical location, e.g. multiple clinics or hospitals.(21) Therefore, list the name and address of the institution where the investigation will take place.
If there is a charge for the device, include the amount and an explanation of why the sale does not constitute commercialization of the device. Note that commercialization, promotion, and misrepresentation of an investigational device are prohibited.
Environmental Impact Considerations. This is no longer required in the IDE.
Include the labeling for the device. Because the use in the trial is investigational the device must be labeled accordingly. The label should include the name and place of business of the manufacturer, packer, or distributor, the quantity of contents if appropriate, and the following statement: “CAUTION--Investigational device. Limited by Federal (or United States) law to investigational use.” If applicable to the investigation include relevant contraindications, hazards, adverse effects, interfering substances or devices, warnings, and precautions. The label should not bear any statement that is false or misleading.
Include a copy of the consent form and any informational materials to be provided to the subjects used to obtain consent.
Provide any other information necessary for review. In discussions with the FDA, the official may indicate a need for additional information. Reference any previously submitted documents.
Submitting an IDE
Submit three identical copies (1 original and 2 copies) on 8½″ by 11″ 3-hole punched bound copies (or in binders, different colors for original and copies). Leave at least a 1½″ wide left margin to allow for binding. Separate into volumes and identify volume number if the submission exceeds 2″ in thickness. Label the binders to identify the submission as an original IDE application. For subsequent submissions identify the FDA assigned document number and the reason for and type of submission. Be certain that the pages are sequentially numbered, that there is a table of contents, and that the sections are separated with labeled tabs. The outer packaging of the submission should likewise identify the contents consistent with the cover letter and binder identification. Table 2 has contact information and mailing address for the CDRH. Additionally, the FDA website has information on the organizational structure and contact information. This includes a list of the divisions and offices. (22)
FOLLOWING RECEIPT OF THE IDE BY THE FDA
The appropriate division for review will handle the IDE application. The FDA will notify the Sponsor-Investigator in writing the date of receipt of the application. They will assign an IDE number and a lead reviewer to address in future submissions under the IDE. There is a 30-day waiting period for review during which the FDA may approve, approve with conditions, or disapprove an IDE application. Note that the FDA may request additional information at any point in the review process. The study may begin 30 days after the date of submission receipt unless the FDA notifies the sponsor otherwise.
Responding to the FDA
All correspondence with the FDA for the IDE should be clearly labeled with the identifying information for the IDE and the type of document and sequentially numbered for your reference and the FDA’s.
Registration with Clinicaltrials.gov
Registration requirements under Public Health Law 110-85 stipulate that controlled device trials with health outcomes are accompanied by registration at the Protocol Registration System (clinicaltrials.gov) with the exception of small feasibility studies. (23) Investigators at academic health centers should also be aware that the agreement of the International Committee of Medical Journal Editors requires prospective registration of all interventional clinical trials, including devices, for all publications derived from the clinical investigation. Note that the registration must be complete before enrolling any subject. (24)
REGULATORY REQUIREMENTS FOR AN IDE DURING THE STUDY AND AT COMPLETION
Submission of an IDE begins the regulatory process for a study. In doing so, the Sponsor-Investigator commits to an ongoing process. The IDE is a dynamic document and must be kept current as the study progresses. In brief, the Sponsor-Investigator agrees to keep the FDA as well as the IRB apprised of any adverse events, any changes or amendments in the protocol; to file annual reports; and to notify the FDA at the completion of the study. The FDA guidance has an explanation and description of required records (21 CFR 812.40) and reports (21 CFR 812.150).(25) For each of these reports, clearly indicate the type of report in the correspondence and documents for the report.
Investigational Plan Modifications (21 CFR 812.35)
It is to be expected that there will be numerous changes to any one of the component parts. However, for a Sponsor-Investigator held IDE investigating an FDA approved device being used in an off-label indication, most modifications will be in the Investigational Plan. If a modification occurs while the IDE application is under review, the change is called an amendment. Conversely, if the IDE is already approved, the modification is called a supplement. Depending upon the nature of the modification, prior approval from the FDA may be required before implementation of the change is made. In general, changes that require prior approval are those that have a significant effect on the validity and scientific soundness of the trial (e.g. changes in the indication, type of study control, primary end point, sample size etc.). Those changes should be submitted as an IDE supplement describing the type and reason for changes as well as necessary supporting documentation. FDA will review those submissions and will issue an approval, approval with conditions, or disapproval letter within 30 days.(26)
Changes that do not require prior FDA approval may nonetheless need to be reported to the FDA within the 5 working days of the change implementation (often referred to as a 5-day notice). As per the FDA guidance, examples of modifications eligible for the 5-day notice submission are modifications of the inclusion/exclusion criteria for better targeting the patient population, modification of the secondary endpoints, additional patient observation, etc. Those supplements should be identified as “Notice of IDE change”, and unless there is disagreement with the implemented change, there will not be any response to the Sponsor-Investigator.(26)
Note that modifications to the investigational plan to protect the life or physical well being of a subject in an emergency must also be reported to the FDA within 5 days. Prior approval of the FDA is not required before implementation due to the necessity of preventing harm to subjects.
Finally, minor changes to the investigational plan that do not affect the validity of the data, risk/benefit ratio to the patient, scientific soundness of the study and the rights, safety and welfare of the subject, can be submitted in the progress report. Examples of those changes would include change to the name of the device, changes to the name and address of the monitors, minor changes to the labeling, informed consent, etc.
Reporting Responsibilities for Sponsor-Investigators (Sec. 812.150)
Regulations require that progress reports be submitted by a Sponsor-Investigator to the Agency on an annual basis, at a minimum. As with all FDA reports, include the IDE number and identifying information. The Progress report should include a summary of progress to date, a summary of results, a summary of anticipated and unanticipated adverse effects, any deviations from the protocol, any minor changes to the protocol, and any changes to the risk analysis. State future plans including any anticipated changes to the investigation. Include any resulting publications from the study. Note that this report will be very similar to the report required by the local IRB and both must be filed well before the annual date of respective approvals.
Other reports include notifying the FDA if the local IRB withdraws approval, any device recalls or withdrawal of FDA approval, changes to the consent form, a current list of investigators, and unanticipated serious adverse device events.(25)
Monitoring Responsibilities for Sponsor-Investigators
The FDA states that submission of written monitoring procedures are not required for studies sponsored by a Sponsor-Investigator where only one investigator is involved in the study.(21) This does not mean that monitoring is not required. Monitoring of the study is an ongoing responsibility and unless otherwise specified in the IDE, the Sponsor-Investigator will be responsible for assuring and documenting that human subjects are adequately protected and that the trial is conducted in compliance with the protocol and regulations. Most institutions have within the research administration a research compliance component or a quality assurance unit that can assist the Sponsor-Investigator in meeting the monitoring requirements. Monitoring for multi-center studies is much more complex and requires a substantial effort on the part of a Sponsor-Investigator to meet the regulatory requirements and is not addressed in this review. (27)
Safety Reports
Sponsor-Investigators are responsible for investigating all safety concerns and reporting to both the FDA and the IRB as needed. An unanticipated adverse device effect is “any serious adverse effect on health or safety or any life-threatening problem or death caused by, or associated with, a device, if that effect, problem, or death was not previously identified in nature, severity, or degree of incidence in the investigational plan or application (including a supplementary plan or application), or any other unanticipated serious problem associated with a device that relates to the rights, safety, or welfare of subjects” (21 CFR 812.3(s)). Any unanticipated adverse device events must be reported to FDA and the reviewing IRB as well as all participating investigators within 10 working days after the Sponsor-Investigator is aware of the occurrence. The FDA and IRB may request additional reports. Such reports should be clearly labeled “IDE Safety Report”.(28)
Likewise, any deviations from the investigational plan that are taken to protect the life or physical well being of a subject in an emergency must be reported to the FDA and reviewing IRB no later than 5 working days after the emergency deviation occurred.
WITHDRAWAL, TERMINATION, AND FINAL REPORT
An IDE will end at some point in time. A Sponsor-Investigator may terminate an IDE study. If the IDE is not approved, it may be withdrawn. If the IDE is approved but there are no subjects enrolled, the IDE may also be withdrawn. Once subjects have been enrolled, the FDA and reviewing IRB should be notified of the end of the study in a written report. In this event, enrollment ceases, but the subjects should be followed as detailed in the investigational plan. If the withdrawal is for safety concerns, the report must provide a full report of the necessity for the action. When all subjects have reached the end of follow-up, a final report is filed. For a SR device study the Sponsor-Investigator must notify the FDA and the reviewing IRB within 30 working days of the termination or completion of the study and submit a final report to the FDA within six months.(16) The FDA may terminate a study, as well, with or without the agreement of the investigator.
CONCLUSIONS
Clinical investigators must meet regulatory requirements in order to conduct research with FDA regulated products. Many investigators see these requirements as too intimidating to attempt to do important and innovative studies. The FDA has made much of the information easily accessible and available on their website. They also have made it easy to contact and work with the officials who are responsible for IDE research. With guidance and direction the requirements for an individual investigator who wants to do research with an FDA approved device are readily met. Indeed, medical device research including the filing and maintenance of an IDE is well within the scope of investigators at academic medical centers. Meeting regulatory requirements for medical device studies should not be regarded as an obstacle to conducting clinical research.
Acknowledgments
This work was conducted with support from UT-STAR, NIH/NCRR Grant Number UL1RR024982 and NIH/NCRR Grant Number UL1RR024128. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, or the National Institutes of Health.
Contributor Information
M. E. Blair Holbein, Email: Blair.Holbein@UTSouthwestern.edu, University of Texas Southwestern Medical Center, Department of Clinical Sciences, 5323 Harry Hines Blvd., Dallas, Texas 75390-9066, Phone: 214-648-5009, Fax: 214-548-3934
Jelena Petrovic Berglund, Email: Jelena.petrovic@duke.edu, Duke University School of Medicine, Duke Translational Medicine Institute (DTMI), 2424 Erwin Rd. Suite 402, P.O. Box 17969, Durham, NC 27715, Phone: 919-668-4639, Fax: 919-668-7868
References
- 1.Protection of Human Subjects. Title 45 C.F.R. Part 46. (2011)
- 2.Medical Devices. Title 21 C.F.R. Part 812 Subchapter H (2011)
- 3.Federal Food, Drug, and Cosmetic Act, 21 U.S.C. (2010 Edition).
- 4.Federal Food, Drug, and Cosmetic Act, 21 U.S.C. Subchapter II – Definitions (2010 Edition).
- 5. [Accessed May 30, 2012.];Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors: Frequently Asked Questions About Medical Devices. 2006 Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM127067.pdf.
- 6. [Accessed May 30, 2012.];Device Classification. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/default.htm.
- 7. [Accessed May 30, 2012.];General and Special Controls. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/GeneralandSpecialControls/default.htm.
- 8. [Accessed May 30, 2012.];Design Control Guidance For Medical Device Manufacturers. http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm070627.htm.
- 9. [Accessed May 30, 2012.];Device Classification Panels. 2011 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/ucm051530.htm.
- 10. [Accessed May 30, 2012.];Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors: Significant Risk and Nonsignificant Risk Medical Device Studies. 2006 Available from: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126418.pdf.
- 11. [Accessed May 30, 2012.];IDE Definitions and Acronyms. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm046698.htm.
- 12.Berro M, Burnett BK, Fromell GJ, Hartman KA, Rubinstein EP, Schuff KG, et al. Support for investigator-initiated clinical research involving investigational drugs or devices: the Clinical and Translational Science Award experience. Acad Med. 2011;86(2):217–23. doi: 10.1097/ACM.0b013e3182045059. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed May 29, 2012.];Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors. 2006 Available from: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM127067.pdf.
- 14. [Accessed May 30, 2012.];Device Advice: Comprehensive Regulatory Advice. 2012 Available from: http://www.fda.gov/medicaldevices/deviceregulationandguidance/default.htm.
- 15. [Accessed May 30, 2012..];Procedures for Handling Inquiries Regarding the Need for an Investigational Device Exemptions Application for Research Involving Medical Devices. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm126598.htm.
- 16. [Accessed May 30, 2012];IDE Application. 2011 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm046706.htm.
- 17.Spurgin EA. Planning for effective interaction with FDA. Diabetes Technol Ther. 2004;6(6):770–5. doi: 10.1089/dia.2004.6.770. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed May 29, 2012.];CDRH Premarket Review Submission Cover Sheet. Available at: http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM080872.pdf.
- 19.Good Laboratory Practice For Nonclinical Laboratory Studies. Title 21 C.F.R. Part 58.
- 20. [Accessed May 30, 2012.];IDE Financial Disclosure. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm051337.htm.
- 21.FDA. [Accessed May 30, 2012.];Guidance on IDE Policies and Procedures. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm080202.htm.
- 22. [Accessed May 30, 2012.];About the Center for Devices and Radiological Health. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/default.htm.
- 23.US Public Law 110-85 (Food and Drug Administraiton Amendments Act of 2007), Title VIII, Section 801.
- 24.DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration. JAMA. 2004;292:1363–4. doi: 10.1001/jama.292.11.1363. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed May 30, 2012.];IDE Reports. 2009 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm046717.htm.
- 26.FDA. [Accessed May 30, 2012.];Changes or Modifications During the Conduct of a Clinical Investigation; Final Guidance for Industry and CDRH Staff. 2011 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm082145.htm.
- 27. [Accessed May 30, 2012.];Guidance for Industry Oversight of Clinical Investigators: Oversight of Clinical Investigations — A Risk-Based Approach to Monitoring. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM269919.pdf.
- 28. [Accessed May 30, 2012.];FAQs about IDE. 2011 Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm051480.htm.