Abstract
Haploinsufficiency of the SCN1A gene encoding voltage-gated sodium channel NaV1.1 causes Dravet Syndrome (DS), a childhood neuropsychiatric disorder including recurrent intractable seizures, cognitive deficit, and autism-spectrum behaviors. The neural mechanisms responsible for cognitive deficit and autism-spectrum behaviors in DS are poorly understood. Here we show that mice with Scn1a haploinsufficiency display hyperactivity, stereotyped behaviors, social interaction deficits, and impaired context-dependent spatial memory. Olfactory sensitivity is retained, but novel food odors and social odors are aversive to Scn1a+/− mice. GABAergic neurotransmission is specifically impaired by this mutation, and selective deletion of NaV1.1 channels in forebrain interneurons is sufficient to cause these behavioral and cognitive impairments. Remarkably, treatment with low-dose clonazepam, a positive allosteric modulator of GABAA receptors, completely rescued the abnormal social behaviors and deficits in fear memory in DS mice, demonstrating that they are caused by impaired GABAergic neurotransmission and not by neuronal damage from recurrent seizures. These results demonstrate a critical role for NaV1.1 channels in neuropsychiatric functions and provide a potential therapeutic strategy for cognitive deficit and autism-spectrum behaviors in DS.
Dravet Syndrome (DS), also called Severe Myoclonic Epilepsy of Infancy, is an intractable developmental epilepsy syndrome with seizure onset in the first year of life1. However, unlike other generalized epilepsy disorders, it is accompanied by characteristic neuropsychiatric comorbidities, including hyperactivity, attention deficit, delayed psychomotor development, sleep disorder, anxiety-like behaviors, impaired social interactions, restricted interests, and severe cognitive deficits1–6. These comorbidities in DS overlap with symptoms of autism-spectrum disorders (ASD), and a recent study suggests that DS patients have autism-spectrum behaviors3. DS is caused by heterozygous loss-of-function mutations in the SCN1A gene7, which encodes the pore-forming α-subunit of the brain voltage-gated sodium channel type-1 (NaV1.1)8. As in DS, mice with heterozygous loss-of-function mutation in Scn1a (Scn1a+/−) have thermally induced and spontaneous seizures, premature death, ataxia and sleep disorder9–13. NaV1.1 channels are expressed in cell bodies and axon initial segments of excitatory and inhibitory neurons in the brain14–16, but deletion of NaV1.1 impairs Na+ currents and action potential firing of GABAergic interneurons specifically because NaV1.1 is the primary Na+ channel in those cells9,15,16. Specific deletion of NaV1.1 channels in forebrain interneurons using a Cre-LoxP strategy recapitulates the symptoms of DS in mice (Cheah, C. S., Yu, F. H., Westenbroek, R. E., Kalume, F. K., Oakley, J. C., Rubenstein, J. L., Catterall, W. A., Soc. Neurosci. Abst. 155.16, 2010)17, confirming that loss of NaV1.1 in GABAergic interneurons causes this disease. Emerging genetic evidence implicates SCN1A in autism18–22, and there is increasing evidence that dysfunction of GABAergic signaling is associated with ASDs23–25, leading to the proposal that elevation of excitation/inhibition ratio in neocortical neurons is the primary cause of ASD26–29. In this study, we have investigated autism-related behaviors in Scn1a+/− mice and shown that they are caused by impaired GABAergic neurotransmission that can be rescued by drug treatment.
Scn1a+/− mice exhibit hyperactivity, anxiety, and excessive stereotyped behaviors
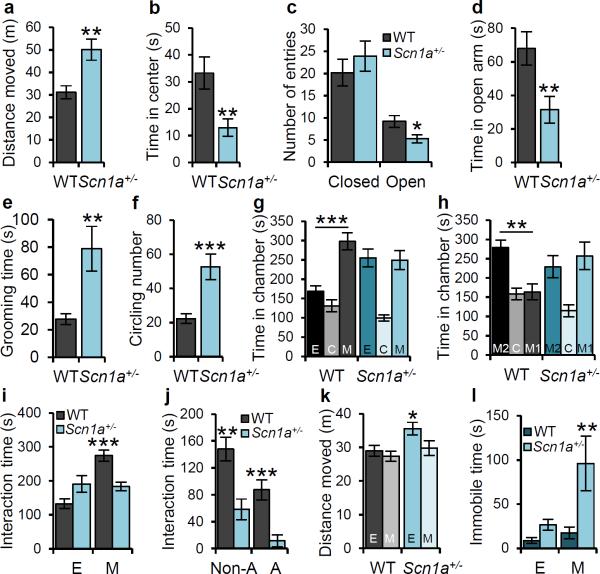
Homozygous Scn1a−/− mice developed severe ataxia and died on postnatal day (P) 15, whereas Scn1a+/− mice had spontaneous seizures and sporadic deaths beginning after P219. Scn1a+/− mice develop multiple behavioral phenotypes, which are phenocopies of comorbidities in DS. During a 10-min open-field test, adult Scn1a+/− mice traveled significantly longer than WT (Fig. 1a) but spent less time in the center of the open field (Fig. 1b, Supplementary Fig. 1). In the elevated plus maze, Scn1a+/− mice entered open arms less frequently compared with WT (Fig. 1c), and spent less time in the open arms (Fig. 1d, Supplementary Fig. 2). Scn1a+/− mice also spent more time self-grooming than WT (Fig. 1e, Supplementary Fig. 3a) and displayed increased circling behavior (Fig. 1f, Supplementary Fig. 3b). These observations indicate that Scn1a+/− mice exhibit hyperactivity, increased anxiety, and increased stereotyped behaviors, which are phenocopies of autistic traits in DS. Scn1a+/− mice also showed decreased nest-building ability compared to WT (Supplementary Fig. 4), which could indicate deficits in social behavior30.
Figure 1. Scn1a+/− mice display hyperactivity, anxiety-like behavior, increased stereotypies, poor nest-building, and impaired social behavior.
In the open field test, Scn1a+/− mice travel longer distances compared with WT mice (a), spend less time in the center (b), spend more time grooming (e) and circling (f) than WT mice. In the elevated plus maze, Scn1a+/− mice enter less frequently in the open arms (c) and spend less time in the open arms (d). In (f) one complete turn is counted as one circle, regardless of direction. g, h, Three-chamber experiment. g, Whereas WT mice spend more time in the chamber housing a stranger mouse (M) than the chamber housing an empty cage (E), Scn1a+/− mice have no preference for either chamber. h, Whereas WT mice spend more time in the chamber housing a novel mouse (M2) than in a chamber housing a familiar mouse (M1), Scn1a+/− mice have no preference for either chamber. i - l, Social interaction test. i, Scn1a+/− mice show decreased interaction with a caged stranger mouse when compared with WT mice. j, In a 10-min reciprocal interaction test, pairs of WT and Scn1a+/− unfamiliar mice had significantly less non-aggressive (Non-A) and aggressive (A) interactions than pairs of WT and WT unfamiliar mice. Aggressive behaviors included attacking, wrestling, and biting the dorsal surface, and non-aggressive behaviors include nose-to-nose sniffing, anogenital sniffing, and grooming. All data shown are means ± s.e.m. from 10 – 12 mice per genotype. *P < 0.05, **P < 0.01, ***P < 0.001. k, Scn1a+/− mice move significantly less when they encountered the stranger mouse compared to an empty cage, whereas there is no difference in movement for WT. l, Scn1a+/− mice, but not WT mice, show increased immobilization behavior in the presence of the caged stranger mouse than in the presence of an empty cage.
Scn1a+/− mice show deficits in social interaction behaviors
We performed behavioral tests to assess deficits in social interaction, a prominent symptom of ASD31. A three-chamber experiment showed that Scn1a+/− mice displayed profound deficits in social interaction. Both Scn1a+/− and WT had no preference for two empty cages, located in the right and the left chambers during a habituation period (Supplementary Fig. 5, 6, 7a). However, when we put a stranger mouse in the cage in one chamber, WT mice spent more time in the mouse-containing chamber than in the empty cage-containing chamber (Fig. 1g, Supplementary Fig. 5), and interacted more extensively with peer mice than with the empty cage (Supplementary Fig. 7b). In contrast, Scn1a+/− mice displayed no preference for the stranger mouse (Fig.1g, Supplementary Fig. 5, 7b). When a second stranger mouse was placed in the unoccupied side chamber to assess the discrimination between a novel and a familiar mouse, WT mice showed strong preference for the novel mouse, but Scn1a+/− mice did not (Fig. 1h, Supplementary Fig. 5, 7c), even though they have preference for novel objects (see below). We observed similar social deficits of Scn1a+/− mice in the social interaction test. Scn1a+/− mice interacted significantly less with a caged stranger mouse in an open field compared with WT (Fig. 1i, Supplementary Fig. 8a). When both the inanimate object and the caged stranger mouse were introduced simultaneously, WT mice interacted significantly more with a caged stranger mouse than with an empty cage, whereas Scn1a+/− mice displayed no preference for the caged mouse (Supplementary Fig. 9a, b). We also examined reciprocal social interactions of freely moving Scn1a+/− and WT littermates with test mice. Scn1a+/− mice displayed decreased duration of both non-aggressive and aggressive interactions (Fig. 1j). We observed that Scn1a+/− mice displayed increased immobilization behavior when they encountered the caged stranger mouse (Supplementary Fig. 8b). Compared to WT, this immobilization decreased distance traveled (Fig. 1k) and increased immobilization time by 400% (Fig. 1l). Taken together, these results indicate that Scn1a+/− mice display profound deficits in social behavior.
In nocturnal rodents, social interaction and olfactory perception are tightly associated32, and impairment of olfactory perception leads to decreased social interaction33. We assessed olfaction in modified three-chamber experiments in which one tightly sealed petri dish containing food pellets and an identical one with holes were placed in the side chambers. Both Scn1a+/− and WT mice spent more time in the food-odor chamber, showed a shorter latency to enter it, and entered it more frequently than the odorless chamber (Supplementary Fig. 10a–d). Alternatively, we used bedding from male or female cages as a social odor. WT mice displayed strong preference for the chamber containing bedding, whereas Scn1a+/− mice displayed no preference for these social odors (Supplementary Fig. 11a, b, d, e). In close-interaction analysis, Scn1a+/− mice avoided interacting with male social cues (Supplementary Fig. 11c), and both WT and Scn1a+/− mice displayed strong avoidance to fox urine (Supplementary Fig. 11f). WT displayed strong habituation and dishabituation to odors of banana, male urine, and standard food, whereas Scn1a+/− mice gave a normal response to food but failed to display habituation/dishabituation to banana or male urine (Supplementary Fig. 12a). However, Scn1a+/− mice displayed greatly increased digging behavior when banana and male urine odors were presented, indicating that they detect these odors (Supplementary Fig. 12b). Moreover, in a Y-maze olfactory choice test, Scn1a+/− mice showed strong avoidance to banana and male urine, whereas WT mice displayed strong preference to both (Supplementary Fig. 12c, d). These data indicate that Scn1a+/− mice perceive food odors and social olfactory cues, but they have no interest or avoid unfamiliar odors and social odors. These results further establish a deficit in social interaction34,35 and avoidance of environmental change36 in Scn1a+/− mice, as in ASDs.
Scn1a+/− mice show deficits in context-dependent spatial memory
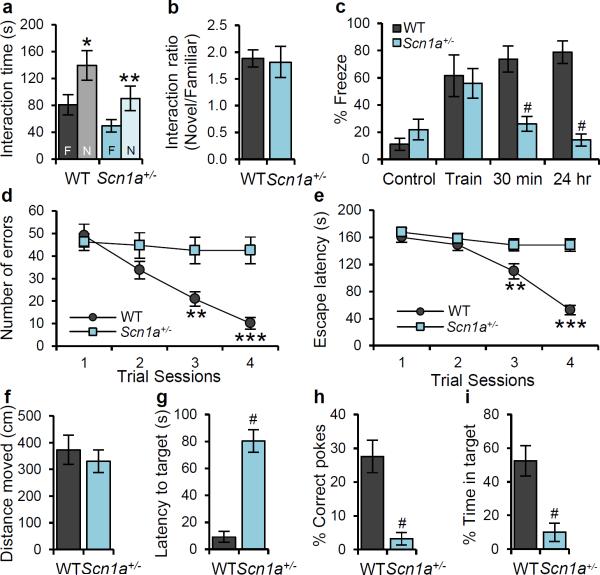
Both WT and Scn1a+/− mice showed a similar ability to recognize a novel object 24 h after training (Fig. 2a, b). In the context-dependent fear-conditioning test, Scn1a+/− and WT mice displayed no freezing behavior during the habituation period in context, and both of them showed similar freezing behavior immediately after a mild foot shock (Fig. 2c). However, whereas WT mice displayed sustained freezing behaviors when returned to the shock cage 30 min and 24 h later, Scn1a+/− mice displayed substantially reduced freezing behavior (Fig. 2c). The loss of fear-associated freezing behavior was specific because measurements of distance and velocity of movement during the fear-conditioning test did not reveal other fear-associated responses such as panic fleeing (Supplementary Fig. 13).
Figure 2. Profound deficits in context-dependent spatial learning and memory formation in Scn1a+/− mice.
a, In the novel object recognition test, Scn1a+/− mice show normal recognition memory for preconditioned object (F: Familiar), which was presented 24 hr before the test, so that they spend more time with novel object (N: Novel). b, Discrimination index, the normalized ratio of time spent with the familiar object and time spent with the novel object, shows that there is no difference between WT and Scn1a+/− mice for novel object recognition ability. c, In the contextual fear conditioning test, Scn1a+/− mice display a profound deficits in short-term (30 min) and long-term (24 hr) memory of 2 s mild foot shock (0.5 mA)-associated context, but normal fear response immediately after the training (Train) when compared to WT mice. d, e, In the Barnes circular maze test, Scn1a+/− mice display profound deficit in spatial learning. WT mice make less errors to find the target hole (d), and display decreased latency to escape the maze (e) during the 4-day repeated training trials, but Scn1a+/− mice display no improved performance for both the number of errors made to find the target hole (d), and the time to escape the maze (e). f - i, During the probe trial at 5th day of trials, Scn1a+/− mice display profound deficit in spatial memory. They spend significantly more time to find the target hole (g), poke target hole with significantly lower correct choice (h), and stay significantly less time in the target area (i) when compared with WT mice, although total moved distance is not significantly different with that of WT mice (f). All data shown are means ± s.e.m. from 6 – 10 mice per genotype. *P < 0.05, #, ***P < 0.001.
To assess spatial learning and memory in the absence of fear, we performed the Barnes circular maze test in which mice learn to rapidly escape a brightly lighted circular field by finding a specific dark hole at its periphery. Scn1a+/− mice failed to improve their learning performance during four days of training (Fig. 2d, e), and displayed substantially reduced spatial memory during the probe trials at day 5 (Fig. 2f – i). These data, together with the results of the context-dependent fear-conditioning test (Fig. 2c), indicate that Scn1a+/− mice have severely impaired spatial learning and memory.
Conditional Scn1a+/− mutant mice show autism-related behaviors
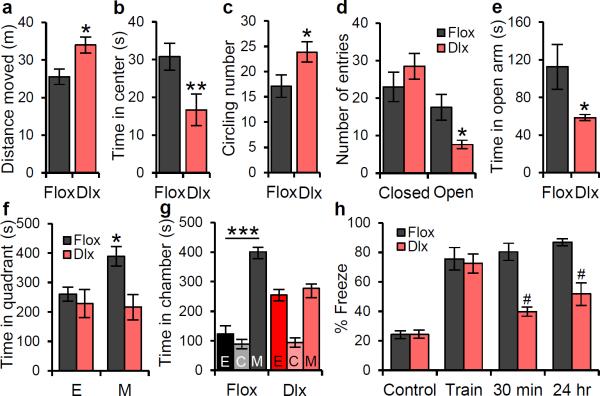
To determine whether the autism-related phenotypes of Scn1a+/− mice emerge specifically from reduced NaV1.1 activity in forebrain GABAergic neurons, we generated forebrain GABAergic neuron-specific conditional Scn1a+/− mutant mice using the Dlxi12b-Cre Cre-recombinase mouse line (Dlx1/2-Cre; Cheah, C. S., Yu, F. H., Westenbroek, R. E., Kalume, F. K., Oakley, J. C., Rubenstein, J. L., Catterall, W. A., Soc. Neurosci. Abst. 155.16, 2010)17,37. These mice have a specific reduction of NaV1.1 channels in forebrain GABAergic neurons and have similar epilepsy and premature death as Scn1a+/− mice38. Dlx1/2+ Scn1a heterozygous mutant mice (Dlx1/2-Scn1a+/−) recapitulated the autism-related phenotypes and spatial learning deficit of Scn1a+/− mice (Fig. 3), whereas control Cre-positive Scn1a+/+ mice did not (Supplementary Fig. 14). In the open field test, Dlx1/2-Scn1a+/− mice traveled more (Fig. 3a), spent less time in the center (Fig. 3b), and displayed increased circling behavior (Fig. 3c) 17when compared with Cre-negative Scn1aloxp/+ mice. In the elevated-plus maze test, Dlx1/2-Scn1a+/− mice entered the open arms less frequently and spent less time in open arms than Cre-negative Scn1aloxp/+ mice (Fig. 3d, e). In the social interaction test, Dlx1/2-Scn1a+/− mice spent less time interacting with the caged stranger mouse compared to Cre-negative Scn1aloxp/+ mice (Fig. 3f). In addition, in the three-chamber test Cre-negative Scn1aloxp/+ mice stayed longer in the mouse chamber vs. the inanimate-object chamber; in contrast, Dlx1/2-Scn1a+/− mice showed no preference (Fig. 3g). Finally, in the contextual fear-conditioning test, Dlx1/2-Scn1a+/− mice displayed similar freezing behavior in control and training sessions, but significantly less freezing behavior in the 30 min and 24 h after the training compared with Scn1aloxp/+ mice (Fig. 3h). These results show that Dlx1/2-Scn1a+/− mice reproduce hyperactive and anxiety-like behaviors, deficits in social interactions, and impaired context-dependent fear conditioning of global Scn1a+/− mice. This evidence indicates that the autism-related phenotype emerges from reduced NaV1.1 activity specifically within forebrain GABAergic interneurons.
Figure 3. Dlx1/2-Scn1a+/− mice have the impaired spatial learning and autism-related phenotypes observed in Scn1a+/− mice.
a, In the open field test, Dlx1/2-Scn1a+/− mice run longer compared with Cre-negative floxed littermate mice. b, In the open field test, Dlx1/2-Scn1a+/− mice spend less time in the center during the open field test. c, Dlx1/2-Scn1a+/− mice show increased circling behavior. One complete turn, regardless of direction is counted as one circling. d, e, In the elevated plus maze, Dlx1/2-Scn1a+/− mice enter less frequently in open arms (d), and spend significantly less time in open arms (e). f, In the social interaction test, Dlx1/2-Scn1a+/− mice display decreased interaction with social cue when compared to negative floxed littermate mice. g , In the 3-chamber test, Dlx1/2-Scn1a+/− mice have no preference for the stranger mouse. h, In the contextual fear conditioning test, Dlx1/2-Scn1a+/− mice display profound deficit in short-term (30 min) and long-term (24 hr) memory of 2 s mild foot shock (0.5 mA)-associated context, but normal fear response immediately after the foot shock during training (Train) when compared to WT mice. Dlx, Dlx1/2-Scn1a+/− mice. Flox, Cre-negative floxed Scn1a+/+ mice. E, Empty cage. C, Center. M, Mouse. All data shown are means ± s.e.m. from 7 – 9 mice per genotype. *P < 0.05; **P < 0.01; #, ***P < 0.001.
Deficit of NaV1.1 channels impairs cortical and hippocampal GABAergic interneuron neurotransmission
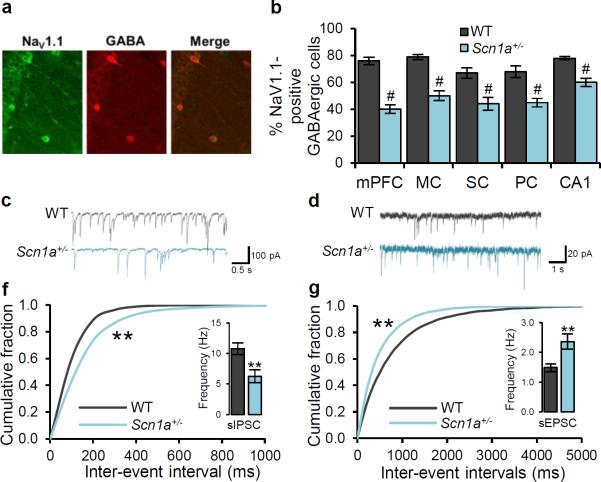
To test our hypothesis that the autism-related phenotypes and spatial learning deficits in Scn1a+/− mice are caused by decreased NaV1.1 activity in GABAergic interneurons in the forebrain, we compared the properties of cortical and hippocampal GABAergic interneurons in WT and Scn1a+/− mice. NaV1.1 protein is expressed in adult hippocampal and neocortical interneurons, as assessed by co-immunolabeling of NaV1.1 channels and GABA in the hippocampal CA1 region (Fig. 4a) and prefrontal cortex (Supplementary Fig. 15). The proportion of GABAergic interneurons expressing NaV1.1 in of Scn1a+/− mice was decreased 20–50% throughout the cortex and hippocampus (Fig. 4b), whereas there was no reduction in the total number of GABA-stained interneurons (Supplementary Fig. 16, legend). The deep layer of prefrontal cortex was the most affected by the Scn1a mutation (Fig. 4b), and the intensity of immunostaining for NaV1.1 in GABAergic cells with detectable staining was reduced 50% in the prefrontal cortex (Supplementary Fig. 16).
Figure 4. Deficit of NaV1.1 channels and GABAergic neurotransmission in Scn1a+/− hippocampal GABAergic interneurons.
Immunocytochemical staining of forebrain neurons from 10-month old mice for NaV1.1 channels. a, Co-immunolabeling of NaV1.1 and GABA reveals co-expression of NaV1.1 and GABA in the hippocampal CA1 region in WT mice. b, Co-immunolabeling of NaV1.1 and GABA reveals a decreased expression of NaV1.1 channels in GABAergic interneurons in various forebrain regions in Scn1a+/− mice. c, Example traces of sIPSC from WT and Scn1a+/− mice hippocampal CA1 region. d, Example traces of sEPSC from WT and Scn1a+/− mice hippocampal CA1 region. f, Cumulative plot and average values (inset) of sIPSC frequency. The frequency of sIPSC is decreased, but the amplitude of sIPSC is unchanged in Scn1a+/− hippocampal CA1 slices when compared to WT slices (Suppl. Fig. 17a). g, Cumulative plot and average values (inset) of sEPSC frequency. The frequency of sEPSC is increased, but the amplitude of sEPSC is unchanged in Scn1a+/− hippocampal CA1 slices when compared to WT slices (Suppl. Fig. 17b). mPFC, medial prefrontal cortex. MC, motor cortex. SC, sensory cortex. PC, parietal cortex. CA1, hippocampal CA1 region. All data shown are means ± s.e.m. from 15 – 19 recordings per genotype. #, **P < 0.01.
Some forms of autism are postulated to be caused by an imbalance of synaptic transmission between excitatory and inhibitory circuits26–29. Scn1a+/− mice have reduced Na+ currents and impaired action potential firing in hippocampal interneurons and cerebellar Purkinje neurons9,10, which are GABAergic neurons. When action potentials were blocked with tetrodotoxin (TTX, 1 μM), recordings of miniature inhibitory postsynaptic currents (IPSC) and miniature excitatory postsynaptic current (EPSC) from the hippocampal CA1 region and the prefrontal cortex showed that amplitude and frequency were not altered, indicating normal synaptic function in Scn1a+/− slices (Supplementary Fig. 17, 18). Similarly, in the absence of TTX, the amplitudes of spontaneous IPSCs and spontaneous EPSCs were unchanged (Fig. 4c, d, Supplementary Fig. 19, 20), indicating that the postsynaptic response to released neurotransmitter was not altered. In contrast, in the absence of TTX, the frequency of spontaneous IPSCs in hippocampal CA1 and prefrontal cortex slices from Scn1a+/− mice was reduced (Fig. 4c, f, Supplementary Fig. 20a, b), and the frequency of spontaneous EPSCs was increased (Fig. 4d, g, Supplementary Fig. 20c, d) compared to WT slices. Because no differences in frequencies of miniature IPSCs or EPSCs were observed when action potentials were blocked by TTX, these changes in frequencies of IPSCs and EPSCs recorded in the absence of TTX must represent differences in action potential-dependent neurotransmission. Therefore, these results indicate that inhibitory synaptic input was decreased because of reduced firing frequency of GABAergic interneurons caused by Scn1a haploinsufficiency, whereas excitatory synaptic activity was increased as an indirect consequence of decreased inhibition.
Treatment of autism-related phenotypes in Scn1a+/− mice with clonazepam
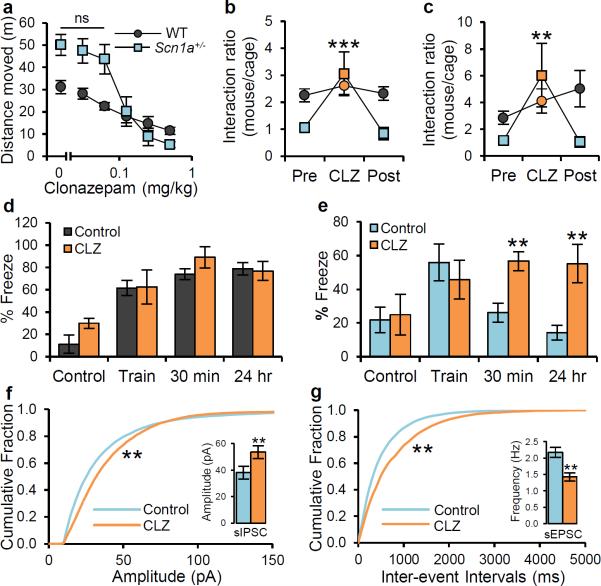
Given that the autism-related phenotype and spatial-learning deficit in Scn1a+/− mice emerge from decreased NaV1.1 activity in GABAergic interneurons, we reasoned that they could be rescued by increasing the strength of GABAergic transmission. To test this idea, we treated Scn1a+/− and WT mice with the benzodiazepine clonazepam, a positive allosteric modulator of the GABAA receptor. Benzodiazepines do not open the GABAA receptor chloride channel in the absence of GABA, but instead boost GABA signaling only when presynaptically released GABA binds to the receptor39. First, we examined the effects of clonazepam in the open-field and elevated plus-maze tests to avoid potential sedative effects in our behavioral experiments, which depend on locomotor activity. The maximal intraperitoneal dose of clonazepam that did not cause significant sedation or anxiolytic effect in the open field and elevated plus maze tests was 0.0625 mg/kg for Scn1a+/− mice (Fig. 5a; Supplementary Fig. 21), 20-fold lower than typical anxiolytic doses40. To test the effect of clonazepam on social behavior, we performed three sets of identical trials at one week intervals with same groups of mice. In the first trial, we performed the social interaction test in the open arena and the three-chamber test without any treatment. In a subsequent trial, the same behavioral tests were performed 30 min after i.p. injection of 0.0625 mg/kg clonazepam. In the last trial, the tests were performed 30 min after i.p. injection of vehicle. The data were analyzed as the ratio of the time of interaction with a stranger mouse over the time of interaction with an empty cage. Both in the open arena and in the three-chamber test, clonazepam treatment completely rescued impaired social behaviors of the Scn1a+/− mice, and this effect was reversed after the one-week clearing period (Fig. 5b, c; Supplementary Fig. 22, 23). In contrast, low-dose clonazepam had no effect on the social behavior of WT mice. Treatment with low-dose clonazepam 30 min before testing also rescued impaired context-dependent fear conditioning. Whereas WT mice were unaffected by clonazepam (Fig. 5d), Scn1a+/− mice displayed a complete reversal of the loss of their 30-min and 24-h contextual fear memory (Fig. 5e). These results indicate that a single low dose of clonazepam can reversibly rescue core autistic traits and cognitive deficit in Scn1a+/− mice.
Figure 5. Complete rescue of impaired social behavior and fear-associated memory deficits by low-dose clonazepam treatment.
a, Both WT and Scn1a+/− mice show dose-dependent sedation by CLZ. Maximal concentration of CLZ without sedative effect is 0.0625 mg/kg. b, c, In the social interaction test (b) and 3-chamber test (c), decreased social interaction in Scn1a+/− mice is completely recovered by a single i.p. injection of 0.0625 mg/kg CLZ, 30 min prior to the test; this CLZ effect on social interaction completely disappears after 1 week of clearance period in the same Scn1a+/− mice. CLZ effects on social interaction are absent in WT mice. d, e, In the contextual fear conditioning test, a single i.p. injection of 0.0625 mg/kg CLZ, 30 min prior to the training, leads to a complete rescue of short-term (30 min) and long-term (24 h) fear-associated contextual memory in Scn1a+/− mice (e), but no significant change of fear-associated contextual memory by CLZ is observed in WT mice (d). Pre, Pre-clonazepam treated. CLZ, Clonazepam. Post, Post-Clonazepam treated. All data shown are means ± s.e.m. from 6 – 12 mice per genotype. ns, not significant. f, Cumulative plot and average value (inset) of sIPSC amplitude. The treatment of 10 μM CLZ increases the amplitude of sIPSC, but the frequency of sIPSC is unchanged by 10 μM CLZ in Scn1a+/− hippocampal CA1 slices (Suppl. Fig. 24a). g, Cumulative plot and average value (inset) of sEPSC frequency. The treatment of 10 μM CLZ decreases the frequency of sEPSC, but the amplitude of sEPSC is unchanged by 10 μM CLZ in Scn1a+/− hippocampal CA1 slices (Suppl. Fig. 24b). All data shown are means ± s.e.m. from 15 – 20 recordings per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001.
We also tested the effects of clonazepam on GABAergic inhibitory transmission in the hippocampal CA1 region in Scn1a+/− mice. As expected, treatment with 10 μM clonazepam increased sIPSC amplitude, but not frequency, in Scn1a+/− hippocampal slices (Fig. 5f, Supplementary Fig. 24a). The increased amplitude of spontaneous IPSCs after treatment with 10 μM clonazepam leads to a decrease in frequency of spontaneous EPSCs, without change in amplitude in Scn1a+/− hippocampal slices (Fig. 5g, Supplementary Fig. 24b). These results support our hypothesis that behavioral rescue by treatment with clonazepam is associated with increased strength of inhibitory transmission.
Discussion
Despite their adverse impacts on quality of life, the neuropsychiatric comorbidities and cognitive deficit in DS have not previously been studied in an animal model, and the role of the NaV1.1 channel in these deficits in brain functions was unknown. Our results show that mice with heterozygous loss-of-function mutation in NaV1.1 channels display both cognitive deficits and autistic traits, including hyperactivity, anxiety, excessive stereotyped behaviors, and social interaction deficits. Together with previously reported phenotypes of epilepsy9, premature death9, thermally induced seizures11, ataxia10, and sleep dysfunction12, these studies show that Scn1a+/− mice phenocopy all the major symptoms of DS.
These cognitive and behavioral deficits in Scn1a+/− mice are caused by decreased action potential firing in forebrain GABAergic interneurons. Our previous studies show that deletion of NaV1.1 channels causes selective reduction in Na+ currents and action potential firing of GABAergic interneurons in hippocampus and cerebellum9,10. This deficit in action potential firing in interneurons in the hippocampus leads to a selective loss of inhibitory neurotransmission compared to excitatory transmission (Fig. 4). Moreover, Dlx1/2-Scn1a+/− mice, which have a specific deficit in NaV1.1 channels in forebrain GABAergic interneurons, reproduce the core autistic features and cognitive deficits (Fig. 3). These results indicate that the autism-related traits in DS mice are caused by decreased inhibitory neurotransmission in GABAergic interneurons as a consequence of Scn1a haploinsufficiency.
To further test this hypothesis, we treated Scn1a+/− with clonazepam, a benzodiazepine, in order to reverse decreased GABAergic tone. High-dose benzodiazepine has been widely used to alleviate epileptic seizure13 and anxiety-like behaviors40, but not for rescuing major autism-related behaviors because of its sedative effects. Remarkably, a single low-dose clonazepam injection completely rescued deficits in social interactions and fear-associated contextual memory without sedative or anxiolytic effects in Scn1a+/− mice. The reversible rescue of cognitive deficit and autism-related behaviors by clonazepam at the time of training implies that these comorbidities in DS mice are not caused by recurrent seizure-induced excitotoxicity, but instead are caused by Scn1a haploinsufficiency and the resulting reduction of GABAergic transmission. These results suggest that low-dose benzodiazepine treatment could be a potential pharmacological intervention for cognitive deficit and autistic symptoms in DS patients.
Genome-wide association studies identified the chromosome 2q24.3 region, where the SCN1A gene is located, as an autism susceptibility locus18,19. Sequencing of the genomes of autistic patients identified mutations of SCN1A gene in familial autism20. Exome sequencing revealed that de novo mutations in the SCN1A gene cause autism21. Our results suggest the hypothesis that DS should be included in the category of ASD-related syndromes, such as Fragile-X Syndrome, Rett Syndrome, and Timothy Syndrome41. With a prevalence of 1:20,000 births for DS and related SCN1A channelopathies42, DS is less frequent than Fragile-X Syndrome (1:5000) or Rett Syndrome (1:10,000) but much more common than Timothy Syndrome (<1:1,000,000). Interestingly, mutations in many ASD susceptibility genes also exhibit cytogenetic dysfunctions in GABAergic interneurons24–29,43,44. Thus, autistic traits in DS and in a broad range of ASDs are likely caused by a reduction of GABAergic signaling. Our results suggest that low-dose benzodiazepine treatment may be effective in alleviating these autistic traits and cognitive impairment in DS and possibly in ASDs more broadly.
METHODS SUMMARY
Animals
The mice used for all behavioral analyses were 6 – 8 month-old adult male mice except DLX1/2 conditional mutant mice which were 3 – 5 months old. Adult mice 10 months old were used for Immunohistochemical staining, and young mice 3 – 4 weeks old were used for electrophysiological recording. All behavioral tests were done blind to genotypes with age-matched littermate pairs of male mice. All experiments with animals were performed according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of Washington Institutional Animal Care and Use Committee.
Statistical analysis
All data are shown as mean ± s.e.m. and analyzed using Student's t-test, one-way ANOVA with Tukey's post hoc comparison, and two-way ANOVA with Bonferroni's post hoc comparison. All the statistical analyses were done using Prism 4 (GraphPad). Details of particular tests in each experiment are described in the supplementary methods section, and full statistical tests and values for behavioral data are presented in Supplementary Table 1.
Supplementary Material
Acknowledgements
This work was supported by Research Grants R01 NS25704 (W. A. C.), R01 MH075016 (H. O. D), and R37 MH049428 (J. L.R.) from the National Institutes of Health and by a grant from the McKnight Foundation (W. A. C.). Authors thank Mr. Eric Strakbein in the Machine Division at the University of Washington for making all the mazes for the behavioral experiments in this study.
Footnotes
Author Contributions. W.A.C. and H.O.D. are co-senior authors. S.H., C.T., R.E.W., C.S.C., T.S., H.O.D., and W.A.C. designed the experiments. S.H., C.T., R.E.W., C.S.C., and T.S. performed the experiments. F.H.Y., C.S.C., G.B.P., and J.L.R., and W.A.C. designed, prepared, and characterized the genetically modified mouse lines. S.H., C.T., J.L.R., H.O.D., and W.A.C. wrote and revised the manuscript.
References
- 1.Wolff M, Casse-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl 2):45–48. doi: 10.1111/j.1528-1167.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 2.Genton P, Velizarova R, Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011;52(Suppl 2):44–49. doi: 10.1111/j.1528-1167.2011.03001.x. [DOI] [PubMed] [Google Scholar]
- 3.Li BM, et al. Autism in Dravet syndrome: prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy Behav. 2011;21:291–295. doi: 10.1016/j.yebeh.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 4.Brunklaus A, Dorris L, Zuberi SM. Comorbidities and predictors of health-related quality of life in Dravet syndrome. Epilepsia. 2011;52:1476–1482. doi: 10.1111/j.1528-1167.2011.03129.x. [DOI] [PubMed] [Google Scholar]
- 5.Besag FM. Behavioral aspects of pediatric epilepsy syndromes. Epilepsy Behav. 2004;5(Suppl 1):S3–13. doi: 10.1016/j.yebeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney K, et al. Variable neurologic phenotype in a GEFS+ family with a novel mutation in SCN1A. Seizure. 2009;18:492–497. doi: 10.1016/j.seizure.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, et al. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 9.Yu FH, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 10.Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SY, Schwartz FH, Linton MD, Bosma JD, Hurley MM, Catterall JB, de la Iglesia WA, H.O. NaV1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and normal circadian rhythms. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1115729109. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588:1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 15.Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- 16.Ogiwara I, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter GB, et al. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167–186. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pescucci C, et al. 2q24-q31 deletion: report of a case and review of the literature. Eur J Med Genet. 2007;50:21–32. doi: 10.1016/j.ejmg.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD. An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1152–1158. doi: 10.1002/ajmg.b.30739. [DOI] [PubMed] [Google Scholar]
- 20.Weiss LA, et al. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Mol Psychiatry. 2003;8:186–194. doi: 10.1038/sj.mp.4001241. [DOI] [PubMed] [Google Scholar]
- 21.O'Roak BJ, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012 doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao HT, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X Syndrome: The GABAergic System and Circuit Dysfunction. Dev Neurosci. 2011 doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penagarikano O, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31:247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markram K, Markram H. The intense world theory - a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 31.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockhorst U, Pietrowsky R. Olfactory perception, communication, and the nose-to-brain pathway. Physiol Behav. 2004;83:3–11. doi: 10.1016/j.physbeh.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, et al. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomot M, et al. Change detection in children with autism: an auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheah CS, et al. Conditional deletion of NaV1.1 channels in inhibitory interneurons is sufficient to cause the seizures and premature death in a mouse model of SMEI. Neuroscience Abstracts 255.216. 2010 [Google Scholar]
- 39.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low K, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 41.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yakoub M, Dulac O, Jambaque I, Chiron C, Plouin P. Early diagnosis of severe myoclonic epilepsy in infancy. Brain Dev. 1992;14:299–303. doi: 10.1016/s0387-7604(12)80147-1. [DOI] [PubMed] [Google Scholar]
- 43.Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011:649325. doi: 10.1155/2011/649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile X syndrome. J Neurophysiol. 2011;106:2264–2272. doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.